Abstract
Introduction
To investigate the structural changes of the brain that correlate with physical frailty and cognitive impairments in Parkinson’s disease (PD) patients.
Methods
Sixty-one PD patients and 59 age- and sex-matched healthy controls were enrolled. For each participant, a frailty assessment using Fried’s criteria and comprehensive neuropsychological testing using the Wechsler Adult Intelligence Scale-III and Cognitive Ability Screening Instrument were conducted, and structural brain MR images were acquired for voxel-based morphometric analysis. The neuropsychological testing includes various tests in these five domains: attention, executive, memory, speech and language, and visuospatial functions. Exploratory group-wise comparisons of gray matter volume (GMV) in the PD patients and controls were conducted. Voxel-wise multiple linear regression analyses were conducted for physical frailty–related and cognitive impairment–related GMV changes in the PD patients. Voxel-wise multiple linear regressions were also performed with the five cognitive domains separated using the same model.
Results
The PD patients exhibited diffuse GMV reductions in comparison to the controls. In the PD patients, physical frailty–related decreases in GMV were observed in the bilateral frontal and occipital cortices, while cognitive impairment–related decreases in GMV were observed in the bilateral frontal, occipital, and temporal cortices. These regions overlap in the lateral occipital cortex. After the five domains of cognitive functions were analyzed separately, physical frailty–related decreases in GMV still overlap in lateral occipital cortices with every domain of cognitive impairment–related decreases in GMV.
Conclusion
Reduced GMV in the lateral occipital cortex is associated with cognitive impairment and physical frailty in PD patients.
Key Points
• Physical frailty in PD was associated with decreased GMV in the frontal and occipital cortices, while cognitive impairment was associated with decreased GMV in the frontal, temporal, and occipital cortices.
• Physical frailty and cognitive impairment were both associated with decreased GMV in the lateral occipital cortex, which is part of the ventral object-based visual pathway.
• Decreased GMV in the lateral occipital cortex may serve as a potential imaging biomarker for physical frailty and cognitive impairment in PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Physical frailty describes a state of increased vulnerability to stressors due to decreases in functions and reserve across multiple physiologic systems [1]. In a commonly used model proposed by Fried et al in the cardiovascular health study, frailty includes the following five domains: weight loss, exhaustion, slow walking speed, low physical activity, and low grip strength [1]. The presence of frailty in the elderly has been linked to increased mortality and other adverse outcomes [2]. Recent studies have also demonstrated that frailty or components of frailty are associated with brain structural or functional alterations in elderly individuals [3,4,5]. Due to shared pathophysiologies such as systemic inflammation and increased oxidative stress, physical frailty and cognitive impairment are interrelated and can aggravate each other through an intricate interplay that leads to a vicious cycle [6]. For example, the presence of physical frailty can lead to decreased social engagement and increased cardiovascular risk factors, which then lead to cognitive decline and depression [6]. Subsequently, these conditions can cause chronic undernutrition, oxidative stress, and systemic inflammation, which then aggravate the physical frailty status [6, 7]. Recently, it was proposed that the assessment of the frailty syndrome should include an evaluation of cognitive impairment [8].
Parkinson’s disease (PD) patients are prone to develop cognitive impairment with cognitive impairment–associated cortical atrophy that progresses as the cognitive impairment worsens [9]. However, while studies have demonstrated a link between cognitive impairments and physical frailty in the normal aging population [10], the role of physical frailty in PD patients and its association with cognitive impairments are less well studied. It has been reported that PD patients have a higher incidence of physical frailty than the normal aging population while the progression of PD is different from the development of physical frailty [11]. Moreover, a recent study of community-dwelling older individuals found that cortical atrophy was associated with frailty in said individuals [3]. However, the brain structural changes related to physical frailty and cognitive impairment in PD patients have not yet been reported.
In this study, we therefore aimed to investigate the structural changes in the brain that are correlated with physical frailty and cognitive impairments in PD patients. Due to the shared pathophysiology of physical frailty and cognitive impairments [6], we hypothesized that there would be distinct but overlapping patterns of physical frailty–associated and cognitive impairment–associated structural changes in the brains of PD patients. Identifying the presence of physical frailty and cognitive impairments, as well as their relationships with PD, might shed light on their possible underlying mechanisms, which may guide clinicians in developing personalized treatments for PD patients in the future.
