Abstract
The volume of the hippocampus decreases more slowly than the volume of the cortex during normal aging. We explored changes in the hippocampus-to-cortex volume (HV:CTV) ratio with increasing age in non-demented Parkinson’s disease (PD) patients as compared to healthy controls (HC). We also evaluated the association between the HV:CTV ratio and cognitive outcomes. Altogether 130 participants without dementia aged 51–88 years were consecutively enrolled, including 54 PD patients (mean age 67, standard deviation (SD) 8 years) and 76 HC (mean age 69, SD 7 years). All participants underwent structural magnetic resonance examination and psychological evaluation. Hippocampal and cortex volumes were determined from T1 and FLAIR scans using FreeSurfer software, and the HV:CTV ratio was calculated. Regression lines for age-dependence of the HV:CTV ratio for PD and HC groups were calculated. We further assessed the association between the HV:CTV ratio and cognitive tests examining hippocampus-related cognitive functions. PD patients and age-matched HC showed a significant difference in age-dependence of HV:CTV ratio (p value = 0.012), with a decreasing slope in PD and increasing slope in HC. In the PD group, a significant correlation (R = 0.561, p = 0.024) was observed between the HV:CTV ratio and the Digit Symbol-Coding test. The reduction of HV:CTV ratio is accelerated in pathological aging due to PD pathology. The HV:CTV ratio was associated with impaired processing speed, i.e., the cognitive function that is linked to subcortical alterations of both associated basal ganglia circuitry and the hippocampus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gray matter (GM) atrophy on magnetic resonance imaging (MRI) is a marker of brain neurodegeneration. A characteristic cortical GM atrophy pattern has not yet been conclusively established for Parkinson’s disease (PD) (Krajcovicova et al. 2019). This may be at least partially due to the fact that PD is a pathologically and genetically heterogeneous entity (Litvan et al. 2012). Early cognitive deficits in PD primarily affect attention and the executive functions (Lawson et al. 2016) due to dopaminergic deficits and dysfunctions of the associated basal ganglia circuitry (Ferrazzoli et al. 2018). Other cognitive functions, such as memory or visuospatial functions, could also be impaired in PD patients. It was reported that mild cognitive impairment (MCI) is present in approximately 20% of PD patients at the time of diagnosis and in about 60% of PD-MCI converted to dementia (PD-D) during 5 years of follow-up (Aarsland et al. 2021). Previous research also showed that PD-D significantly differed from PD-MCI in tests of executive functions and visuospatial functions (Biundo et al. 2014). While frontal lobe (i.e., an output region of the associated basal ganglia circuitry) atrophy alone may predict dementia in the PD population (Chung et al. 2019), both fronto-parietal GM loss and temporal region atrophy patterns involving the hippocampus, entorhinal cortex, and inferior temporal lobe seem to be characteristic for PD-dementia (Rektorova et al. 2014). Moreover, medial temporal atrophy and baseline cerebrospinal fluid (CSF) Aβ were shown to independently predict subsequent cognitive impairment in PD (Tropea et al. 2018; Weintraub et al. 2012).
Indeed, hippocampal atrophy on structural MRI is probably the best documented specific pathological feature in both typical and limbic variants of Alzheimer’s disease (AD) (Jack et al. 2018). It has been shown that the hippocampus-to-cortex volume (HV:CTV) ratio serves as a valuable MRI biomarker for identifying various AD subtypes, closely following the spatial distribution of tau pathology (Risacher et al. 2017; Whitwell et al. 2012).
However, both cortical and subcortical atrophy also occur physiologically due to aging (Fjell et al. 2014; Thomann et al. 2013). The rate of cortical and subcortical atrophy is not the same; in healthy subjects, the volume of the hippocampus decreases more slowly than the volume of the cortex (Nobis et al. 2019). Since both cortical and medial temporal lobe atrophy are documented in PD subjects, our first objective was to explore the relationship between volume reduction of the hippocampus and volume reduction of the cortex relative to age in PD patients without any major cognitive impairment as compared to age-matched healthy controls (HC). We hypothesize that the HV:CTV ratio reduction with age is higher in PD patients than in age-matched controls.
