Abstract
Background
FDG PET has not yet found a role in the clinical evaluation of the tumor extent of breast cancer. FDG PET has been reported to be useful for evaluating the prognoses of breast cancer patients with more accuracy than conventional imaging modalities. The purpose of this study was to compare the accuracy of FDG PET and MRI for the preoperative assessment of the tumor extent of breast cancer, for evaluating the impact of FDG PET on systemic staging, and also for predicting the prognosis of patients who are candidates for breast-conserving therapy.
Methods
The study was a prospective series of 23 breasts with breast cancer that underwent both FDG PET and MRI before surgery. Systemic staging with FDG PET was also performed. The correlation between the results of these examinations and histological findings was thus examined. The maximum standardized uptake value (SUVmax) of the tumors was investigated in association with the patient prognoses.
Results
When evaluating the local tumor extent, the accuracy of FDG PET (43.5%) was significantly lower than that of MRI (91%) (P < 0.001). The sensitivity, specificity, and accuracy of FDG PET regarding the nodal status were 60, 94, and 87%, respectively. No patients demonstrated any distant metastasis, whereas FDG PET gave a false positive in one patient. The mean follow-up period was 61 months. The SUVmax value of the worse prognosis patient group was significantly higher than that of the good prognosis patient group (P = 0.032).
Conclusions
FDG PET is not a breast imaging modality for evaluating the local tumor extent, but it is useful for predicting the prognoses of patients who are candidates for breast-conserving therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast-conserving therapy (breast-conserving surgery with radiation therapy) (BCT) is an accepted alternative to a mastectomy for the treatment of many women with early-stage invasive breast cancer [1]. The recommended technique is the local excision of the primary tumor with clear margins, which has provided good cosmetic results [1]. Although various factors have been identified that affect the rate of local recurrence after BCT [2], negative margins of BCT are necessary to obtain optimal local tumor control [3, 4]. A preoperative breast imaging evaluation of the intraductal spread of breast cancer (ductal carcinoma in situ: DCIS) is essential to determine a patient’s eligibility for BCT [5, 6]. Although MRI, multidetector row CT and ultrasonography can complement mammography for the preoperative evaluation of patients who are candidates for BCT, MRI has been reported to be the most accurate breast imaging modality for determining the tumor extent of breast cancer [6]. FDG PET has a relatively low diagnostic accuracy for small breast cancer because of the limited spatial resolution of PET devices [7]. Therefore, it cannot be expected FDG PET will detect the intraductal spread of breast cancer. Avril et al. [8] reported that only nine out of 18 patients who had multifocal or multicentric breast cancer were identified by FDG PET, whereas Schirrmeister et al. [9] reported that FDG PET was twice as sensitive at detecting multifocal lesions than a combination of mammography and ultrasound. Moreover, there are still very few reports in the literature that report a comparison between FDG PET and MRI findings regarding the tumor extension of breast cancer [10, 11]. FDG PET exhibited a sensitivity of 92.5%, which, though slightly lower than that of MRI (95.2%), was superior to that of conventional modalities [10]. Heinisch et al. [11] reported that MRI was more accurate than FDG PET in the assessment of multifocal disease, but the study had only three patients with multifocal disease. Therefore, FDG PET has not yet found a role in the clinical evaluation of the tumor extent of breast cancer.
Recently, FDG PET has been reported to be useful for evaluating the prognoses of breast cancer patients, yielding more accuracy than conventional imaging modalities [12–14]. A high maximum standardized uptake value (SUV max) is suggested to be useful in the preoperative evaluation of the patient prognosis, and the findings have also been reported to be more accurate that with other modalities [13, 14]. The purpose this study was to compare the accuracy of FDG PET and MRI for preoperative assessment of the tumor extent of breast cancer and to evaluate the impact of FDG PET on systemic staging and for predicting the prognoses of patients who are candidates for BCT.
Subjects and methods
This study comprised a prospective series of 22 patients with breast cancer who underwent both FDG PET and MRI within 34 days (mean, 10 days). One patient had bilateral breast cancer. Therefore, a total of 23 breasts with histologically proven breast cancer were eligible for this study. Our institutional review board approved the protocol, and written informed consent was obtained from all patients.