Materials and methods
Participants
Sixty-one patients (24 males and 37 females; mean age 62.61 ± 8.59 years) diagnosed with idiopathic PD according to the United Kingdom Brain Bank criteria [12] and without other neurological disorders or psychiatric diseases were prospectively enrolled in a single tertiary medical center. The Unified Parkinson’s Disease Rating Scale (UPDRS), the modified Hoehn and Yahr Staging Scale, and the Schwab and England Activities of Daily Living Scale were utilized to assess the functional status and disease severity of the patients [13, 14]. Fifty-nine sex- and age-matched healthy subjects (22 males and 37 females; mean age 60.9 ± 6.3 years) with similar education levels and without neurologic disease, psychiatric illness, alcohol or substance abuse, or head injury were recruited from the local community and family members of patients as a control group. The hospital’s Institutional Review Committee on Human Research approved the study protocol, and all of the participants or their guardians provided written informed consent.
Frailty assessment
For each PD patient, a frailty assessment based on Fried’s criteria of weight loss, self-described exhaustion, low physical activity, slowness, and weakness was conducted [1]. Weight loss was defined as unintentional weight loss of more than 3 kg over the previous year. Self-described exhaustion was defined as fatigue or exhaustion for more than 3 days in the previous week. Physical activity was assessed by calorie consumption using the Taiwan International Physical Activity Questionnaire (Taiwanese version of the IPAQ) [15]. Slowness was measured based on the time it took to walk 5 m. Weakness was assessed by measuring the grip strength of both hands with a hand-held dynamometer.
Neuropsychological testing
A clinical psychologist blinded to each participant’s status performed the Mini-Mental State Examination (MMSE) and a neuropsychological battery of tests focusing on the attention, executive, speech and language, memory, and visuospatial functions. The Chinese versions of the Wechsler Adult Intelligence Scale-III (WAIS-III) [16] and the Cognitive Ability Screening Instrument (CASI) [17] were both used so that there would be more than three tests for each of the five cognitive domains. Attention functions were evaluated using the digit span and letter number sequencing subtest scores for the WAIS-III and by the scores for attention and orientation from the CASI. Executive functions were evaluated using the digit symbol coding, arithmetic, picture arrangement, and matrix reasoning scores from the WAIS-III and by the abstract thinking scores from the CASI.
Memory functions were evaluated using the short- and long-term memory scores from the CASI and the information scores from the WAIS-III. Speech and language ability were evaluated using the vocabulary, comprehension, and similarity scores from the WAIS-III and the language and semantic fluency scores from the CASI. Visuospatial functions were evaluated using the picture completion and block design scores from the WAIS-III and the drawing scores from the CASI.
Structural MR imaging
Image acquisition
The MR data were acquired using a 3.0T whole body GE Signa MRI system (General Electric Healthcare). For each subject, the subject’s head was immobilized with foam pillows inside the coil to diminish motion artifacts. The T1-weighted structured images were acquired using a 3D-FSPGR sequence (repetition time (TR) = 9.492 ms, echo time (TE) = 3.888 ms, flip angle = 20°, field of view (FOV) = 24 × 24 cm, matrix size = 512 × 512, 110 continuous slices with a slice thickness of 1.3 mm and in-plane spatial resolution of 0.47 × 0.47 mm) through the whole head parallel to the anterior-posterior commissure (AC-PC).
Imaging data pre-processing
All images were pre-processed using SPM12 (Statistical Parametric Mapping; http://www.fil.ion.ucl.ac.uk/spm/; University College London) running on Matlab R2012a (Mathworks).
