Abstract
Objectives
The purpose of this study was to investigate whether a correlation exists between 18F-fluorodeoxyglucose (FDG) uptake and prognostic factors in triple-negative breast cancer (TNBC).
Methods
Between January 2009 and December 2013, 103 patients (mean age, 50.6 years) with primary TNBC (mean, 2.6 cm; range, 1.0–6.5 cm) underwent 18F-FDG PET/CT for initial staging. Correlations between maximum standardized uptake value (SUVmax) on PET/CT and prognostic factors including tumour size, nodal status, histological grade, Ki-67 proliferation index, tumour suppressor p53, and ‘basal-like’ markers (epidermal growth factor receptor and CK 5/6) were assessed.
Results
The mean SUVmax of the 103 tumours was 10.94 ± 5.25 (range: 2–32.8). There was a positive correlation between SUVmax and Ki-67 (Spearman’s rho = 0.29, P = 0.003) and tumour size (Spearman’s rho = 0.27, P = 0.006), whereas this relationship was not observed in the nodal status, histological grade, p53 status and ‘basal-like’ phenotypes. In a multivariate regression analysis, Ki-67 (P < 0.001) and tumour size (P = 0.009) were significantly associated with SUVmax in TNBCs.
Conclusions
Increased 18F-FDG uptake on PET/CT was correlated with a high Ki-67 proliferation index and larger tumour size in TNBC. These results suggest a potential role of 18F-FDG PET/CT in identifying TNBC with more aggressive behaviour.
Key points
• A wide range of FDG uptake reflected heterogeneity of cancer metabolism.
• FDG uptake was correlated with the Ki-67 proliferation index in TNBC.
• FDG uptake was correlated with tumour size in TNBC.
• FDG uptake was not correlated with ‘basal-like’ phenotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triple-negative breast cancer (TNBC), which is defined by a lack of oestrogen receptor (ER) and progesterone receptor (PR) expression, as well as a lack of human epidermal growth factor receptor-2 (HER2) amplification, represents approximately 15–20 % of invasive breast cancers and is associated with higher rates of relapse and worse overall survival than ER-positive, PR-positive and HER2-negative breast cancer [1–3]. The reasons for this unfavourable prognosis include the aggressive nature of the disease and the absence of effective targeted therapy [4]. TNBC is a heterogeneous disease, and the identification of biomarkers to reliably select high- and low- risk subsets of patients at the time of surgery is important for treatment decision-making [4, 5]. In addition to tumour size, axillary lymph node status, histological grade, immunohistochemically defined molecular markers, such as the Ki-67 proliferation index, the tumour suppressor p53, and basal-like phenotype markers [e.g., epidermal growth factor receptor (EGFR) and cytokeratin (CK5/6)] have been reported to be prognostic factors in TNBC [5]. A basal-like phenotype is common in breast cancers in young women or in BRCA1 mutation carriers [6].
In breast cancer patients, 18F-fluorodeoxyglucose (FDG) PET/CT is a valuable tool for the initial staging or restaging of recurrence and for monitoring the response to chemotherapy [7–9]. Previous studies have correlated 18F-FDG uptake expressed as the maximum standardized uptake value (SUVmax) with tumour size, histological grade or hormonal receptor expression status, all of which are important prognostic indicators for long-term survival in breast cancer patients [10]. Several studies have investigated the correlation between tumour 18F- FDG uptake and breast cancer subtypes [11–13]. TNBCs with aggressive biology are associated with higher SUVmax than ER-positive or HER2-positive breast cancers. Recent data suggest that 18F-FDG PET/CT in TNBC can help predict a patient’s response to chemotherapy and a risk of early relapse [14]. In a study of 30 patients with TNBC, the 3-year disease-free survival rates were 90.9 % for patients with a tumour of SUVmax < 8.6 and 42.9 % for patients with a tumour of SUVmax > 8.6 (P < 0.002); high SUVmax was the only significant independent prognostic factor (P < 0.047) [15]. However, very few studies have investigated the association between molecular markers and 18F-FDG uptake in TNBC [12]. There is a need for imaging biomarkers that can identify TNBCs with more aggressive biological behaviour and direct cancer therapy for clinical trials [16, 17].
