Abstract
Purpose
To determine whether CAIPIRINHA-Dixon-TWIST (CDT) volume-interpolated breath-hold examination (VIBE) improves image quality by reducing gadoxetate-disodium-associated transient arterial-phase motion artefacts in magnetic resonance imaging (MRI) of the liver.
Materials and methods
MRI studies of the liver from 270 patients who had received gadoxetate disodium were retrospectively evaluated in regard to arterial timing accuracy and arterial phase motion artefact severity (VIBE: 90/270, CAIPIRINHA-VIBE: 90/270 and CDT-VIBE: 90/270 cases). Three independent and blinded readers assessed arterial phase timing and motion artefact severity (5-point scale). Interrater agreement was calculated by weighted kappa. Continuous variables were compared via a two-sided ANOVA, categorical variables via a χ2 test. An ordinal regression analysis was performed to identify other predictors of motion artefacts.
Results
CDT-VIBE improved correct late arterial timing rates and reduced motion-related image deterioration rates. Successful late arterial liver visualisation was achieved in 56.7% (VIBE) compared with 66.7% (CAIPIRINHA-VIBE) and 84.4% (CDT-VIBE) (P < 0.0001). Good/excellent image quality was achieved in 56.7% vs. 66.7% and 73.3%, respectively (P = 0.03). Male sex negatively influenced image quality (P = 0.03).
Conclusion
CDT-VIBE increases the diagnostic utility of gadoxetate disodium-based liver MRI by reducing respiratory motion artefacts and optimising late arterial visualisation compared with VIBE and CAIPIRINHA-VIBE.
Key Points
• CAIPIRINHA-Dixon-TWIST-VIBE-MRI (CDT) mitigates effects of acute transient dyspnoea caused by gadoxetate disodium.
• CDT improves late arterial imaging compared with VIBE and CAIPIRINHA-VIBE.
• The rate of ideal late arterial images is higher with CDT-VIBE vs. VIBE or CAIPI-VIBE.
• The impact of respiratory motion artefacts on arterial phase images can be reduced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently gadoxetate disodium (Eovist® in North America, Primovist® in Europe; BayerSchering Pharma AG, Berlin, Germany)—an intravenously administered hepatobiliary contrast agent for magnetic resonance imaging (MRI) of the liver—has been associated with acute transient dyspnoea, which is a temporary, self-limiting phenomenon lasting for 10-20 s [1]. It is widely used for both standard dynamic images and late hepatobiliary phase images. Despite additional information provided in the hepatobiliary phase, arterial phase enhancement still remains essential for the detection and characterisation of liver lesions [1, 2]. Especially in patients with liver cirrhosis, arterial hyperenhancement is still a hallmark and a surrogate in the diagnosis of hepatocellular carcinoma [3,4,5,6,7]. Therefore the correct timing of the so-called late arterial phase can be crucial for the correct diagnosis [8].
Davenport et al. reported that the rate of transient severe motion (TSM) was significantly higher with gadoxetate disodium than with gadobenate dimeglumine (Multihance; Bracco, Milan, Italy). Hence, significantly more arterial phase images were degraded by respiratory motion-related artefacts than with gadobenate dimeglumine. Another study by the same group supported these results 1 year later in matched patients and suggested that results are not solely explicable by selection bias [9]. These results were further confirmed by several authors with the conclusion that the described artefacts are associated with breath-hold failure [10,11,12].
Manufacturers are continuously working on development and optimisation of their MR sequences with emphasis on the acquisition time and signal-to-noise ratio. Controlled Aliasing In Parallel Imaging Results In Higher Acceleration Factor (CAIPIRINHA) allows for reduction of acquisition times while still maintaining adequate spatial resolution [13]. The further developed CAIPIRINHA-Dixon Time-resolved angiography With Interleaved Stochastic Trajectories (TWIST, [14]) (CDT, [15]) volume-interpolated breath-hold examination (VIBE [16]) is promoted as a high-end sequence for abdominal imaging [15, 17]. This 3D gradient-echo sequence with Dixon fat-suppression technique and view-sharing combines incomplete interpolated k-space filling with a parallel imaging technique to achieve a fourfold acceleration factor [18] and allows acquiring multiple data sets during one breath-hold. Therefore, in contrast-enhanced dynamic liver imaging, it can be ascertained that at least one data set is available with ideal arterial enhancement [19].