FDG PET imaging
FDG PET imaging was performed using a modern PET camera (Advance NXi; GE Medical Systems, Milwaukee, WI, USA). The patients fasted for 4 h before the PET scan. The emission scan was started 60 min after the injection of 220–240 MBq 18F-fluorodeoxyglucose. The FDG PET scans included 6–7 bed positions (5 min acquisition time per position; total acquisition time, 30–35 min) while covering the area from the skull to the proximal femora. The results of post-emission transmission scans were used to correct for attenuation. The standardized uptake values were calculated.
MRI
MRI examinations were performed with the patients in the prone position. The instrument used was a 1.5 T commercially available system (Gyroscan Intera; Philips Medical Systems, Best, The Netherlands) with double breast-surface coils. Our imaging protocol included a localizing sequence followed by sagittal fast-spin echo T2-weighted imaging (TR/TE, 5056/90; ETL, 15; matrix, 158 × 320) with fat suppression (SPIR; spectral presaturation inversion recovery) of the affected breast. Other parameters were: field-of-view, 18 cm; section thickness, 4 mm; interslice gaps, 0.8 mm. This examination was followed by a dynamic study of the affected breast, consisting of serial imaging of a three-dimensional (3D) sagittal turbo-field echo T1-weighted sequence (TR/TE, 11/5.4; flip angle, 20; matrix, 143 × 256) with fat suppression (ProSet; principle of selective excitation technique). The parameters were field-of-view, 18 cm; section thickness, 2 mm; interslice gap, −1 mm. Gadopentetate dimeglumine (Magnevist; Nihon Schering, Osaka, Japan) was administered as a bolus intravenous injection (2 mL/s) at a dose of 0.1 mmol/kg body weight. This was followed by a 20-mL saline solution flush. For dynamic studies, we acquired one pre- and three post-enhancement scans; the scan time was 2 min per scan.
MMG examination
Bilateral digital MMG was performed (Senographe 2000D unit; GE Medical Systems, Milwaukee, WI, USA), including routine craniocaudal and mediolateral oblique views of the breasts and spot-magnification views of the area of the lesion.
Breast imaging interpretations
The digital mammograms on soft-copy reading were independently double-read using BI-RADS assessment categories [15] by two radiologists with 8–12 years of experience in mammography. The readers also rated breast density according to the standard BI-RADS scale (extremely dense, heterogeneously dense, scattered fibroglandular densities, and almost completely fat). When different BI-RADS assessment categories and BI-RADS breast density scales were assigned by the two readers, then a consensus was reached after discussing the findings. MR images were independently interpreted by one of two radiologists with knowledge of the clinical and mammographic findings using the BI-RADS MR lexicon [15]. Any contrast enhancement contiguous with the index tumor was considered to be positive [6, 16, 17]. A diffuse enhancement in the bilateral breasts was considered to be negative. Following the BI-RADS MR lexicon [15], the morphology and kinetics at MRI were evaluated for all enhanced lesions. FDG PET images were independently interpreted by one of two radiologists with knowledge of the clinical and mammographic findings. The focally marked increased FDG uptake was defined as an index tumor. A segmentally increased FDG uptake in a single quadrant was defined as a multifocal lesion. A case with double focally marked increased FDG uptakes in greater than one quadrant was defined as a multicentric lesion. The FDG PET images and MRI images of a given individual were not interpreted by the same radiologist.
Histopathological examination
The samples for histopathological examination were prepared by making serial 5-mm slices of breast-conserving surgical specimens and 5–10-mm slices of mastectomy specimens according to the technique of Egan [18]. Histological diagnoses were made by one pathologist with 16 years of experience in breast histology. The correlations between the histological and the breast imaging findings were examined by the radiologists and pathologist in all cases.