Therefore, the purpose of this study was to investigate whether a correlation exists between quantitative 18F- FDG uptake on PET/CT expressed as SUVmax and prognostic factors in operable primary TNBCs.
Materials and methods
Patient selection
The institutional review board of our hospital approved this retrospective study; the requirement for informed consent was waived. Between January 2009 and December 2013, a consecutive series of 1194 patients with newly diagnosed invasive breast cancer who had not received neoadjuvant chemotherapy underwent whole-body 18F-FDG PET/CT for initial staging followed by surgical treatment. We reviewed the medical records and pathology reports of 186 patients who had been surgically diagnosed with TNBC during the study period. We excluded 36 patients who underwent whole-body 18F-FDG PET/CT on a different PET/CT system, two patients who had undergone previous excisional biopsy, 21 patients with an invasive tumour size of less than 1 cm because of partial volume effects, and 24 patients who lacked available data on immunohistochemical profiles of their tumours. In total, 103 index breast cancers in 103 female patients (mean age, 50.6 years) were included in this study. The size of tumours based on histopathology ranged from 1.0–6.5 cm (mean, 2.6 cm).
PET/CT
Before intravenous administration of 18F-FDG (5.2 MBq/kg of body weight), the patients were instructed to fast for at least 4 hours to ensure a serum glucose level of less than 7.8 mmol/L. One hour after the intravenous administration of 18F-FDG, images from the base of the skull to the proximal thigh were acquired using commercially available PET/CT systems (Biograph, Siemens Medical Solutions, Hoffmann Estates, IL, USA) in 3D mode. During image acquisition, CT images were obtained first for attenuation correction and anatomical correlation without the use of intravenous contrast media (tube voltage, 120 kVp; current intensity, 50–160 mAs; transaxial FOV, 56.5 cm; axial FOV, 18 cm). Emission data were acquired for 2 min per bed. PET images were reconstructed using an iterative algorithm with two iterations, 21 subsets, a matrix size of 168 × 168, a 60.5-cm transaxial field of view and a 21.6-cm axial field of view. Specifically, the ordered-subset expectation maximization (OSEM) algorithm was used.
PET, CT and fused PET/CT images were generated and then reviewed on a computer workstation. All PET/CT images were interpreted by two institutional nuclear medicine physicians (10 years’ and 8 years’ experience, respectively, in PET/CT imaging) individually as a standard-of-care examination. For the quantitative analysis of 18F-FDG uptake, a region of interest (ROI) was placed over the most intense area of 18F-FDG accumulation for each patient. The SUV was calculated as follows:
SUV = region’s radioactivity concentration (Bq/mL)/injected dose (Bq)/patient’s weight (g). The SUVmax, which was defined as the peak SUV in the pixel with the highest count within the ROI, was measured and recorded to determine the focal areas of uptake.
Histological evaluation
Mastectomy (n = 25) or breast-conserving surgery (n = 78) was performed for all breast cancers. Tumour size, histological grade, lymph node involvement status, and the presence of ductal carcinoma in situ (DCIS) were determined from the surgical specimens. The expression of ER, PR, HER2, Ki-67, p53, EGFR and cytokeratin (CK) 5/6 was evaluated using immunohistochemical (IHC) analysis as part of the routine pathological assessment at our institution. All primary antibodies were monoclonal antibodies: ER (1:100, 1D5; Dako, Glostrup, Denmark), PR (1:200, PgR636; Dako), c-erbB2 (1: 200, CB11; Leica Microsystems, Berlin, Germany), Ki-67 (1:300, MIB-1; Dako), p53 (1:500, DO-7; Dako), EGFR (1:40, 2-18c9; Dako) and CK 5/6 (1:50, D5/16B4; Dako).