In theory, CDT-VIBE, as a multiple arterial phase imaging technique [20], could provide at least one image set with reduced or limited motion artefacts and ideally with late arterial opacification. The purpose of this study was to determine whether the use of CDT-VIBE in imaging the liver with gadoxetate disodium provides improved late arterial phase imaging with adequate image quality by reducing the effect of transient motion artefacts. Therefore CDT-VIBE was compared with clinically approved single (VIBE) and double (CAIPIRINHA-VIBE) arterial phase imaging techniques regarding phase timing and image quality.
Materials and methods
Study population
Institutional review board approval was obtained with patient consent waived for this retrospective study (Ethikkommission, Medical University Innsbruck).
For this retrospective study, a keyword search of the radiology reports in the medical record database at our centre (Department of Radiology, Medical University Innsbruck) was performed to identify patients who met the following inclusion criteria: (1) dynamic gadoxetate disodium enhanced MRI of the liver on a 3.0-T scanner and (2) successful, intravenous administration of gadoxetate disodium. No age limit was applied. In addition, the MRI protocol had to be either a single arterial phase VIBE, a double arterial phase CAIPIRINHA-VIBE or a multiple arterial phase CDT; for each sequence type the patient number was limited to 90.
Two hundred seventy MR imaging studies between March 2011 and April 2016 could be identified and were included. Demographic data on age, gender and cause of referral (cirrhosis, liver metastasis, other, unknown) were collected.
MR imaging
All studies were performed on a 3.0-T MR system (MAGENTOM Skyra; Siemens Healthcare, Erlangen, Germany) using an 18-element body matrix coil and a 32-element spine coil. Patients were examined in supine position. All images were acquired by predefined institutional protocols for contrast-enhanced liver imaging in which three types of dynamic axial imaging sequences were used: single arterial VIBE (n = 90), double arterial phase CAIPIRINHA-VIBE (n = 90) and multiple arterial phase CDT-VIBE (n = 90). The respective sequence parameters are summarised in Table 1. All patients also received a T2-weighted Half Fourier Acquisition Single-shot Turbo Spin Echo (HASTE) sequence of the liver before administration of the contrast agent (see Table 1 for details). This sequence was used for further morphological assessment described below. After one unenhanced imaging sequence, patients received 10 ml of gadoxetate disodium by manual injection through a peripheral intravenous line at the cubita or forearm at an approximate injection rate of 1.5-2.0 ml/s followed by a 20-ml saline flush. In all patients dynamic imaging was started after a predefined delay of 15 s after the start of the contrast agent injection to acquire an optimally timed late arterial phase, defined as having “strong hepatic arterial, substantial portal venous, slight parenchymal, and no hepatic venous enhancement” [21]. Portal venous phase and later venous phase images were acquired at 45 s and 120 s after contrast injection; late phase imaging was performed 30-45 min after contrast injection. Late phase images were, however, not evaluated for this study.
Image interpretation
Three radiologists with 8 (reader 1), 6 (reader 2) and 2 (reader 3) years of experience in the field of abdominal MRI performed independent readings in an anonymised fashion and blinded to the cause of referral, final diagnosis and other patient information. Reading was performed on an Agfa Impax EE (Agfa Healthcare; Mortsel, Belgium) workstation. All readers were aware of the difference between true motion-related artefacts and rapid bolus-related ring artefacts [10].
Each reader rated each imaging study (unenhanced T2w HASTE and unenhanced VIBE, respectively) regarding the presence of morphological signs of liver cirrhosis, presence and severity of ascites (none/moderate/severe) and pleural effusion (none/moderate/severe). For CAIPI-VIBE and CDT-VIBE studies the best sequence phase was then chosen regarding the lowest degree of motion artefacts and optimal arterial timing. This was done in consensus among all three readers to ensure that for further evaluation every reader evaluated the same arterial phase.