Disease extent criteria
In an effort to avoid artificially inflating or deflating the performance of any imaging modality, we sought to identify a practical definition of disease extent. In this study, we focused on the intraductal spread and the extent of invasive tumor to evaluate the disease extent. The extent of disease was classified into the following three types: the breast carcinoma of limited extent (BCLE) type was defined as having no invasive carcinoma or DCIS beyond 1 cm from the edge of the dominant mass [6, 17, 19]; the multifocal non-BCLE type, defined as a mass having tumor foci beyond 1 cm from the edge of the index tumor in a single quadrant; and the multicentric non-BCLE type, defined as non-BCLE in more than one quadrant. Concerning MRI images, non-mass enhancement (focal, linear, ductal, segmental, and regional) as defined in the BI-RADS MR lexicon [15] corresponded to non-BCLE. Moreover, a mass with non-mass enhancement corresponded to non-BCLE. Concerning the mammograms, suspicious microcalcifications, focal asymmetric density, architectural distortion, or masses with an indistinct margin as defined in the BI-RADS mammography lexicon [15] corresponded to non-BCLE. Moreover, the presence of any microcalcifications and/or densities around and at a distance from the mass corresponded to non-BCLE [6, 17, 19]. Concerning FDG PET, a focally marked increased FDG uptake was defined as BCLE. A segmentally increased FDG uptake in a single quadrant was defined as a multifocal non-BCLE. A case with double focally marked increased FDG uptakes in greater than one quadrant was defined as a multicentric non-BCLE. Figure 1 is a schematic representation of the disease extent criteria used in this study.
Analysis
The findings from each breast imaging modality were compared with mapping data from the histopathological findings according to the prospectively defined three extent criteria: the BCLE type, the multifocal non-BCLE type, and the multicentric non-BCLE type. The chi-square and Fisher exact tests for statistical significance, with a value of P < 0.05 considered to be significant, were performed to compare each modality regarding its accuracy for detecting the tumor extent of breast cancer using a statistical software package (SPSS Inc., Chicago, IL, USA). The Mann–Whitney test was used to determine whether the SUVmax of tumors was related to the association with the patient prognoses.
Results
Among the 23 breast cancers, pathological examination revealed four BCLE (17%), 16 multifocal non-BCLE (70%) (Fig. 2), and three multicentric non-BCLE (13%). The histological results and the disease extents are summarized in Table 1. This study had 20 palpable tumors (87%) and three non-palpable tumors (13%). The mean age was 55 years, with a range of 35–75 years. Four breasts underwent a wide excision, nine breasts underwent a quadrantectomy, and ten breasts underwent a mastectomy. According to the standard BI-RADS scale, 13 of these 23 breasts (56.5%) were dense breasts (breast pattern BI-RADS; extremely dense and heterogeneously dense), and ten (43.5%) of them were fatty breasts (breast pattern BI-RADS; scattered fibroglandular densities and almost completely fat). In addition, nine (39%) of the 23 breast cancers had microcalcification findings on the mammograms.
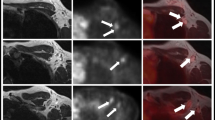
54-year-old woman with multifocal non-breast carcinoma of limited extent. A mastectomy revealed a high-grade DCIS. a, b Axial FDG PET slice and 3D maximum-intensity-projection FDG PET images showing the segmentally increased FDG uptake (arrows). c, d Sagittal T1-weighted contrast-enhanced MRI image and 3D maximum-intensity-projection MRI image showing segmental enhancement in the right upper quadrant. e Mediolateral mammogram showing segmental distribution of pleomorphic and coarse heterogeneous calcifications in the right upper quadrant
Detection of index tumor
The mean histological size of the index tumor was 28 mm (range 10–80 mm); the mean histological size of the invasive ductal carcinoma (IDC) was 16.4 mm (range 10–30 mm), the mean histological size of the DCIS was 52.5 mm (range 15–80 mm), and the mean histological size of the invasive lobular carcinoma (ILC) was 31.7 mm (range 20–50 mm). Of the 23 index breast tumors, 23 (100%) were detected on MRI, 21 (91%) were detected on FDG PET, and 20 (87%) were detected on mammograms. The pathological types of the index tumors missed by each modality are shown in Table 2. All index tumors missed on mammography were for dense breasts. One index tumor missed on mammography was also missed on FDG PET, but the other index tumors missed on mammography were visualized on FDG PET. The median size of the index tumors missed on FDG PET was 17.5 mm (range 15–20 mm). The median size of the index tumors missed on mammography was 30.7 mm (range 20–50 mm).