Our second objective was to explore the association between the HV:CTV ratio and the variability of cognitive tests scores of interest in PD patients, i.e., the scores of tests examining cognitive functions that are linked to the hippocampus and that are known to be impaired in early PD. Our hypothesis is that the HV:CTV ratio reduction is correlated with specific cognitive deficits in PD patients.
Methods
Participants
We enrolled clinically established PD patients (Postuma et al. 2016) and age-matched healthy controls in this study. The disease severity was assessed using the Unified Parkinson’s Disease Rating Scale (UPDRS), part III (Motor Examination) scale. Any subject experiencing a current depressive episode, based on the Geriatric Depression Scale (GDS < 10) and a clinician’s interview with the subject, was excluded. Exclusion criteria also included alcohol or drug abuse, hallucinations, and any diagnosed psychiatric disorder. We included only subjects without dementia, based on the Montreal Cognitive Assessment (MoCA) test for dementia, MoCA > 20 (Biundo et al. 2014), and on a clinician’s interview with a caregiver. These exclusion criteria were the same for the HC and PD groups. Only clinically established PD participants (Postuma et al. 2016) on stable dopaminergic medication and without dyskinesias were included in this study.
The study was conducted at the Central European Institute of Technology (CEITEC) Neuroscience Center, at Masaryk University in Brno. The research was approved by the ethics committee at Masaryk University; informed consent was obtained from all participants.
Neuropsychological assessment
We were specifically interested in evaluating cognitive functions in which the hippocampus plays a role, including memory encoding (Hardcastle et al. 2020; Zatorre et al. 2012), attention (Ruiz et al. 2020), cognitive processing speed (O’Shea et al. 2016; Papp et al. 2014), and visuospatial functions (Kravitz et al. 2011; Yamamoto et al. 2014). Specific neuropsychological tests were chosen based on a recommendation for the cognitive assessment of PD patients by Litvan et al. (2012). To assess processing speed, we used the Digit Symbol-Coding test from the Wechsler Adult Intelligence Scale III (David 1997); to assess hippocampus-related memory deficits, we used the Word List recognition from the Wechsler Memory Scale III (David 1997), and to assess visuospatial functions, we used the Judgment of Line Orientation (JLO) (Benton et al. 1994). The z-scores were computed for each neuropsychological test mentioned above.
MRI imaging
Data acquisition
MRI examinations were performed on a Siemens Prisma 3 T scanner using a 64-channel head coil. The MRI protocol for morphometry included 3D T1-weighted magnetization prepared rapid gradient echo (MPRAGE) sequence with TR = 2.3 s, TE = 2.33 ms, TI = 0.9 s, FA = 8°, isometric voxel size 1 mm in FOV 224 × 224 mm, and 240 slices and 3D Fluid-attenuated inversion recovery (FLAIR) sequence with TR 6000 ms, TE 387 ms, TI1 1900 ms, vox 1 × 1 × 1 × mm.
Data from all participants were manually checked for artifacts, and pathology was checked by an experienced radiologist. Participants who did not meet quality criteria were excluded from the study (scans with technical artifacts; scans with movement artifacts, or scans with inaccurate segmentation). Participants with intracranial pathology, such as tumors or stroke, were excluded.
Data processing
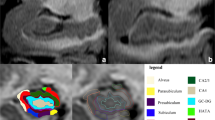
Anatomical MRI data were segmented using FreeSurfer 7. Cortical structures were defined using the Desikan–Killiany cortical atlas (Desikan et al. 2006).
The HV:CTV ratio was calculated in accordance with Risacher et al. (2017) as a sum of left and right hippocampal volumes divided by a sum of left and right lateral frontal cortices (caudal middle frontal gyrus, pars opercularis, pars triangularis, rostral middle frontal gyrus), lateral parietal cortices (superior parietal gyrus, inferior parietal gyrus, supra-marginal gyrus), and superior temporal gyri.
Statistical analysis
Differences between the HC and PD study groups in age, education in years, and cognitive assessment (MoCA, GDS, Word List Recognition, Digit Symbol-Coding, and JLO) were explored using a two-sample t test.