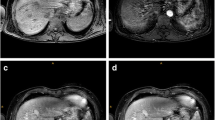
Arterial timing phases were independently graded by each reader as aortic (aortic, yet no intrahepatic arterial enhancement), early arterial (enhancement of hepatic artery, but not of the portal or hepatic veins), late arterial (enhancement of hepatic artery and portal vein branches, yet not of the hepatic veins) and venous (enhancement of hepatic veins) (according to [8]). Examples are given in Fig. 1. Finally, each reader rated the overall quality of the chosen sequence in regard to motion artefacts on a scale of 1 (no motion artefacts, optimal image quality), 2 (virtually no motion artefacts, good image quality), 3 (moderate motion artefacts, but still usable), 4 (severe motion artefacts, barely usable) and 5 (heavy motion artefacts, images not usable for diagnostic purposes) (see also exemplary Fig. 2).
Illustration of liver contrast enhancement phases: aortal (VIBE) with no attenuation of the hepatic artery, portal vein or hepatic veins (a), early arterial (CDT-VIBE) with sole enhancement of the hepatic artery (b), late arterial (CDT-VIBE) with enhancement of the hepatic artery and portal vein (c) and venous (CDT-VIBE) with enhancement of attenuation of the hepatic veins and signal increase of the liver parenchyma (d)
Illustration of the five distinct respiratory motion grades: (a) CDT-VIBE in a 65-year-old female patient after liver transplantation with no noticeable artefacts (grade 1), (b) VIBE in a 52-year-old male patient referred because of an unclear elevation of liver enzymes 7 years after a Whipple procedure with very subtle breathing artefacts—magnification (white outline) is provided in image (c) (grade 2), (d) CAIPI-VIBE in a 69-year-old male patient evaluated for the presence of a hepatocellular carcinoma (HCC) with moderate breathing artefacts (grade 3), (e) VIBE in a 72-year-old male patient with known liver cirrhosis referred to exclude an HCC with severe breathing artefacts substantially decreasing readability (grade 4) and f) CDT-VIBE of a 64-year-old female patient referred for the assessment of an unknown liver lesion with non-diagnostic scans due to severe breathing artefacts (grade 5)
Statistical analysis
All data were analysed in GraphPad Prism Pro 6.05 (GraphPad Software Inc., La Jolla, CA, USA) or SPSS version 23.0 (SPSS Inc., Chicago, IL, USA). P values < 0.05 were considered significant and 95% confidence intervals are given where appropriate.
Interrater agreement concerning the timing of the arterial phase and image quality is given as weighted kappa between all readers; results were rated according to [22].
Relative and absolute frequencies of arterial phases were calculated. Mean values for image quality for VIBE, CAIPIRINHA and CDT were compared via a two-sided ANOVA with Dunnett’s correction for multiple testing. Contingency tables were analysed via χ2 tests.
To determine the extent of gadoxetate disodium-associated breathing artefacts in patients with liver cirrhosis, the analysis was repeated for the subgroup of patients with imaging findings in line with manifest liver cirrhosis (defined by a fibronodular appearance of the liver).
An ordinal regression analysis was carried out to determine the influence of the presence of liver cirrhosis, ascites, pleural effusion as well as age and sex on the final classification of the image quality. Multicollinearity between predictors was excluded by means of the variance inflation factor and tolerance value. Results are given as B-value, 95% confidence intervals (95% CI) and P values.
Results
Study population
Table 2 provides an overview of the three patient groups imaged with VIBE, CAIPI-VIBE and CDT-VIBE. Mean patient age was 56.3 ± 16.3 years; 51.4% of patients were male. There was no significant age difference between groups: mean patient age was 55.0 ± 16.6 years for VIBE, 58.2 ± 15.0 years for CAIPI-VIBE (P = 0.30) and 57.2 ± 15.5 years for CDT-VIBE (P = 0.53, both compared with VIBE). Furthermore, there was no significant difference in regard to gender distribution (P = 0.84), cause of referral (P = 0.37), rates of liver cirrhosis (P = 0.22) or ascites (P = 0.17) among the three groups. Mild pleural effusion was less common in the CAIPI-VIBE group compared with the other two groups (P = 0.03).
Among cirrhotic patients (Table 3), the mean age was 60.4 ± 14.4 years; 69.2% of patients were male. There was no significant difference in age (P = 0.89 for CAIPI-VIBE/0.99 for CDT-VIBE compared with VIBE, respectively), rate of male gender (P = 0.35), referral cause (P = 0.78), ascites (P = 0.75) or pleural effusion (P = 0.31).