First, each T1-weighted structural MR image was intensity-bias corrected and tissue-segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) compartments. Then, these segmented compartments were rigid-aligned (6 degrees of freedom (DOF)) to the MNI space tissue templates. The Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) algorithm was then used to generate study-specific tissue templates by iteratively registering the rigid-aligned tissue segments of all the participants and further warping the individual tissue segments to the constructed template. Afterwards, 12-parameter affine transformation was conducted to transform the rigid-aligned DARTEL space tissue templates to MNI space and then to modulate the corresponding effects of the affine transformation (global scaling of overall brain size) and the non-linear warping effect (local volume change) for each individual. Finally, the GM probability values were smoothed using an 8-mm full width at half-maximum Gaussian kernel, and a probability threshold of 0.15 was used to remove voxels with lower corresponding tissue probability from the analysis to avoid a possible edge effect.
Total intracranial volumes (TIV) were calculated by adding up the total voxels of GM, WM, and CSF in the native space separately.
Statistical analysis
Analysis of demographic data
The demographic data, including the age and sex data, were compared among the study groups using the two sample Student’s t test and Pearson chi-square test, where appropriate, and were reported as mean ± the standard deviation (SD). The significance of differences in the MMSE, disease severity, and neuropsychological test scores were analyzed by analysis of covariance (ANCOVA), with the participant’s age, sex, and education level as covariates. Statistical analysis was performed using the Statistical Product and Service Solutions software version 19 (IBM SPSS), and a p value < 0.05 was considered significant.
Group comparison of gray matter volume (GMV) between patients and controls
Voxel-wise group comparisons of GMV were conducted using the SPM12 (Statistical Parametric Mapping; http://www.fil.ion.ucl.ac.uk/spm/; University College London) software package in Matlab R2012a (Mathworks). A full factorial design was performed with age, sex, and TIV as covariates to detect regional GMV differences between the groups. Results were considered significant under the criteria of family-wise-error (FWE)-corrected p value < 0.05 based on the results of the Monte Carlo simulation (3dClusterSim with the following parameters: single voxel p value < 0.005, FWHM = 8 mm with GM mask, and 10,000 simulations).
Effects of physical frailty and cognitive performance on GMV
Due to the different scoring systems and numbers of tests in each domain of the neuropsychological testing, we constructed a summarized cognitive score for each patient that put equal weights on each of the five domains. The test scores of every neuropsychological assessment were normalized to have group means equal to zero and standard deviations equal to one. The normalized scores of every test within each cognitive domain were then averaged to make a weighted domain score. The five weighted domain scores were then summed up to make a summarized cognitive performance score. A summarized physical frailty score was also constructed in a similar way. Higher summarized cognitive scores indicated better cognitive performance, while higher summarized physical frailty scores indicated increased physical frailty.
Voxel-wise multiple linear regressions were performed on the warped GM segments of the PD patients with a regression model including the summarized cognitive score or frailty score, age, sex, and TIV to account for the confounding effects of overall brain size caused by age, sex, and TIV.
Voxel-wise multiple linear regressions were also performed with the five cognitive domains separated using the same model that included age, sex, and TIV as covariates.
A stepwise multiple regression was performed on the GMV of the overlapping region between cognitive impairment and physical frailty that included summarized physical frailty score, summarized cognitive performance score, age, sex, education level, UPDRS, and levodopa equivalent dose as covariates.
Results
Baseline clinical characteristics of PD patients and controls
The baseline clinical demographics and neuropsychological assessment scores of all the subjects and the physical frailty assessment sores of the PD patients are listed in Table 1. Statistical analysis of the clinical demographics showed a lower mean MMSE score in the PD group. The patients with PD also performed significantly worse than the controls on some of the neuropsychological assessments of attention function (orientation), executive function (digit symbol coding and arithmetic), speech and language function (vocabulary and comprehension), and visuospatial function (block design).
Group comparison of regional GMV
Voxel-wise analysis results of the whole brain with full factorial design are shown in Supplementary Table 1. Compared with the control group, extensive decreases in the GMV of the bilateral cerebellum were observed in the PD group. Scattered regions of decreased GMV in the PD patients were also present in the bilateral frontal, temporal, parietal, and occipital lobes (Supplementary Fig. 1).