Positivity for ER and PR was defined as the presence of 1 % or more positively stained nuclei in ten high-power fields [18]. The intensity of HER2 staining was scored as 0, 1+, 2+ or 3+. Tumours with a score of 3+ were classified as HER2-positive, and tumours with a score of 0 or 1+ were classified as HER2-negative. Gene amplification using fluorescence in situ hybridization (FISH) was used to determine HER2 status in tumours with a score of 2+ (n = 9). HER2 expression was considered positive if the ratio of HER2 gene copies to chromosome 17 signals was greater than 2.2 [19]. Based on immunohistochemistry or FISH, ER-negative, PR-negative and HER2-negative tumours were classified as triple-negative. The Ki-67 of each case was evaluated based on the percentage of Ki-67-positive cells among at least 200 tumour cells [20]. The percentage of tumour cells with nuclear staining for p53 was graded semi-quantitatively: 0 %, 1–25 %, 26–50 %, 51–75 % or >75 %. For statistical analysis, p53 expression was considered as negative if ≤25 % of tumour cells were stained and as positive if >25 % of tumour cells were stained [21]. Cases were considered EGFR-positive or CK 5/6-positive if any cytoplasmic and/or membranous staining of the tumour cells was observed [22].
Data and statistical analysis
All cases were assigned to one of two groups according to the dichotomized clinicopathological variables and prognostic markers: age (≤50 years vs. >50 years), tumour size (≤2 cm vs. >2 cm), axillary nodal status (negative vs. positive metastasis), histological grade (grades 1 and 2 vs. grade 3), the presence of associated DCIS (negative vs. positive), the Ki-67 proliferation index (≤20 % vs. >20 %) and the p53 status (negative vs. positive). TNBCs were categorised as ‘basal’ (positive for EGFR and/or CK 5/6) or ‘non-basal’ (negative for EGFR and CK 5/6) [22, 23]. Characteristics of the ‘basal’ and ‘non-basal’ tumours were compared using the Chi-squared test and Fisher’s exact test.
The SUVmax values were not normally distributed; therefore, the distributions of the SUVmax values between groups according to the prognostic factors were compared using the nonparametric Wilcoxon rank-sum test. We used linear regression analysis to evaluate the association between the SUVmax and the prognostic markers. Variables with P < 0.05 in the univariate analysis were applied to a multivariate analysis to determine which variables were independently associated with SUVmax. The correlations among SUVmax, tumour size (cm) and Ki-67 (%) were assessed using Spearman correlation analysis. The data were analysed using statistical software (SAS version 9.2; SAS Institute, Cary, NC, USA). Statistical significance was defined as P < 0.05.
Results
Tumour characteristics
The mean size of the invasive tumours was 2.6 ± 1.0 cm (range: 1.0–6.5 cm); 35 patients had a pT1 (≤2 cm), 65 patients had a pT2 (>2 cm but ≤5 cm) and three patients had a pT3 (>5 cm) tumour. Positive axillary lymph nodes were detected in 23 patients (overall incidence of 22.3 %). The stage distribution was 30 IA, 52 IIA, 15 IIB, 5 IIIA, and 1 IIIB; the histological cancer types were 100 invasive ductal carcinomas of the usual type, one mucinous carcinoma, one metaplastic carcinoma and one adenoid cystic carcinoma; and the histological grades were grade 1 (n = 2, 1.9 %), grade 2 (n = 7, 6.8 %) and grade 3 (n = 94, 91.3 %). Sixty-four patients had a DCIS component (62.1 %). Eighty patients (77.7 %) had low Ki-67 (≤20 %) scores and 23 patients (22.3 %) had high Ki-67 (>20 %) scores. P53 was positive in 57 patients (55.3 %). EGFR and CK5/6 were positive in 35 (34 %) and 41 (39.8 %) patients, respectively.