Interrater agreement
Arterial phase timing interrater agreement was ‘very good’ between reader 1 and 2 (weighted kappa = 0.979), between reader 1 and 3 (weighted kappa = 0.990) and between reader 2 and 3 (weighted kappa = 0.989).
Motion score interrater agreement was slightly lower between reader 1 and 2 (weighted kappa = 0.815, ‘very good’), between reader 1 and 3 (weighted kappa = 0.774, ‘good’) and between reader 2 and 3 (weighted kappa = 0.780, ‘good’).
Arterial phase timing and motion scores
Ideal arterial timing, i.e. late arterial, was achieved in 56.7% with VIBE, in 66.7% with CAIPI-VIBE and in 84.4% with CDT-VIBE (P < 0.0001, Fig. 3a). While 18.9% of imaging studies were either non-diagnostic (grade 5) or severely affected by motion artefacts (grade 4) with VIBE, this rate was 7.8% for CAIPI-VIBE and 6.7% for CDT-VIBE (Fig. 3b). Accordingly, the rate of excellent (grade 1) or good imaging studies (grade 2) was 56.7% for VIBE, 66.7% for CAIPI-VIBE and 73.3% for CDT-VIBE (P = 0.0298).
Regarding consensus values of all three readers, relative arterial phase timings (aortic/early arterial/late arterial/venous) were 0%/28.9%/56.7%/14.4% for VIBE, 0%/30.0%/66.7%/3.3% for CAIPI-VIBE and 0%/13.3%/84.4%/2.2% for CDT-VIBE (P < 0.0001, Fig. 3a).
There was a significant difference regarding motion scores: Average quality values were 2.70 ± 1.10 for VIBE, 2.39 ± 0.70 for CAIPI-VIBE (P = 0.034 vs. VIBE) and 2.29 ± 0.75 for CDT-VIBE (P = 0.012) when calculating mean values for all three readers (lower is better). This also applied to consensus values with 2.71 ± 1.11 for VIBE, 2.37 ± 0.73 for CAIPI-VIBE (P = 0.026) and 2.26 ± 0.78 for CDT-VIBE (P = 0.007).
Ordinal regression analysis with image quality as the outcome revealed there was a non-significant trend toward lower image quality grades in the presence of liver cirrhosis (B = 0.388, 95% CI -0.215–0.991, P = 0.207) and pleural effusion (B = 0.784, 95% CI -0.147–1.714, P = 0.099), yet no influence of moderate (B = 0.623, 95% CI -0.331–1.578, P = 0.200) or severe ascites (B = 0.070, 95% CI -0.832–0.973, P = 0.878) or age (B = 0.07, 95% CI -0.008–0.022, P = 0.329). Male sex on the other hand was associated with significantly decreased image quality grades (B = 0.532, 95% CI 1.012–0.051, P = 0.03).
While CAIPI-VIBE (B = -0.279, 95% CI -0.849–0.290, P = 0.336) did not increase image quality grades compared with VIBE, CDT-VIBE was associated with improved motion scores (B = -0.674, 95% CI -1.241–-0.108, P = 0.02).
Subgroup analysis in cirrhotic patients
There was a significant increase of successful late arterial phase visualisation rates (Fig. 4a) from 37.5% (VIBE) to 54.5% (CAIPI-VIBE) and 79.2% (CDT-VIBE) in patients with a cirrhotic liver (P < 0.0001). This effect was even more pronounced than for the overall population because of the comparably low rate of late arterial phases successfully imaged with VIBE in the cirrhotic population (37.5% vs. 56.7%). Relative arterial phase timings (aortic/early arterial/late arterial/venous) were 0%/40.7%/37.5%/21.9% for VIBE, 0%/36.4%/54.5%/9.1% for CAIPI-VIBE and 0%/20.8%/79.2%/0% for CDT-VIBE (P < 0.0001, Fig. 4a).
The overall consensus quality was improved from 2.91 ± 1.09 for VIBE to 2.50 ± 0.80 for CAIPI-VIBE (P = 0.14) and 2.33 ± 0.96 (P = 0.06; both compared with VIBE). The rate of excellent and good studies was 40.7% (VIBE) compared with 68.2% (CAIPI-VIBE) and 58.3% (CDT-VIBE), while the corresponding rate of non-diagnostic or barely usable studies was 28.1%, 18.2% and 12.5% (VIBE, CAIPI-VIBE and CDT-VIBE, respectively) (Fig. 4b).