Overall accuracy of tumor extent
Table 3 summarizes the overall accuracy of the tumor extent, estimated using each of the imaging modalities. MRI was more accurate than FDG PET for the tumor extent of breast cancer. The accuracy of FDG PET (43.5%) was significantly lower than that of MRI (91%) (P < 0.001). The accuracy of mammography (30.4%) was significantly lower than that of MRI (91%) (P < 0.001). The accuracy of FDG PET was slightly, but not significantly, higher than the accuracy of mammography (P = 0.542). There was no overestimation of the tumor extent in these breast modalities in this study.
Axillary lymph node involvement
An axillary lymph node dissection was performed for 20 of the 23 (87%) patients. The remaining three patients did not undergo an axillary lymph node dissection because one had DCIS and the others were elderly persons. However, no false-negative diagnoses occurred in the three patients because there was no axillary lymph node metastasis within a clinical follow-up period of more than 60 months.
Overall, 22% (5/23) of the patients had positive lymph nodes in this study. The sensitivity, specificity, accuracy, positive and negative predictive value of FDG PET for nodal status were 60% (3/5), 94% (17/18), 87% (20/23), 75% (3/4), and 89% (17/19), respectively. The mean diameter of false-negative lymph node involvement was 7.5 mm (range 7–8 mm), whereas the mean diameter of true-positive lymph node involvement was 23.3 mm (range 20–30 mm). One false-positive lymph node involvement was a case with reactive lymph node swelling.
Distant metastases
There was no patient with distant metastases in the initial staging, although FDG PET was suggestive of metastasis in one patient, thus corresponding to radiological studies; the clinical examinations and follow-up were considered to be consistent with lung sarcoidosis.
SUVmax and prognoses
Two DCIS were not visualized by FDG-PET. The remaining 21 index tumors were visualized by FDG-PET. The mean SUVmax value for IDC was higher than those for DCIS (P = 0.405) or ILC (P = 0.08), but not significantly so (Table 4).
The mean follow-up period was 61 months (range 50–64 months). A recurrence of breast cancer was diagnosed in two (9%) patients with disseminated disease. Of those two patients, one died 56 months after the first surgery, while the other has survived with recurrent breast cancer for 64 months following the first surgery. The patient who died had triple negative breast cancer with axillary lymph node metastasis. The surviving patient with recurrent breast cancer had non-triple negative breast cancer without axillary lymph node metastasis. The remaining 20 patients have been free of recurrence. The SUVmax value of the worse prognosis patient group (n = 2) was significantly higher than that of the good prognosis patient group (n = 20) (P = 0.032).
Discussion
To our knowledge, this is the first study to prospectively assess the accuracy of MRI, FDG PET, and mammography for the preoperative assessment of the local extent of intraductal spread of breast cancer. Although two small studies have reported comparisons of FDG PET and MRI for multifocal or multicentric lesions [10, 11], they did not report any clear definition or criterion for the diagnosis of multifocal or multicentric lesions. In this study, the use of the simple “BCLE” or “non-BCLE” tumor extent system was considered to be useful and objective because the 1-cm margin was not chosen arbitrarily but in accordance with the current practice of surgeons to excise tumors with at least a 1-cm margin of macroscopically uninvolved tissue based on the established BCT procedures [6, 17, 19]. The staging system represents a compromise between usefulness and convenience [6]. This study showed that MRI was significantly more accurate than FDG PET for assessing the tumor extent of breast cancer. This result was not surprising, because it cannot be expected that FDG PET will detect the intraductal spread of breast cancer due to the limited spatial resolution of PET devices. Avril et al. [8] reported that only nine out of 18 patients who had multifocal or multicentric breast cancer were identified by FDG PET. Heinisch et al. [11] reported that MRI was more accurate than FDG PET in the assessment of multifocal disease, but the study had only three patients with multifocal disease. The results of the present study and these studies show that FDG PET is not the optimal breast imaging modality for evaluating the local tumor extent for the preoperative evaluation of patients who are candidates for BCT. However, another previous study reported that FDG PET had a good sensitivity of 92.5% for multifocal lesions, which was slightly lower than that of MRI (95.2%) [9]. This difference may be the result of the different populations investigated in these studies, or the different