The first objective of our study was to test whether the dependence of the HV:CTV ratio on age differed between HC and PD. We used a general linear model that modeled factor groups with two levels for HC and PD, and the interaction of age and group. As we cannot disprove the potential effects of gender, education in years, or GDS on the ratio, we added these variables to the model as covariates of no interest. We hypothesized a potential effect of UPDRS III and disease duration on patient ratio; therefore, we added these two covariates as well (filled by zeros for HC). The model was estimated using the ordinary least squares approach. Finally, we tested the following effects of interest with appropriate contrasts and t tests (Friston et al. 2007). The level of statistical significance was set to p < 0.05. Effects of interests:
1. the mean difference between HC and PD,
2. age interaction difference between HC and PD,
3. effect of the disease duration and UPDRS III.
Pearson’s partial correlation was used to assess the second objective. The partial correlation of Word List Recognition, Digit Symbol-Coding, and JLO was used to investigate links between HV:CTV ratio and the cognition of PD patients. Age, sex, education (in years), and GDS score were used as covariates of no interest.
Results
Altogether 54 clinically established PD patients (18 women) and 76 healthy controls (55 women) aged 51–88 years meeting the inclusive criteria were enrolled. See Table 1 for demographic and clinical data.
The PD and HC groups were not significantly different in age and years of education, but they differed in sex (more males in the PD group than in the HC group). The PD patients had significantly more depressive symptoms (however, the subjects were not experiencing a current depressive episode) and lower z-scores for the Digit Symbol-Coding test than HC; see Table 1 for details.
HV:CTV ratio comparison between PD and HC groups
The PD (mean = 0.0688; standard deviation (SD) = 0.0084) and HC (mean = 0.0722; SD = 0.0092) groups were significantly different in HV:CTV ratio with a p value = 0.0390.
Age-dependence of HV:CTV ratio for PD and HC groups
The age-dependence of HV:CTV ratio for HC (blue) and PD (red) is plotted in Fig. 1. Linear regression lines are calculated for each group. The blue line increases at a slope of 0.0001 for HC; the red line decreases at a slope of − 0.0004 for PD. The difference in regression slopes between groups was significant with a p value = 0.0124.
We were additionally interested in the impact of the disease duration and severity on the HV:CTV ratio. The appropriate p values were 0.2014 and 0.4548, respectively. The effect of neither variable was significant.
Relationship between HV:CTV ratio and psychological tests
We found a significant partial correlation with the Digit Symbol-Coding test (r = 0.561; p = 0.024). For details see Table 2.
Discussion
Our study investigated the impact of PD pathology on changes in the HV:CTV ratio with increasing age. We found that the hippocampal volume loss relative to cortical volume loss was significantly higher in PD than in HC. While there is a significant acceleration in the rate of hippocampal volume loss in middle age even in HC (Nobis et al. 2019; Thomann et al. 2013), we demonstrated that this was more pronounced in the PD group. In AD, clinico-pathological studies demonstrated that the HV:CTV ratio decreases reflect the spatial distribution of tau pathology (Risacher et al. 2017; Whitwell et al. 2012), and predicted faster clinical decline in AD patients who were clinically indistinguishable at baseline except for a greater dysexecutive syndrome. The pathological cause of the accelerated decline of the hippocampus relative to cortical atrophy in the PD population with increasing age is unknown. It has been hypothesized that posterior temporo-parietal changes are mainly caused by structural pathology, such as Lewy body and amyloid deposits (Halliday and McCann, 2010; Irwin et al. 2012; Kehagia et al. 2013); however, cholinergic neuron losses in the nucleus basalis of Meynert and other cholinergic structures of the basal forebrain may also impact the AD pattern of atrophy in PD (Ray et al. 2018). Of note, solely hippocampal volume was shown to be a major predictor for developing MCI and dementia in PD (Kandiah et al. 2014).
We showed that the HV:CTV ratio decreases were related to impaired performance on a test that particularly reflects cognitive processing speed (Rektorova et al. 2005) and is known to be linked to both hippocampal integrity(O’Shea et al. 2016; Papp et al. 2014; Ruiz et al. 2020) and dopaminergic deficiency in PD (Ferrazzoli et al. 2018). This distinct association may due to the fact that the variability in the Digit Symbol-Coding test was higher than in other evaluated tests in the PD group and it was significantly more impaired in PD than in HC. The major results of the HV:CTV ratio decreases were not influenced or explained by the disease severity or by the disease duration.