Discussion
Our results demonstrate that CDT-VIBE, a sequence primarily aimed at optimising phase timing with the ability to capture multiple (arterial) phases during one breath-hold [20], can reduce the impact of transient respiratory motion artefacts on arterial phase image quality compared with other evaluated sequences. As assessed by three independent readers, an increase in image quality and optimal timing rate of the late arterial phase was observed. Further, more imaging studies were rated as good or excellent in respect to readability.
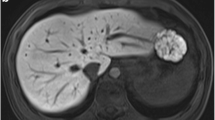
In recent years, research into transient arterial phase dyspnoea after administration of gadoxetate disodium has increased. The most frequent cause of image degradation has been attributed to breath-hold failure after a feeling of dyspnoea [10] with some authors suggesting to focus on informing patients before the examination [9]. This may not be successful in all patients, though, especially if pre-existing conditions with dyspnoea or reduced compliance are present. Another successful approach encompassed the pre-dilution of gadoxetate disodium [23, 24]. By acquiring multiple closely timed images through reducing breath-hold times [25] and a rapid (abdominal) imaging sequence such as CAIPIRINHA-VIBE or CDT-VIBE (see also exemplary Fig. 5), on the other hand, the chance to acquire at least one usable arterial phase sequence should also increase [24, 26].
Five-phase single breath-hold CAIPIRINHA Dixon-TWIST VIBE acquisitions (a–e) in a 70-year-old male patient referred for a follow-up examination after liver transplantation to illustrate temporal resolution. Ideal late arterial-phase depiction (black arrowhead: hepatic artery; white arrowhead: portal vein) is achieved in (b) with no noticeable respiratory-associated motion artefacts (grade 1). Magnification is provided in image (c). The diffuse left liver border is due to partial volume effects and peristaltic movement of the adjacent stomach
Comparable to previous studies, a significant rate of series ranking high regarding motion artefact image degradation using a single-phase VIBE imaging protocol was observed: Roughly 19% of scans were affected severely (grade 4) or were unusable (grade 5). This is in accordance with Davenport et al., who reported 17 out of 99 scans (18%) severely affected after administration of gadoxetate disodium when not using an optimised sequence [1, 9]. Similar results were reported by Haradome et al. even after employing a triggered imaging protocol (18.5%) [27]. Motosugi et al. reported a lower rate of image degradation by motion artefacts of 7.7% [10], while another study found 40.9% of studies affected to some degree by motion artefacts [28]. Especially patients suffering from liver cirrhosis may have a lower rate of successful late arterial phase imaging, as was seen in our study at 37.5% (compared with 56.7% for the overall study population).
A recent retrospective study by Li et al. in 28 patients with focal liver lesions imaged using a five-arterial phase TWIST-VIBE protocol after administration of gadoxetic acid demonstrated a degradation of motion scores over increasing phases with a ‘late middle arterial phase’ (21.8 s after administration) being affected the most [29]. This effect was significantly less pronounced—yet still existent—in unenhanced scans or patients who received gadopentetic acid.
Obviously, some means to counter gadoxetate disodium-associated image degradation would be of benefit. Pietryga et al. reported that by using triple-phase arterial CAIPIRINHA-accelerated imaging at least one arterial phase acquisition was usable in 30 out 37 cases (81%) [28]. Our results seem to confirm the idea that an increase in temporal resolution using multiple acquisitions can yield optimal phase timing [26], even when using a fixed delay. The increase of imaging studies scored as excellent or good acquired with CAIPI-VIBE or CDT-VIBE was substantial compared with a single-phase VIBE protocol.
This has clear clinical implications, as sufficient arterial phase imaging is of utmost importance to characterise liver lesions, especially in cirrhotic patients [4, 30]. Our results substantiate the potential benefit of using multiple-acquisition sequences in a cirrhotic subpopulation, as the average quality score was higher with CAIPI-VIBE and CDT-VIBE and the rate of non-diagnostic or barely usable scans was substantially reduced. As computed tomography (CT) and MRI suffer from a reduction in sensitivity in HCCs smaller than 2 cm [31], any further reduction of imaging reliability such as motion-related image degradation should be avoided. Arterial hyperenhancement is considered a hallmark in the diagnosis of hepatocellular carcinoma [32,33,34,35] and reliable detection of contrast enhancement dynamics is of utmost importance especially in small lesions.