definitions and criteria for diagnosis used for multifocal lesions. However, it is very important to recognize that FDG PET is not a powerful tool for discovering microscopic disease, such as the intraductal spread of breast cancer. This study showed that the accuracy of mammography was significantly lower than that of MRI. Our results also showed that the accuracy of FDG PET was slightly, but not significantly, higher than the accuracy of mammography. However, Schirrmeister et al. [9] reported that FDG PET was twice as sensitive at detecting multifocal lesions than a combination of mammography and ultrasound. The main cause of these different results may be the number of dense breast cases in the studies, because two index tumors missed on mammography were visualized by FDG PET in this study. FDG PET may not be affected by the dense breast tissue, which reduces the diagnostic value of mammography, because FDG PET can provide 3D visualization of the breast, like MRI. However, dense breast tissue may be unlikely to affect the ability of FDG PET to detect tumors [20]. In this study, two cases with DCIS were not visualized by FDG PET. Little information is available about the ability of FDG PET to detect DCIS. Previous studies suggest that FDG PET cannot contribute to an improved diagnosis of DCIS [8, 21]. This may be linked to tumor biology, because DCIS does not demonstrate either an increased vascularity or glycolytic activity. This study also showed that the mean SUVmax value of IDC was higher than those of DCIS or ILC, but not significantly so. Previous studies reported a higher glucose metabolism for IDC in comparison to ILC [22, 23]. The lower SUV in ILC might thus be explained by a lower tumor cell density and diffuse surrounding breast tissue infiltration, because partial-volume effects can affect the lower SUV.
In this study, the sensitivity, specificity, positive and negative predictive value of FDG PET for the nodal status were 60, 94, 75, and 89%, respectively. These results are similar to those of a previous study [24], which reported the sensitivity, specificity, positive and negative predictive value to be 61, 80, 62, and 79%, respectively, for FDG PET in axillary staging in 360 patients. This study showed the mean diameter of false-negative lymph node involvement to be 7.5 mm, whereas the mean diameter of true-positive lymph node involvement was 23.3 mm. The previous study also showed that FDG PET failed to detect small and few axillary lymph node metastases [24]. Wahl et al. [24] concluded that FDG PET was not routinely recommended for axillary staging of patients with newly diagnosed breast cancer, although it was highly predictive for nodal tumor involvement. Other previous studies suggested that a positive FDG PET can identify patients who require axillary lymph node dissection and could forego sentinel lymph node biopsy because of the low rate of false-positive findings [25, 26]. However, this study showed that one case of false-positive lymph node involvement was actually reactive lymph node swelling. Therefore, this aspect still requires further study.
Systemic staging is not routinely performed in patients who are candidates for BCT, due to the low likelihood of distant metastases. In fact, this study showed that no patient had distant metastases in the initial staging, although FDG PET was suggestive of metastasis in one patient with lung sarcoidosis. FDG PET is recommended as an option for patients with either recurrent or stage 4 breast cancer [27–29].
A previous study reported that the high uptake of FDG by tumor may be useful as a prognostic indicator for patients with primary breast cancer [12]. Another previous study reported that FDG PET was useful in the preoperative evaluation of the prognosis for breast cancer patients, yielding greater accuracy than conventional TNM staging [13]. This study showed that high SUVmax was significantly associated with a poor prognosis in breast cancer patients who are candidates for BCT. In this study, the dead patient had triple negative breast cancer with an axillary lymph node metastasis. Inoue et al. [13] reported that the prognosis for breast cancer patients with high SUVmax and FDG PET positive axillary lymph node involvement was significantly poorer than that for the other patients. Moreover, Basu et al. [30] reported that triple negative breast cancer with a poor prognosis was associated with enhanced FDG uptake, commensurate with its aggressive biology, and was detected with a very high sensitivity using FDG PET. The results from this study and previous studies suggest that FDG PET may therefore be useful for predicting the prognoses of patients who are candidates for BCT, and that it can also be a noninvasive imaging technique for selecting high-risk and low-risk patients at the time of primary surgery.