Effects of physical frailty on regional GMV in PD patients
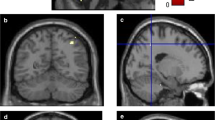
The results of a voxel-wise multiple linear regression to determine the effects of physical frailty on GMV in PD patients are shown in Supplementary Table 2A. Physical frailty–related decreases in GMV of PD patients were mostly observed in the bilateral frontal and occipital cortices. After overlaying the regions of physical frailty–related decreases in GMV over regions of the PD-related decrease in GMV in the same template, overlapping regions were observed in the bilateral frontal and left occipital regions (Fig. 1).
Effects of physical frailty and cognitive impairment on regional GMV in PD patients. Blue voxels indicate regions with significantly lower GMVs in the PD patients vs. the healthy controls. Red voxels indicate regions with significantly reduced physical frailty–associated GMVs in the PD patients. Green voxels indicate regions with significantly reduced cognitive impairment–associated GMVs in the PD patients (FWE-corrected p < 0.05)
Effects of cognitive impairments on regional GMV in PD patients
The results of a voxel-wise multiple linear regression to determine the effects of cognitive impairments on the GMV in PD patients are shown in Supplementary Table 2B. Cognitive impairment–related decreases in GMV of PD patients were mostly observed in the bilateral frontal, occipital, and temporal cortices. After overlaying the regions of cognitive impairment–related decreases in GMV over regions of the PD-related decrease in GMV in the same template, overlapping regions were observed in the bilateral temporal, occipital, and left frontal regions (Fig. 1).
Effects of physical frailty and cognitive impairments on regional GMV in PD patients
The significant regions of decreased GMV due to physical frailty and cognitive impairments were overlaid on the significant regions of decreased GMV in PD patients, as shown in Fig. 2. An overlapping region was observed in the left lateral occipital cortex. Stepwise multiple regression showed that the physical frailty and cognitive performance were optimal for the prediction of average GMV level in the left lateral occipital cortex (F = 15.849, R2 = 0.353, adjusted R2 = 0.331, p < 0.001). The model explained 33% of the variance observed, and each predictor significantly affected the GMV in left lateral occipital cortex, as shown in Table 2.
Effects of both physical frailty and cognitive impairment on regional GMV in PD patients. Blue voxels indicate regions with significantly lower GMVs in the PD patients vs. the healthy controls. Red voxels indicate regions with significantly reduced physical frailty–associated GMVs in the PD patients. Green voxels indicate regions with significantly reduced cognitive impairment–associated GMVs in the PD patients
Effects of physical frailty and different domains of cognitive impairments on regional GMV in PD patients
The significant regions of decreased GMV due to physical frailty were overlaid on different domains of cognitive impairments associated decreased GMV separately in PD patients, as shown in Fig. 3. An overlapping region was observed in the left lateral occipital cortex in every domain. Additionally, an overlap over left calcarine cortex was observed in attention function while superior frontal cortex was observed in executive function.
Effects of physical frailty and individual domains of cognitive impairments. Red voxels indicate regions with significantly reduced physical frailty–associated GMVs in the PD patients. Green voxels indicate regions with significantly reduced cognitive impairment–associated GMVs of each domain in the PD patients. Yellow voxels indicate the regions of overlap
Discussion
In this study, we found cognitive impairment–associated reduced GMV in bilateral frontal, occipital, and temporal cortices; physical frailty was associated with reduced GMV in bilateral frontal and occipital cortices. To the best of our knowledge, we are the first to report physical frailty–associated reduced GMV in PD patients. In clinical practice, physical frailty is not routinely assessed in PD patients, while cognitive impairments are often tested using simple measures such as the MMSE. Comprehensive neuropsychological tests are very time-consuming and may exhibit inter-rater variability. By using voxel-based morphometry, we were able to assess the brain correlates of physical frailty and cognitive impairment in a quick and objective manner, and the results of these assessments may provide further information regarding the potential underlying pathophysiological mechanisms.