Every patient in our study underwent a standardised imaging protocol and received the same dose of 10 ml gadoxetate disodium with the injection start beginning 15 s before the first arterial phase acquisition, similar to Pietryga et al. [28]. Previous studies have not always used a standardised dose and applied up to 20 ml of gadoxetate disodium—through either fixed or body weight adaptation [9, 36]. On this basis, the claim was made that there was no relevant influence of the dosing regimen on the artefact rate [9], although more thorough pharmacokinetic modelling would probably be required to assess the effects of the applied dose and application rate.
Our study did not focus on the diagnostic performance of the CDT sequence. We are aware that there may be disadvantages of the CDT-VIBE concerning the image quality and therefore on lesion conspicuity. Furthermore, we used different fat-saturation techniques for our sequences. VIBE and CAIPI-VIBE were acquired with chemical shift selective (CHESS) fat-suppression and CDT with the Dixon method. Our study did not consider the quality of fat saturation, although suppression of the fat signal is more uniform and less affected by artefacts when using the Dixon method with only slightly longer acquisition times [37]. Nevertheless, the advantages of CDT-VIBE have already been addressed in other studies and an evaluation of its diagnostic performance would go beyond the goal of this study [20].
Limitations
This study was retrospective without any randomisation. As our department’s routine protocols for liver imaging were changed from VIBE to CAIPI-VIBE and then to CDT-VIBE over time, technicians might have developed an increased awareness of the occurrence of artefacts even though patients were not routinely instructed on the potential feeling of dyspnoea. The selection bias should be low among all groups as all patients were specifically referred for liver imaging and subgroup analysis revealed no significant differences. In some instances, due to potential overlap between ring and motion artefacts, motion artefact scores might have been overestimated. Unfortunately, data on some pre-existing conditions such as pulmonary diseases were not collected. Interestingly, there was no correlation (as assessed by multicollinearity) between predictors such as ascites and cirrhosis in our population, probably due to heterogeneous underlying causes.
In contrast to other studies, which mostly used power injectors [1, 9, 28], contrast injection was performed manually, with a fixed off-label dose of 10 ml, as the gadoxetate disodium injection syringes do not fit our institution’s injector system and contrast agent exchange might be problematic in regard to hygiene and time efficiency. Potentially, a variability of injection rates might have been introduced. To counter this, technicians are routinely instructed on the exact injection rates. Furthermore, manual injection may differ from power injection regarding the immediacy of the saline flush, yet all patients underwent the same injection procedure, thus guaranteeing comparability between cohorts. The fixed off-label dose was used by many other groups as also mentioned above [9, 12].We did not use bolus tracking, which might further improve late arterial timing yield, especially in sequences with lower temporal resolution such as VIBE. Additionally, further measures to prevent breath-hold failure in the first place such as contrast agent dilution [23, 38]—which we have since incorporated into our institution’s standardised protocol— are certainly of importance, yet not within the focus of this manuscript.
In conclusion, the use of a multiple arterial phase imaging technique such as CDT-VIBE increases the likelihood to acquire satisfactory late arterial imaging studies and reduces the impact of transient arterial phase respiratory motion-related artefact on imaging quality. This can consequently increase the diagnostic utility of MRI in patients undergoing liver MRI with gadoxetate disodium.