The ‘basal’ group accounted for 56.3 % (n = 58) of the total 103 TNBCs. The ‘basal’ and ‘non-basal’ subgroups were similar in terms of mean age at diagnosis (51.7 ± 11.3 vs. 49.2 ± 8.4 years), invasive tumour size (2.6 ± 1.0 vs 2.7 ± 1.0 cm), lymph node involvement status (22.4 % vs. 22.2 %), histological grade (89.7 % vs. 93.3 %), the presence of DCIS (60.3 % vs. 64.4 %) and p53 status (58.6 % vs. 51.1 %) (all P values > 0.2). However, more tumours with high Ki-67 scores were identified in the ‘basal’ group than in the ‘non-basal’ group (29.3 % [17/58] vs. 13.3 % [6/45]); this finding was of borderline significance (P = 0.053).
Correlation between SUVmax and prognostic markers
The mean SUVmax value of the 103 tumours was 10.94 ± 5.25 (range: 2–32.8). The mean SUVmax value for tumours >2 cm (11.7 ± 5.6) was higher than that of tumours ≤2 cm (9.2 ± 4.0, P = 0.031). The mean SUVmax value for tumours with Ki-67 > 20 % (14.2 ± 6.1) was higher than that of tumours with Ki-67 ≤ 20 % (10.0 ± 4.6, P = 0.003). However, the SUVmax values did not differ significantly between the ‘non-basal’ and the ‘basal’ subgroups (11.4 ± 6.0 vs. 10.6 ± 4.7, P = 0.778). In the univariate analysis, tumour size (cm) and Ki-67 scores were significantly associated with SUVmax (P = 0.022 and P = 0.001), whereas this relationship was not observed for age (≤50 years vs. >50 years), the axillary lymph node status (negative vs. positive), the histological grade (grade 1 and 2 vs. grade 3), the presence of DCIS (no vs. yes), p53 status (negative vs. positive) and ‘basal’ or ‘non-basal’ type (Table 1).
In the multivariate regression analysis, Ki-67 scores and tumour size were significantly associated with SUVmax (Table 2). TNBCs measuring >2 cm and Ki-67 > 20 % had 1.31-fold (P = 0.009) and 1.51-fold (P < 0.001) higher SUVmax values, respectively, compared with TNBCs measuring ≤2 cm and Ki-67 ≤ 20 %, after adjustment for age, ‘basal’ or ‘non-basal’ type, axillary lymph node involvement status, histological grade, the presence of DCIS and p53 status.
There was a positive correlation between 18F-FDG uptake, as measured by SUVmax, and tumour size (Spearman’s rho = 0.27, P = 0.006) as well as Ki-67 (Spearman’s rho = 0.29, P = 0.003) (Figs. 1, 2 and 3). Ki-67 was not correlated with tumour size (Spearman’s rho = 0.03, P = 0.789).
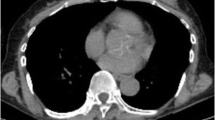
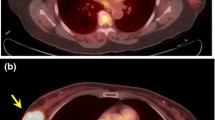
PET/CT images of three patients with TNBC. (a) A 72-year-old woman with a 1.8-cm, invasive ductal carcinoma of the left breast (Ki-67 3 %) showing increased fluorodeoxyglucose (FDG) uptake (maximum standardized uptake value (SUVmax) 5.2); (b) a 55-year-old woman with a 2.0-cm, invasive ductal carcinoma of the left breast (Ki-67 50 %) showing intense FDG uptake (SUVmax 14.5); (c) a 42-year-old woman with a 2.5-cm, invasive ductal carcinoma of the left breast (Ki-67 70 %) showing very intense FDG uptake (SUVmax 32.8)
Discussion
In this study, we investigated whether a correlation existed among tumour 18F-FDG uptake on PET/CT, expressed as SUVmax, clinicopathological variables and molecular markers in 103 operable, stage I–III TNBCs. We observed that SUVmax was higher in TNBCs with larger invasive tumour size and higher Ki-67 scores. There was a wide range of 18F-FDG uptake values (SUVmax 2–32.8), reflecting the heterogeneity of glucose metabolism within TNBCs. In a previous study of 282 TNBCs, tumour size was found to have independent prognostic significance in the lymph node-positive subgroup [5]. A study by Tchou et al. [12] indicated that SUVmax was significantly correlated with Ki-67 in 22 women with TNBC. Our results, as well as the results of previous studies, support the idea that 18F-FDG PET/CT has the potential to be used as a noninvasive tool to assess the Ki-67 proliferation index in TNBCs and to select TNBCs with more aggressive biological behaviour. The present study involves the largest sample ever used to evaluate 18F-FDG uptake in TNBC and is the first to correlate 18F-FDG uptake with various molecular markers, including p53, EGFR and CK 5/6.