The study limitation is a heterogeneous group of PD patients with variability in disease duration and severity and an unbalanced number of men and women. Future prospective studies will be required to investigate whether the HV:CTV ratio and its temporal change can be used as biomarkers of cognitive impairment progression and of dementia conversion in PD.
In conclusion, we demonstrated that the reduction of HV:CTV ratio is accelerated in pathological aging due to PD pathology. The HV:CTV ratio was associated with impaired processing speed, i.e., the cognitive function that is linked to subcortical alterations of both associated basal ganglia circuitry and the hippocampus.
Availability of data and material
All data are available upon request at the Repository CEITEC Masaryk University, MAFIL CF.
Code availability
Not applicable.
Abbreviations
- AD:
-
Alzheimer’s disease
- CSF:
-
Cerebrospinal fluid
- GDS:
-
Geriatric Depression Scale
- GLM:
-
General linear model
- GM:
-
Gray matter
- HC:
-
Healthy controls
- HV:CTV ratio:
-
Hippocampus-to-cortex volume ratio
- JLO:
-
Judgment of Line Orientation
- MCI:
-
Mild cognitive impairment
- MoCA:
-
Montreal cognitive assessment
- MRI:
-
Magnetic resonance imaging
- PD:
-
Parkinson’s disease
- SD:
-
Standard deviation
- UPDRS:
-
Unified Parkinson’s Disease Rating Scale
References
Aarsland D, Batzu L, Halliday GM, Geurtsen GJ, Ballard C, Ray Chaudhuri K, Weintraub D (2021) Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers 7(1):47. https://doi.org/10.1038/s41572-021-00280-3
Benton AL, Sivan AB, de Hamsher KS, Varney NR, Spreen O (1994) Contributions to neuropsychological assessment: a clinical manual, 2nd edn. Oxford University Press, Oxford
Biundo R, Weis L, Facchini S, Formento-Dojot P, Vallelunga A, Pilleri M, Antonini A (2014) Cognitive profiling of Parkinson disease patients with mild cognitive impairment and dementia. Parkinsonism Relat Disord 20(4):394–399. https://doi.org/10.1016/j.parkreldis.2014.01.009
Chung SJ, Yoo HS, Lee YH, Lee HS, Ye BS, Sohn YH, Kwon H, Lee PH (2019) Frontal atrophy as a marker for dementia conversion in Parkinson’s disease with mild cognitive impairment. Hum Brain Mapp. https://doi.org/10.1002/hbm.24631
David W (1997). In: Tulsky David LM, Zhu J (eds) WAIS-III WMS-III technical manual. Harcourt Brace and Company, San Diego
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31(3):968–980. https://doi.org/10.1016/j.neuroimage.2006.01.021
Ferrazzoli D, Ortelli P, Madeo G, Giladi N, Petzinger GM, Frazzitta G (2018) Basal ganglia and beyond: The interplay between motor and cognitive aspects in Parkinson’s disease rehabilitation. Neurosci Biobehav Rev 90:294–308. https://doi.org/10.1016/j.neubiorev.2018.05.007
Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB (2014) What is normal in normal aging? Effects of aging, amyloid and Alzheimer’s disease on the cerebral cortex and the hippocampus. Prog Neurobiol 117:20–40. https://doi.org/10.1016/J.PNEUROBIO.2014.02.004
Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, Penny WD (2007) Statistical parametric mapping, 1st edn. Elsevier Press, Amsterdam
Halliday GM, McCann H (2010) The progression of pathology in Parkinson’s disease. Ann N Y Acad Sci 1184(1):188–195. https://doi.org/10.1111/j.1749-6632.2009.05118.x
Hardcastle C, O’Shea A, Kraft JN, Albizu A, Evangelista ND, Hausman HK, Boutzoukas EM, Van Etten EJ, Bharadwaj PK, Song H, Smith SG, Porges EC, Dekosky S, Hishaw GA, Wu SS, Marsiske M, Cohen R, Alexander GE, Woods AJ (2020) Contributions of hippocampal volume to cognition in healthy older adults. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2020.593833