One problem with the present study is that the number of patients studied was small. Therefore, the statistical significance of these findings may be insufficient. Uematsu et al. [6] reported that MR imaging tended to result in an overestimation of the tumor extent. The small number of patients included in this study may therefore have masked the risk for such potential overestimation. Further validation is thus warranted, using a larger study population. Another problem with the present study is that relatively small injected doses of FDG were administered. It is possible that our injected doses (in the 220–240 MBq range) may have reduced the sensitivity of FDG PET. However, lower injected doses of FDG are typically given in Japan because most Japanese patients are smaller than Western patients. Finally, 13 of the 23 breasts studied underwent breast-conserving surgery. Therefore, pathologically, we could not determine whether these cancers were multicentric. However, no false-positive diagnoses occurred within a clinical follow-up period of more than 60 months.
In conclusion, FDG PET is not a breast imaging modality for evaluating tumor extent in patients who are candidates for BCT, but it may be useful for predicting the prognoses of patients who are candidates for BCT, and it is also a useful noninvasive imaging technique for selecting high-risk and low-risk patients at the time of primary surgery.
References
NIH Consensus Conference. Treatment of early-stage breast cancer. J Am Med Assoc. 1991;265:391–5.
Vicini FA, Eberlein TL, Connolly JL, Recht A, Abner A, Schnitt SJ, et al. The optimal extent of resection for patients with stages 1 or 2 breast cancer treated with conservative surgery and radiotherapy. Ann Sur. 1991;214:200–4.
Smitt MC, Nowels KW, Zdeblick MJ, Jeffrey S, Carlson RW, Stockdale FE, et al. The importance of the lumpectomy surgical margin status in long term results of breast conservation. Cancer. 1995;76:259–67.
Gage I, Schnitt SJ, Nixon AJ, Silver B, Recht A, Troyan SL, et al. Pathologic margin involvement and the risk of recurrence in patients treated with breast-conserving therapy. Cancer. 1996;78:1921–8.
Connolly JL, Boyages J, Nixon AJ, Peiro G, Silver B, Recht A, et al. Predictors of breast recurrence after conservative surgery and radiation therapy for invasive breast cancer. Mod Pathol. 1998;11:134–9.
Uematsu T, Yuen S, Kasami M, Uchida Y. Comparison of magnetic resonance imaging, multidetector row computed tomography, ultrasonography, and mammography for tumor extension of breast cancer. Breast Cancer Res Treat 2008; Epub ahead of print.
Avril N, Adler LP. F-18 fluorodeoxyglucose-positron emission tomography imaging for primary breast cancer and loco-regional staging. Radiol Clin North Am. 2007;45:645–57.
Avril N, Rose CA, Schelling M, Dose J, Kuhn W, Bense S, et al. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol. 2000;18:3495–502.
Schirrmeister H, Kuhn T, Guhlmann A, Santjohaser C, Horster T, Nuessle K, et al. Fluorine-18 2-deoxy-2 fluoro-D-glucose PET in the preoperative staging of breast cancer: comparison with the standard staging procedures. Eur J Nucl Med. 2001;28:351–8.
Rieber A, Schirrmeister H, Gabelmann A, Nuessle K, Reske S, Kreienberg R, et al. Pre-operative staging of invasive breast cancer with MR mammography and/or PET: boon or bunk? Br J Radiol. 2002;75:789–98.
Heinisch M, Gallowitsch HJ, Mikosch P, Kresnik E, Kumnig G, Gomez I, et al. Comparison of FDG-PET and dynamic contrast-enhanced MRI in the evaluation of suggestive breast lesions. Breast. 2003;12:17–22.
Oshida M, Uno K, Suzuki M, Nagashima T, Hashimoto H, Yagata H, et al. Predicting the prognoses of breast carcinoma patients with positron emission tomography using 2-deoxy-2-fluoro[18F]-D-glucose. Cancer. 1998;82:2227–34.
Inoue T, Yutani K, Taguchi T, Tamaki Y, Shiba E, Noguchi S. Preoperative evaluation of prognosis in breast cancer patients by [18F]2-deoxy-2-fluoro-D-glucose-positron emission tomography. J Cancer Res Clin Oncol. 2004;130:273–8.
Cermik TF, Mavi A, Basu S, Alavi A. Impact of FDG PET on the preoperative staging of newly diagnosed breast cancer. Eur J Nucl Med Mol Imaging 2007;35(3):475–83.