In this study, we found extensive cerebral and cerebellar atrophy in the PD patients compared with the healthy controls, findings which are consistent with those of previous studies [9]. The brain degeneration in PD patients extends far beyond the nigrostriatal system and causes additional maladies, such as cognitive impairments, in addition to its well-known motor symptoms [18]. Cognitive impairments in PD patients are well-documented and range from mild cognitive impairment to PD dementia [19, 20]. It is recommended that a cognitive assessment of PD patients include these five domains: attention, executive, memory, speech and language, and visuospatial domains [19]. Recent studies have shown that cognitive impairments in PD patients are associated with diffuse cortical atrophy and that more extensive cortical atrophy is seen in PD patients with poorer cognitive status [9, 21]. Our results showed that the cognitive impairments in PD patients are associated with atrophy of the frontal, temporal, and occipital cortices.
PD patients have a 5-fold higher prevalence rate of physical frailty than the normal aging population [11]. This may partly be explained by the potential shared pathophysiologies of physical frailty and PD, such as increased systemic inflammation [11]. The presence of frailty, however, is different from the progression of PD [11]. In this study, the prediction model for GMV in the lateral occipital cortex includes physical frailty but not UPDRS score. This result supports the notion that PD progression is different from physical frailty. Assessing the GMV changes in PD patients may aid in the identification of frailty status, which is important because it is potentially treatable [22, 23]. Treatments for physical frailty include nutritional support, identifying and treating depressive status, and increased exercise [22].
Physical frailty has recently been linked to brain architectural changes in community-dwelling elderly individuals, suggesting the role of the central nervous system in the pathophysiology of physical frailty [3]. Chen et al reported that in the community-dwelling elderly individuals, physical frailty status is associated with decreased GMV in the cerebellum, hippocampi, frontal, occipital, and parietal cortices [3]. Among these areas, the cerebellum showed the highest correlation with physical frailty. Our results showed that physical frailty is associated with decreased GMV in the frontal and occipital regions in PD patients. Although cerebellum seemed to be the most significant neural signature of physical frailty in the community-dwelling elderly individuals [3], our results did not show such an association. When the analysis of frailty was separated into motor (slowness, weakness, grip strength) and non-motor (weight loss, exhaustion) components, the results still showed no significant cluster in the cerebellum for either the motor or non-motor components (results not shown). These discrepancies may be explained by the differences in the study populations. In fact, PD-related cerebellar degeneration is different from cerebellar degeneration in the normal aging population [24, 25]. The cerebellum plays an important role in both motor and cognitive symptoms in PD, and the resulting effects may be mediated by both pathological and compensatory effects [25, 26]. The pathological effects result in progressive functional decline, while the compensatory effects aid in maintaining functions at relatively early stages of the disease but eventually fail as the disease progresses [25]. As our patients already showed significant cerebellar atrophy, the pathological effects and the failure in the compensatory effects may explain why no association was found between physical frailty and cerebellar GMV change in these PD patients. As opposed to frailty in members of the normal aging population, in which cerebellar atrophy may be an important imaging biomarker [3], our results support the notion that frailty syndrome in PD patients may be mediated by other brain regions.
Stacking the regions of decreased GMV associated with cognitive impairment and physical frailty together showed that the cognitive impairment-related decreases in GMV involved the occipital and inferior temporal lobes while the physical frailty–related decreases in GMV involved the occipital lobe. The overlapping region was in the lateral occipital cortex. These regions are part of the ventral object-based visual pathway, which starts from the visual cortex that propagates to the inferior temporal lobe, processing object information [27]. We also found that although distinct atrophy maps are present among different cognitive domains, there is consistent overlap over the lateral occipital cortex with the frailty associated GM atrophy map. Additionally, there are overlaps in the primary visual cortex between frailty and attention function and in the superior frontal cortex between frailty and executive function. The primary visual cortex is also part of the ventral object–based visual pathway. Aside from receiving signals from the retina, it is also involved in the attention and awareness [28]. The superior frontal cortex is involved in working memory and executive function [29]. Currently, most studies addressing physical frailty and cognitive function assess the cognitive function using simple cognitive measurements such as MMSE [10]. In contrast, our study used comprehensive neuropsychological battery of tests that include multiple tests among different domains of cognitive function. To the best of our knowledge, we are the first to evaluate the GM structural changes between physical frailty and cognitive impairment in PD patients. Although we found distinct atrophy maps among different domains of cognitive impairment in PD patients, further conclusion cannot be drawn until future studies dedicated specifically to each cognitive domain are done.