Irwin DJ, White MT, Toledo JB, Xie SX, Robinson JL, Van Deerlin V, Lee VM-Y, Leverenz JB, Montine TJ, Duda JE, Hurtig HI, Trojanowski JQ (2012) Neuropathologic substrates of Parkinson disease dementia. Ann Neurol 72(4):587–598. https://doi.org/10.1002/ana.23659
Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Silverberg N (2018) NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement 14(4):535–562. https://doi.org/10.1016/j.jalz.2018.02.018
Kandiah N, Zainal NH, Narasimhalu K, Chander RJ, Ng A, Mak E, Au WL, Sitoh YY, Nadkarni N, Tan LCS (2014) Hippocampal volume and white matter disease in the prediction of dementia in Parkinson’s disease. Parkinsonism Relat Disord 20(11):1203–1208. https://doi.org/10.1016/j.parkreldis.2014.08.024
Kehagia AA, Barker RA, Robbins TW (2013) Cognitive impairment in Parkinson’s disease: the dual syndrome hypothesis. Neurodegener Dis 11(2):79–92. https://doi.org/10.1159/000341998
Krajcovicova L, Klobusiakova P, Rektorova I (2019) Gray matter changes in Parkinson’s and Alzheimer’s disease and relation to cognition. Curr Neurol Neurosci Rep 19(11):85. https://doi.org/10.1007/s11910-019-1006-z
Kravitz DJ, Saleem KS, Baker CI, Mishkin M (2011) A new neural framework for visuospatial processing. Nat Rev Neurosci 12(4):217–230. https://doi.org/10.1038/nrn3008
Lawson RA, Yarnall AJ, Duncan GW, Breen DP, Khoo TK, Williams-Gray CH, Barker RA, Collerton D, Taylor J-P, Burn DJ (2016) Cognitive decline and quality of life in incident Parkinson’s disease: the role of attention. Parkinsonism Relat Disord 27:47–53. https://doi.org/10.1016/j.parkreldis.2016.04.009
Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams-Gray CH, Aarsland D, Kulisevsky J, Rodriguez-Oroz MC, Burn DJ, Barker RA, Emre M (2012) Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord 27(3):349–356. https://doi.org/10.1002/mds.24893
Nobis L, Manohar SG, Smith SM, Alfaro-Almagro F, Jenkinson M, Mackay CE, Husain M (2019) Hippocampal volume across age: Nomograms derived from over 19,700 people in UK Biobank. Neuroimage 23:101904. https://doi.org/10.1016/j.nicl.2019.101904
O’Shea A, Cohen RA, Porges EC, Nissim NR, Woods AJ (2016) Cognitive aging and the hippocampus in older adults. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2016.00298
Papp KV, Kaplan RF, Springate B, Moscufo N, Wakefield DB, Guttmann CRG, Wolfson L (2014) Processing speed in normal aging: Effects of white matter hyperintensities and hippocampal volume loss. Aging Neuropsychol Cogn 21(2):197–213. https://doi.org/10.1080/13825585.2013.795513
Postuma RB, Berg D, Adler CH, Bloem BR, Chan P, Deuschl G, Gasser T, Goetz CG, Halliday G, Joseph L, Lang AE, Liepelt-Scarfone I, Litvan I, Marek K, Oertel W, Olanow CW, Poewe W, Stern M (2016) The new definition and diagnostic criteria of Parkinson’s disease. Lancet Neurol 15(6):546–548. https://doi.org/10.1016/S1474-4422(16)00116-2
Ray NJ, Bradburn S, Murgatroyd C, Toseeb U, Mir P, Kountouriotis GK, Teipel SJ, Grothe MJ (2018) In vivo cholinergic basal forebrain atrophy predicts cognitive decline in de novo Parkinson’s disease. Brain 141(1):165–176. https://doi.org/10.1093/brain/awx310
Rektorova I, Rektor I, Bares M, Dostal V, Ehler E, Fanfrdlova Z, Fiedler J, Klajblova H, Kulistak P, Ressner P, Svatova J, Urbanek K, Veliskova J (2005) Cognitive performance in people with Parkinson’s disease and mild or moderate depression: effects of dopamine agonists in an add-on to l-dopa therapy. Eur J Neurol 12(1):9–15. https://doi.org/10.1111/j.1468-1331.2004.00966.x