Ki-67 has been used as a marker of proliferative activity of cells in various cancers [24], and a high Ki-67 value is associated with worse prognosis in breast cancer patients [25]. The St. Gallen International Expert Consensus on Primary Therapy for Early Breast Cancer (2009) adopted Ki-67 as a proliferation index to indirectly support the augmentation of chemotherapy with endocrine therapy in patients with ER-positive breast cancer [26]. Keam et al. [27] evaluated Ki-67 in 105 patients with TNBC who were treated with neoadjuvant chemotherapy; they demonstrated that two subgroups of TNBC can be defined based on Ki-67, with different response rates and prognoses. A recent meta-analysis confirmed the prognostic function of Ki-67 in relation to pathological complete response rates in patients receiving neoadjuvant chemotherapy for TNBC [28]. The main disadvantage of Ki-67, however, is the high degree of interobserver variability in its assessment [29]. Ki-67 values can vary as a function of several critical factors, including human error, selection of the tumour areas to be evaluated for Ki-67, and the specific antibody used. An international Ki-67 study group showed improved consistency of Ki-67 interpretation after a standardisation and training process [30].
Cheang et al. [23] demonstrated that a ‘core basal’ group (ER -, PR -, HER2 -, positivity for either EGFR or CK5/6) had significantly worse outcomes (10-year breast cancer-specific survival, 62 %) with an absolute 10 % lower survival than a five marker-negative phenotype group (ER -, PR -, HER2 -, EGFR -, CK5/6 -), and suggested that the ‘core basal’ classification identifies a subset of particularly high-risk patients. However, there was no significant difference in SUVmax between the ‘basal’ and ‘non-basal’ group in this study. Additionally, there was a wide range of 18F-FDG uptake within two subgroups, reflecting the heterogeneity of glucose metabolism within TNBC. This discrepancy suggests that grouping TNBC into ‘basal’ and ‘non-basal’ subtypes according to IHC may oversimplify the inter- or intra-tumour heterogeneity of TNBC. Recent gene profiling studies revealed six subtypes of TNBC, including two basal-like, an immunomodulatory, a mesenchymal, a mesenchymal stem-like and a luminal androgen receptor subtype [31]. Further studies are needed to investigate whether 18F-FDG PET/CT can be used as a non-invasive tool to study the metabolic phenotypes of the TNBC subtypes [32].
We found no correlation between 18F-FDG uptake and the presence of DCIS. A potential explanation is that DCIS does not demonstrate either an increased vascularity or glycolytic activity [33]. In this study, the SUVmax data did not show a correlation with the axillary lymph node involvement status and p53 status. According to a recent study, the tumour SUVmax on preoperative PET/CT correlated well with axillary lymph node metastasis for ER-positive/HER2-negative and HER2-positive tumours, but not for triple-negative tumours [34]. Prior breast cancer studies have demonstrated a correlation between 18F-FDG uptake and p53 status [10, 35], but there is also conflicting data in a study of 61 patients that failed to show a correlation [36]. Silwal-Pandit et al. [37] reported that p53 mutations were associated with a worse outcome in luminal B, HER2-enriched and normal-like subtypes, whereas no significant effect was observed in the basal-like and luminal A subtypes. Further investigation is warranted to identify the association between tumour metabolism and clinicopathological factors for improved prediction of clinical outcome in TNBC patients.