American College of Radiology. Breast imaging reporting and data system (BI-RADS), 4th ed. American College of Radiology, Reston; 2003.
Berg WA, Gutierrez L, NessAiver MS, Carter WB, Bhargavan M, Lewis RS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233:830–49.
Uematsu T, Sano M, Homma K, Sato N. Comparison between high-resolution helical CT and pathology in breast examination. Acta Radiol. 2002;43:385–90.
Egan RL. Multicentric breast carcinomas: clinical-radiographic–pathologic whole organ studies and 10-year survival. Cancer. 1982;49:1123–30.
Faverly DRG, Hendriks JHCL, Holland R. Breast carcinomas of limited extend. Cancer. 2001;91:647–59.
Vranjesevic D, Schiepers C, Silverman DH, Quon A, Villalpando J, Dahlbom M, et al. Relationship between 18F-FDG uptake and breast density in women with normal breast tissue. J Nucl Med. 2003;44:1238–42.
Tse NY, Hoh CK, Hawkins RA, Zinner MJ, Dahlbom M, Choi Y, Maddahi J, Brunicardi FC, Phelps ME, Glaspy. The application of positron emission tomographic imaging with fluorodeoxyglucose to the evaluation of breast disease. Ann Surg. 1992;216:27–34.
Crippa F, Seregni E, Agresti R, Chiesa C, Bogri A, Decise D, et al. Association between F-18 fluorodeoxyglucose uptake and postoperative histopathology, hormone receptor status, thymidine labeling index and p53 in primary breast cancer: a preliminary observation. Eur J Nucl Med. 1998;25:1429–34.
Avril N, Menzel M, Dose J, Schelling M, Weber W, Janicke F, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med. 2001;42:9–16.
Wahl RL, Siegel BA, Coleman E, Gatsonis CG. Prospective multicenter study of axillary nodal staging by positron emission tomography in breast cancer: a report of the staging breast cancer with PET study group. J Clin Oncol. 2004;22:277–85.
Lovrics PJ, Chen V, Coates G, Cornacchi SD, Goldsmith CH, Law C, et al. A prospective evaluation of positron emission tomography scanning sentinel lymph node biopsy, and standard axillary dissection for axillary staging in patients with early stage breast cancer. Ann Surg Oncol. 2004;11:846–53.
Barranger E, Grahek D, Antoine M, Montravers F, Talbot JN, Uzan S. Evaluation of fluorodeoxyglucose positron emission tomography in the detection of axillary lymph node metastases in patients with early-stage breast cancer. Ann Surg Oncol. 2004;10:622–7.
Eubank WB, Mankoff D, Bhattacharya M, Gralow J, Linden H, Ellis G, et al. Impact of FDG PET on defining the extent of disease and on the treatment of patients with recurrent or metastatic breast cancer. Am J Roentgenol. 2004;183:479–86.
Vranjesevic D, Filmont JE, Meta J, Silverman DH, Phelps ME, Rao J, et al. Whole-body (18) F-FDG PET and conventional imaging for predicting outcome in previously treated breast cancer patients. J Nucl Med. 2002;43:325–9.
Monn DH, Maddahi J, Silverman DH, Glaspy JA, Phelps ME, Hoh CK. Accuracy of whole-body fluorine-18-FDG PET for the detection of recurrent or metastatic breast carcinoma. J Nucl Med. 1998;39:431–5.
Basu S, Chen W, Tchou J, Mavi A, Cermik T, Czerniecki B, et al. Comparison of triple-negative and estrogen receptor-positive/progesterone receptor-positive/HER2-negative breast carcinoma using quantitative fluorine-18 fluorodeoxyglucose/positron emission tomography imaging parameters. Cancer. 2008;112:995–1000.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Uematsu, T., Kasami, M. & Yuen, S. Comparison of FDG PET and MRI for evaluating the tumor extent of breast cancer and the impact of FDG PET on the systemic staging and prognosis of patients who are candidates for breast-conserving therapy. Breast Cancer 16, 97–104 (2009). https://doi.org/10.1007/s12282-008-0065-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-008-0065-9