Rektorova I, Biundo R, Marecek R, Weis L, Aarsland D, Antonini A (2014) Grey matter changes in cognitively impaired Parkinson’s disease patients. PLoS ONE 9(1):e85595. https://doi.org/10.1371/journal.pone.0085595
Risacher SL, Anderson WH, Charil A, Castelluccio PF, Shcherbinin S, Saykin AJ, Schwarz AJ (2017) Alzheimer disease brain atrophy subtypes are associated with cognition and rate of decline. Neurology 89(21):2176–2186. https://doi.org/10.1212/WNL.0000000000004670
Ruiz NA, Meager MR, Agarwal S, Aly M (2020) The medial temporal lobe is critical for spatial relational perception. J Cogn Neurosci 32(9):1780–1795. https://doi.org/10.1162/jocn_a_01583
Thomann PA, Wüstenberg T, Nolte HM, Menzel PB, Wolf RC, Essig M, Schröder J (2013) Hippocampal and entorhinal cortex volume decline in cognitively intact elderly. Psychiatry Res 211(1):31–36. https://doi.org/10.1016/J.PSCYCHRESNS.2012.06.002
Tropea TF, Xie SX, Rick J, Chahine LM, Dahodwala N, Doshi J, Davatzikos C, Shaw LM, Van Deerlin V, Trojanowski JQ, Weintraub D, Chen-Plotkin AS (2018) APOE, thought disorder, and SPARE-AD predict cognitive decline in established Parkinson’s disease. Mov Disord 33(2):289–297. https://doi.org/10.1002/mds.27204
Weintraub D, Dietz N, Duda JE, Wolk DA, Doshi J, Xie SX, Davatzikos C, Clark CM, Siderowf A (2012) Alzheimer’s disease pattern of brain atrophy predicts cognitive decline in Parkinson’s disease. Brain 135(1):170–180. https://doi.org/10.1093/brain/awr277
Whitwell JL, Dickson DW, Murray ME, Weigand SD, Tosakulwong N, Senjem ML, Knopman DS, Boeve BF, Parisi JE, Petersen RC, Jack CR, Josephs KA (2012) Neuroimaging correlates of pathologically defined subtypes of Alzheimer’s disease: a case-control study. Lancet Neurol 11(10):868–877. https://doi.org/10.1016/S1474-4422(12)70200-4
Yamamoto N, Philbeck JW, Woods AJ, Gajewski DA, Arthur JC, Potolicchio SJ, Levy L, Caputy AJ (2014) Medial temporal lobe roles in human path integration. PLoS ONE 9(5):e96583. https://doi.org/10.1371/journal.pone.0096583
Zatorre RJ, Fields RD, Johansen-Berg H (2012) Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci 15(4):528–536. https://doi.org/10.1038/nn.3045
Acknowledgements
Thanks to Michal Mikl for statistics assistance and Anne Johnson for English language assistance. This project received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no. 734718 (CoBeN), from a grant from the Czech Ministry of Health, NU20-04-00294, and from the Czech Science Foundation GAČR 21-25953S. We acknowledge the help of the core facility multimodal and functional imaging laboratory (MAFIL) of the Central European Institute for Technology – Masaryk University (CEITEC MU), supported by the Czech Ministry of Education, Youth and Sports (MEYS CR) (LM2018129 Czech-BioImaging).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
The study was approved by ethics committees of the participating institutions.
Consent to participate
All participants gave their informed consent before the study started.
Consent for publication
Corresponding author has the right to publish any and all data separate and apart from any sponsor.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Říha, P., Brabenec, L., Mareček, R. et al. The reduction of hippocampal volume in Parkinson’s disease. J Neural Transm 129, 575–580 (2022). https://doi.org/10.1007/s00702-021-02451-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-021-02451-8