Our study has several limitations. First, this was a retrospective analysis of a cohort of 103 women who underwent preoperative 18F-FDG PET/CT at a single cancer centre, which unavoidably introduces selection bias. Second, interobserver variation was not assessed in this study. We performed 18F-FDG PET/CT scans using a single scanner and used region of interest (ROI) drawing and SUVmax for the measurement of 18F-FDG uptake intensity. Further measurements, including texture analysis of 3D matrices, may have reflected the intra- and inter-tumoral heterogeneity of TNBC [38]. Finally, we did not attempt to perform a survival analysis because the follow-up duration in the study population was relatively short (median follow-up duration: 29 months).
In conclusion, we have found that increased 18F-FDG uptake on PET/CT correlated with higher Ki-67 and larger tumour size in patients with TNBC. These results suggest a potential role of PET/CT in identifying TNBCs with more aggressive behaviour in clinical practice. In the future, larger prospective studies are needed to define the role of 18F-FDG PET/CT in the treatment decision for women with TNBC.
References
Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V (2007) Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer 109:1721–1728
Dent R, Trudeau M, Pritchard KI et al (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13:4429–4434
Hudis CA, Gianni L (2011) Triple-negative breast cancer: an unmet medical need. Oncologist 16:1–11
Lehmann BD, Pietenpol JA (2014) Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J Pathol 232:142–150
Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO (2007) Prognostic markers in triple-negative breast cancer. Cancer 109:25–32
Prat A, Adamo B, Cheang MC, Anders CK, Carey LA, Perou CM (2013) Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist 18:123–133
Koolen BB, Vrancken Peeters MJ, Aukema TS et al (2012) 18F-FDG PET/CT as a staging procedure in primary stage II and III breast cancer: comparison with conventional imaging techniques. Breast Cancer Res Treat 131:117–126
Aukema TS, Rutgers EJ, Vogel WV et al (2010) The role of FDG PET/CT in patients with locoregional breast cancer recurrence: a comparison to conventional imaging techniques. Eur J Surg Oncol 36:387–392
Hatt M, Groheux D, Martineau A et al (2013) Comparison between 18F-FDG PET image-derived indices for early prediction of response to neoadjuvant chemotherapy in breast cancer. J Nucl Med 54:341–349
Groheux D, Giacchetti S, Moretti JL et al (2011) Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging 38:426–435
Basu S, Chen W, Tchou J et al (2008) Comparison of triple-negative and estrogen receptor-positive/progesterone receptor-positive/HER2-negative breast carcinoma using quantitative fluorine-18 fluorodeoxyglucose/positron emission tomography imaging parameters: a potentially useful method for disease characterization. Cancer 112:995–1000
Tchou J, Sonnad SS, Bergey MR et al (2010) Degree of tumor FDG uptake correlates with proliferation index in triple negative breast cancer. Mol Imaging Biol 12:657–662
Koo HR, Park JS, Kang KW et al (2014) 18F-FDG uptake in breast cancer correlates with immunohistochemically defined subtypes. Eur Radiol 24:610–618
Groheux D, Hindie E, Giacchetti S et al (2012) Triple-negative breast cancer: early assessment with 18F-FDG PET/CT during neoadjuvant chemotherapy identifies patients who are unlikely to achieve a pathologic complete response and are at a high risk of early relapse. J Nucl Med 53:249–254
Ohara M, Shigematsu H, Tsutani Y et al (2013) Role of FDG-PET/CT in evaluating surgical outcomes of operable breast cancer: usefulness for malignant grade of triple-negative breast cancer. Breast 22:958–963
Mountz JM, Yankeelov TE, Rubin DL et al (2014) Letter to cancer center directors: progress in quantitative imaging as a means to predict and/or measure tumor response in cancer therapy trials. J Clin Oncol 32:2115–2116