The ventral visual pathway is an occipitotemporal network that processes the stable aspects of visual information, such as shape, color, size, and brightness [30]. Damage to the ventral visual pathway can result in impairment of object recognition [31], and studies have shown that poor visual function is associated with frailty status, poor physical outcomes, and cognitive impairments in the normal population [32, 33]. In PD patients, decreased cortical thickness involving the ventral visual pathway is associated with poor executive, visuospatial, and memory function [34], which may be mediated by increased oxidative stress [35]. The results of the current study support the notion that physical frailty and cognitive impairments are interrelated in the brain and suggest the role of the ventral visual pathway, especially the lateral occipital cortex, as a potential imaging biomarker for physical frailty and cognitive impairment in PD patients. Although increased oxidative stress is an important underlying mechanism for PD [36] and is associated with physical frailty and cognitive decline [6], future longitudinal studies are needed to delineate their causal relationships and connections with the structural changes of the brain. By combining other clinical parameters, future longitudinal studies could also assess the possibility of using this imaging biomarker as a potential predictor for the development of physical frailty and cognitive impairment in PD.
Limitations
This study does have several limitations. First, the patients who participated were recruited from a single tertiary center and so may not be representative of all PD populations. Second, this study was a cross-sectional study, such that readers should interpret the results with caution. Further causal relationships among GMV, cognitive impairment, and physical frailty may thus need to be delineated by future longitudinal studies. Lastly, the frailty status was not assessed in the normal control subjects. Therefore, we could not assess the differences in frailty between the PD patients and the normal controls.
Conclusion
Reduced GMV in the lateral occipital cortex is associated with cognitive impairment and physical frailty in PD patients. Identification of structural changes to the lateral occipital cortex may aid in detecting physical frailty and cognitive impairments in PD patients.
Abbreviations
- GMV:
-
Gray matter volume
- MMSE:
-
Mini-Mental State Examination
- PD:
-
Parkinson’s disease
- TIV:
-
Total intracranial volume
- UPDRS:
-
Unified Parkinson’s Disease Rating Scale
References
Fried LP, Tangen CM, Walston J et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–M156
Rothman MD, Leo-Summers L, Gill TM (2008) Prognostic significance of potential frailty criteria. J Am Geriatr Soc 56:2211–2216
Chen WT, Chou KH, Liu LK et al (2015) Reduced cerebellar gray matter is a neural signature of physical frailty. Hum Brain Mapp 36:3666–3676
Nadkarni NK, Nunley KA, Aizenstein H et al (2014) Association between cerebellar gray matter volumes, gait speed, and information-processing ability in older adults enrolled in the Health ABC study. J Gerontol A Biol Sci Med Sci 69:996–1003
Rosano C, Studenski SA, Aizenstein HJ, Boudreau RM, Longstreth WT Jr, Newman AB (2012) Slower gait, slower information processing and smaller prefrontal area in older adults. Age Ageing 41:58–64
Robertson DA, Savva GM, Kenny RA (2013) Frailty and cognitive impairment--a review of the evidence and causal mechanisms. Ageing Res Rev 12:840–851
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K (2013) Frailty in elderly people. Lancet 381:752–762
Kelaiditi E, Cesari M, Canevelli M et al (2013) Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging 17:726–734
Weintraub D, Doshi J, Koka D et al (2011) Neurodegeneration across stages of cognitive decline in Parkinson disease. Arch Neurol 68:1562–1568
Panza F, Seripa D, Solfrizzi V et al (2015) Targeting cognitive frailty: clinical and neurobiological roadmap for a single complex phenotype. J Alzheimers Dis 47:793–813
Ahmed NN, Sherman SJ, Vanwyck D (2008) Frailty in Parkinson’s disease and its clinical implications. Parkinsonism Relat Disord 14:334–337
Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51:745–752
Zhao YJ, Wee HL, Chan YH et al (2010) Progression of Parkinson’s disease as evaluated by Hoehn and Yahr stage transition times. Mov Disord 25:710–716
Ramaker C, Marinus J, Stiggelbout AM, Van Hilten BJ (2002) Systematic evaluation of rating scales for impairment and disability in Parkinson’s disease. Mov Disord 17:867–876
Qu NN, Li KJ (2004) Study on the reliability and validity of international physical activity questionnaire (Chinese vision, IPAQ). Zhonghua Liu Xing Bing Xue Za Zhi 25:265–268
Wechsler D, Chen Y, Chen X (2002) WAIS-III Chinese version technical manual. Psychological Corporation, San Antonio
Lin KN, Wang PN, Liu HC, Teng EL (2012) Cognitive abilities screening instrument, Chinese version 2.0 (CASI C-2.0): administration and clinical application. Acta Neurol Taiwan 21:180–189
Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Litvan I, Goldman JG, Tröster AI et al (2012) Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord 27:349–356
Emre M, Aarsland D, Brown R et al (2007) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22:1689–1707 quiz 1837
Burton EJ, McKeith IG, Burn DJ, Williams ED, O'Brien JT (2004) Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain 127:791–800
Fairhall N, Langron C, Sherrington C et al (2011) Treating frailty--a practical guide. BMC Med 9:83
Gill TM, Gahbauer EA, Allore HG, Han L (2006) Transitions between frailty states among community-living older persons. Arch Intern Med 166:418–423
Luft AR, Skalej M, Schulz JB et al (1999) Patterns of age-related shrinkage in cerebellum and brainstem observed in vivo using three-dimensional MRI volumetry. Cereb Cortex 9:712–721
Wu T, Hallett M (2013) The cerebellum in Parkinson’s disease. Brain 136:696–709
Mirdamadi JL (2016) Cerebellar role in Parkinson’s disease. J Neurophysiol 116:917–919
Mishkin M, Ungerleider LG, Macko KA (1983) Object vision and spatial vision: two cortical pathways. Trends Neurosci 6:414–417
Kamitani Y, Tong F (2005) Decoding the visual and subjective contents of the human brain. Nat Neurosci 8:679–685
du Boisgueheneuc F, Levy R, Volle E et al (2006) Functions of the left superior frontal gyrus in humans: a lesion study. Brain 129:3315–3328
Kravitz DJ, Saleem KS, Baker CI, Ungerleider LG, Mishkin M (2013) The ventral visual pathway: an expanded neural framework for the processing of object quality. Trends Cogn Sci 17:26–49
James TW, Culham J, Humphrey GK, Milner AD, Goodale MA (2003) Ventral occipital lesions impair object recognition but not object-directed grasping: an fMRI study. Brain 126:2463–2475
Whitson HE, Cousins SW, Burchett BM, Hybels CF, Pieper CF, Cohen HJ (2007) The combined effect of visual impairment and cognitive impairment on disability in older people. J Am Geriatr Soc 55:885–891
Lin MY, Gutierrez PR, Stone KL et al (2004) Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc 52:1996–2002
Pereira JB, Svenningsson P, Weintraub D et al (2014) Initial cognitive decline is associated with cortical thinning in early Parkinson disease. Neurology 82:2017–2025
Lin WC, Chou KH, Lee PL et al (2015) Brain mediators of systemic oxidative stress on perceptual impairments in Parkinson’s disease. J Transl Med 13:386
Hwang O (2013) Role of oxidative stress in Parkinson’s disease. Exp Neurobiol 22:11–17
Funding
This study has received funding by National Science Council (MOST 106-2314-B-182A-031-MY2 to W-C Lin).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Wei-Che Lin.
Conflict of interest
The authors declare that they have no conflict of interest.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Prospective
• Cross-sectional study
• Performed at one institution
Electronic supplementary material
ESM 1
(DOCX 208 kb)
Rights and permissions
About this article
Cite this article
Chen, YS., Chen, HL., Lu, CH. et al. Reduced lateral occipital gray matter volume is associated with physical frailty and cognitive impairment in Parkinson’s disease. Eur Radiol 29, 2659–2668 (2019). https://doi.org/10.1007/s00330-018-5855-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5855-7