Abbreviations
- CAIPIRINHA:
-
Controlled Aliasing In Parallel Imaging Results In Higher Acceleration Factor
- TWIST:
-
Time-resolved Angiography With Interleaved Stochastic Trajectories
- CDT:
-
CAIPIRINHA-Dixon TWIST
- VIBE:
-
Volume-interpolated Breath-hold Examination
- HASTE:
-
Half Fourier Acquisition Single-shot Turbo Spin Echo
- MRI:
-
Magnetic Resonance Imaging
- CT:
-
Computed Tomography
- TSM:
-
Transient Severe Motion
- T2w:
-
T2 Weighted
- HCC:
-
Hepatocellular Carcinoma
- PACS:
-
Picture Archiving And Communication System
References
Davenport MS, Viglianti BL, Al-Hawary MM et al (2013) Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology 266:452–461
Frericks BB, Loddenkemper C, Huppertz A et al (2009) Qualitative and quantitative evaluation of hepatocellular carcinoma and cirrhotic liver enhancement using Gd-EOB-DTPA. Am J Roentgenol 193:1053–1060
Darnell A, Forner A, Rimola J et al (2015) Liver Imaging Reporting and Data System with MR Imaging: Evaluation in Nodules 20 mm or Smaller Detected in Cirrhosis at Screening US. Radiology 275:141132
Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: An update. Hepatology 53(3):1020–1022
Yu JS, Kim KW, Kim EK, Lee JT, Yoo HS (1999) Contrast enhancement of small hepatocellular carcinoma: usefulness of three successive early image acquisitions during multiphase dynamic MR imaging. Am J Roentgenol 173:597–604
Chanyaputhipong J, Low S-CA, Chow PKH (2011) Gadoxetate Acid-Enhanced MR Imaging for HCC: A Review for Clinicians. Int J Hepatol 2011:1–13
Khosa F, Khan AN, Eisenberg RL (2011) Hypervascular liver lesions on MRI. Am J Roentgenol 197:204–220
Choi J-Y, Lee J, Sirlin CB (2014) CT and MR Imaging diagnosis and staging of hepatocellular carcinoma: Part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology 272:635–654
Davenport MS, Caoili EM, Kaza RK, Hussain HK (2014) Matched within-patient cohort study of transient arterial phase respiratory motion-related artifact in MR imaging of the liver: gadoxetate disodium versus gadobenate dimeglumine. Radiology 272:123–131
Motosugi U, Bannas P, Bookwalter CA, Sano K, Reeder SB (2016) An investigation of transient severe motion related to gadoxetic acid–enhanced MR Imaging. Radiology 279:93–102
McClellan TR, Motosugi U, Middleton MS et al (2017) Intravenous gadoxetate disodium administration reduces breath-holding capacity in the hepatic arterial phase: a multi-center randomized placebo-controlled trial. Radiology 282:361–368
Davenport MS, Malyarenko DI, Pang Y, Hussain HK, Chenevert TL. (2016) Effect of gadoxetate disodium on arterial phase respiratory waveforms using a quantitative fast Fourier transformatio-based analysis. AJR Am J Roentgenol 208(2):328–336
Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM (2005) Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn Reson Med 53:684–691
Hennig J, Scheffler K, Laubenberger J, Strecker R (1997) Time-resolved projection angiography after bolus injection of contrast agent. Magn Reson Med 37:341–345
Le Y, Kroeker R, Kipfer HD, Lin C (2012) Development and evaluation of TWIST Dixon for dynamic contrast-enhanced (DCE) MRI with improved acquisition efficiency and fat suppression. J Magn Reson Imaging 36:483–491
Rofsky NM, Lee VS, Laub G et al (1999) Abdominal MR imaging with a volumetric interpolated breath-hold examination. Radiology 212:876–884
Michaely HJ, Morelli JN, Budjan J et al (2013) CAIPIRINHA-Dixon-TWIST (CDT)–Volume-Interpolated Breath-Hold Examination (VIBE). Investig Radiol 48:590–597
Budjan J, Schoenberg SO, Riffel P (2016) Fast abdominal magnetic resonance Imaging. RöFo: Fortschr Auf Gebiete Rontgenstrahlen Nuklearmedizin 188:551–558
Budjan J, Ong M, Riffel P et al (2014) CAIPIRINHA-Dixon-TWIST (CDT)-volume-interpolated breath-hold examination (VIBE) for dynamic liver imaging: Comparison of gadoterate meglumine, gadobutrol and gadoxetic acid. Eur J Radiol 83:2007–2012
Kazmierczak PM, Theisen D, Thierfelder KM et al (2015) Improved detection of hypervascular liver lesions with CAIPIRINHA-Dixon-TWIST-volume-interpolated breath-hold examination. Investig Radiol 50:153–160
Hussain HK, Londy FJ, Francis IR et al (2003) Hepatic arterial phase MR imaging with automated bolus-detection three-dimensional fast gradient-recalled-echo sequence: comparison with test-bolus method. Radiology 226:558–566