Mankoff DA, Pryma DA, Clark AS (2014) Molecular imaging biomarkers for oncology clinical trials. J Nucl Med 55:525–528
Hammond ME, Hayes DF, Dowsett M et al (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28:2784–2795
Wolff AC, Hammond ME, Schwartz JN et al (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145
Zabaglo L, Salter J, Anderson H et al (2010) Comparative validation of the SP6 antibody to Ki67 in breast cancer. J Clin Pathol 63:800–804
Zellars RC, Hilsenbeck SG, Clark GM et al (2000) Prognostic value of p53 for local failure in mastectomy-treated breast cancer patients. J Clin Oncol 18:1906–1913
Nielsen TO, Hsu FD, Jensen K et al (2004) Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 10:5367–5374
Cheang MC, Voduc D, Bajdik C et al (2008) Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res 14:1368–1376
Gerdes J, Li L, Schlueter C et al (1991) Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol 138:867–873
Inwald EC, Klinkhammer-Schalke M, Hofstadter F et al (2013) Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat 139:539–552
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ (2011) Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 22:1736–1747
Keam B, Im SA, Lee KH et al (2011) Ki-67 can be used for further classification of triple negative breast cancer into two subtypes with different response and prognosis. Breast Cancer Res 13:R22
Zhang G, Xie W, Liu Z et al (2014) Prognostic function of Ki-67 for pathological complete response rate of neoadjuvant chemotherapy in triple-negative breast cancer. Tumori 100:136–142
Urruticoechea A, Smith IE, Dowsett M (2005) Proliferation marker Ki-67 in early breast cancer. J Clin Oncol 23:7212–7220
Polley MY, Leung SC, McShane LM et al (2013) An international Ki67 reproducibility study. J Natl Cancer Inst 105:1897–1906
Lehmann BD, Bauer JA, Chen X et al (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Investig 121:2750–2767
Kim S, Kim do H, Jung WH, Koo JS (2013) Metabolic phenotypes in triple-negative breast cancer. Tumour Biol 34:1699–1712
Uematsu T, Kasami M, Yuen S (2009) Comparison of FDG PET and MRI for evaluating the tumor extent of breast cancer and the impact of FDG PET on the systemic staging and prognosis of patients who are candidates for breast-conserving therapy. Breast Cancer 16:97–104
Kim JY, Lee SH, Kim S, Kang T, Bae YT (2015) Tumour 18F-FDG Uptake on preoperative PET/CT may predict axillary lymph node metastasis in ER-positive/HER2-negative and HER2-positive breast cancer subtypes. Eur Radiol 25:1172–1181
Gil-Rendo A, Martinez-Regueira F, Zornoza G, Garcia-Velloso MJ, Beorlegui C, Rodriguez-Spiteri N (2009) Association between [18F]fluorodeoxyglucose uptake and prognostic parameters in breast cancer. Br J Surg 96:166–170
Buck A, Schirrmeister H, Kuhn T et al (2002) FDG uptake in breast cancer: correlation with biological and clinical prognostic parameters. Eur J Nucl Med Mol Imaging 29:1317–1323
Silwal-Pandit L, Vollan HK, Chin SF et al (2014) TP53 mutation spectrum in breast cancer is subtype specific and has distinct prognostic relevance. Clin Cancer Res 20:3569–3580
Soussan M, Orlhac F, Boubaya M et al (2014) Relationship between tumor heterogeneity measured on FDG-PET/CT and pathological prognostic factors in invasive breast cancer. PLoS One 9:e94017
Acknowledgments
The scientific guarantor of this publication is Woo Kyung Moon. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. 2012R1A2A1A01010846). No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Methodology: retrospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koo, H.R., Park, J.S., Kang, K.W. et al. Correlation between 18F-FDG uptake on PET/CT and prognostic factors in triple-negative breast cancer. Eur Radiol 25, 3314–3321 (2015). https://doi.org/10.1007/s00330-015-3734-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3734-z