Altman DG (1990) Practical statistics for medical research. Chapman and Hall, London
Kim YK, Lin W-C, Sung K et al (2017) Reducing artifacts during arterial phase of gadoxetate disodium-enhanced MR imaging: dilution method versus reduced injection rate. Radiology 283(2):429–437
Huh J, Kim SY, Yeh BM et al (2015) Troubleshooting arterial-phase MR images of gadoxetate disodium-enhanced liver. Korean J Radiol 16:1207–1215
Park CM, Park YS, Yoo JL et al (2016) The short breath-hold technique, controlled aliasing in parallel imaging results in higher acceleration, can be the first step to overcoming a degraded hepatic arterial phase in liver magnetic resonance imaging: A Prospective Randomized Control Study. Investig Radiol 51:440–446
Yoon JH, Lee JM, Yu MH, Kim EJ, Han JK (2016) Triple arterial phase MR imaging with gadoxetic acid using a combination of contrast enhanced time robust angiography, keyhole, and viewsharing techniques and two-dimensional parallel imaging in comparison with conventional single arterial phase. Korean J Radiol 17:522–532
Haradome H, Grazioli L, Tsunoo M et al (2010) Can MR fluoroscopic triggering technique and slow rate injection provide appropriate arterial phase images with reducing artifacts on gadoxetic acid-DTPA (Gd-EOB-DTPA) -enhanced hepatic MR imaging? J Magn Reson Imaging 32:334–340
Pietryga JA, Burke LMB, Marin D, Jaffe TA, Bashir MR (2014) Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium: examination recovery with a multiple arterial phase acquisition. Radiology 271:426–434
Li H, Xiao Y, Wang S et al (2017) TWIST-VIBE five-arterial-phase technology decreases transient severe motion after bolus injection of Gd-EOB-DTPA. Clin Radiol 72:800.e1–800.e6
Seale MK, Catalano OA, Saini S, Hahn PF, Sahani DV (2009) Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics 29:1725–1748
Choi J-Y, Lee J, Sirlin CB (2014) CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology 273:30–50
Purysko AS, Remer EM, Coppa CP, Leão Filho HM, Thupili CR, Veniero JC (2012) LI-RADS: a case-based review of the new categorization of liver findings in patients with end-stage liver disease. Radiographics 32:1977–1995
An C, Rakhmonova G, Choi J-Y, Kim M-J (2016) Liver imaging reporting and data system (LI-RADS) version 2014: understanding and application of the diagnostic algorithm. Clin Mol Hepatol 22:296–307
Mitchell DG, Bruix J, Sherman M, Sirlin CB (2015) LI-RADS (Liver Imaging Reporting and Data System): Summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology 61:1056–1065
Chen N, Motosugi U, Morisaka H et al (2015) Added value of a gadoxetic acid-enhanced hepatocyte-phase image to the LI-RADS system for diagnosing hepatocellular carcinoma. Magn Reson Med Sci 15:49–59
Ding Y, Rao SX, Chen CZ, Li RC, Zeng MS (2015) Usefulness of two-point Dixon fat-water separation technique in gadoxetic acid-enhanced liver magnetic resonance imaging. World J Gastroenterol 21:5017–5022
Rosenkrantz AB, Mannelli L, Kim S, Babb JS (2011) Gadolinium-enhanced liver magnetic resonance imaging using a 2-point Dixon fat-water separation technique: impact upon image quality and lesion detection. J Comput Assist Tomogr 35:96–101
Polanec SH, Bickel H, Baltzer PAT et al (2017) Respiratory motion artifacts during arterial phase imaging with gadoxetic acid: Can the injection protocol minimize this drawback? J Magn Reson Imaging 46:1107–1114
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Benjamin Henninger.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Two of the authors have significant statistical expertise.
Informed consent
Written informed consent was not required for this study because of its retrospective nature
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Rights and permissions
About this article
Cite this article
Gruber, L., Rainer, V., Plaikner, M. et al. CAIPIRINHA-Dixon-TWIST (CDT)-VIBE MR imaging of the liver at 3.0T with gadoxetate disodium: a solution for transient arterial-phase respiratory motion-related artifacts?. Eur Radiol 28, 2013–2021 (2018). https://doi.org/10.1007/s00330-017-5210-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-5210-4