Abstract
Objectives
To evaluate the association between tumour FDG uptake on preoperative PET/CT and axillary lymph node metastasis (ALNM) according to breast cancer subtype.
Methods
The records of 671 patients with invasive breast cancer who underwent 18 F-FDG PET/CT and surgery were reviewed. Using immunohistochemistry, tumours were divided into three subtypes: oestrogen receptor (ER)-positive/human epidermal growth factor receptor 2 (HER2)-negative, HER2-positive, and triple-negative. Tumour FDG uptake, expressed as maximum standardized uptake value (SUVmax), and clinicopathological variables were analysed.
Results
ALNM was present in 187 of 461 ER-positive/HER2-negative, 54 of 97 HER2-positive, and 38 of 113 triple-negative tumours. On multivariate analysis, high tumour SUVmax (≥4.25) (P < 0.001), large tumour size (>2 cm) (P = 0.003) and presence of lymphovascular invasion (P < 0.001) were independent variables associated with ALNM. On subset analyses, tumour SUVmax maintained independent significance for predicting ALNM in ER-positive/HER2-negative (adjusted odds ratio: 3.277, P < 0.001) and HER2-positive tumours (adjusted odds ratio: 14.637, P = 0.004). No association was found for triple-negative tumours (P = 0.161).
Conclusions
Tumour SUVmax may be an independent prognostic factor for ALNM in patients with invasive breast cancer, especially in ER-positive/HER2-negative and HER2-positive subtypes, but not in those with triple-negative subtype.
Key points
• Tumour SUVmax could be an imaging biomarker for predicting ALNM
• Tumour SUVmax predicting ALNM is effective in ER-positive/HER2-negative and HER2-positive subtypes
• Tumour SUVmax predicting ALNM is inaccurate in triple-negative subtypes
• Accurate prognostic prediction based on molecular subtype may facilitate individualized management
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The axillary lymph node (ALN) status is the most powerful prognostic factor and an important guide for treatment planning in patients with breast cancer [1, 2]. Although lymphovascular invasion, tumour size, and histological grade are well known for their association with ALN involvement [3–5], these established prognosticators are not always sufficient to justify omission of axillary dissection in patients at low risk of ALN metastasis. Efforts for preoperative risk assessment regarding ALN status are warranted and ongoing.
Breast cancer is a heterogeneous disease consisting of biologically distinct tumour subtypes. The breast molecular subtype has been correlated with tumour aggressiveness and identified as an independent prognostic factor for survival in patients with breast cancer [6, 7]. Immunohistochemical (IHC) classification based on expression of the oestrogen receptor (ER), the progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) is the most frequently used molecular marker in clinical practice and is valuable in predicting the clinical outcome as well as guiding targeted treatments [8]. IHC classification categorizes breast tumours into three major tumour subtypes: ER-positive (i.e., ER positive, HER2 negative, PR may be positive or negative), HER2-positive (i.e., HER2 positive; ER and PR may be positive or negative), and triple-negative (i.e., ER negative, PR negative and HER2 negative) [9, 10]; these correspond roughly to the intrinsic molecular subtypes of luminal, HER2-positive, and basal-like forms, respectively [11].
18 F-fluorodeoxyglucose positron emission tomography/computed tomography (18 F-FDG PET/CT) is becoming increasingly important in the diagnosis and management of patients with breast cancer [12–14]. Recent studies found that 18 F-FDG uptake in breast cancer is associated with typical prognostic factors, including tumour size, lymph node status, and histological grade, as well as tumour subtype, which is also an independent predictor for prognosis [15–17]. However, controversy remains concerning the prognostic value of tumour 18 F-FDG uptake with regard to ALN metastasis, and little is known about potential differences in its value among molecular breast cancer subtypes.
Therefore, the purpose of the current study was to evaluate the association between tumour 18 F-FDG uptake on preoperative PET/CT and ALN metastasis in patients with various subtypes of invasive breast cancer.
Materials and methods
Patients
The Institutional Review Board of our hospital approved this study, and the requirement for informed consent was waived. Between January 2011 and December 2013, the medical records of 785 consecutive women diagnosed with invasive breast cancer who underwent whole-body 18 F-FDG PET/CT before breast surgery were evaluated retrospectively. Among these 785 patients, 114 were excluded: 39 who underwent surgical excision or vacuum-assisted breast biopsy for diagnosis prior to 18 F-FDG PET/CT, 42 with no focal uptake on 18 F-FDG PET/CT, 25 with an invasive tumour size <1 cm, and eight with no immunohistochemical data available. In women with bilateral cancer, only one tumour was included, and in women with multifocal or multicentric cancer, the largest tumour was included. After the exclusions, a total of 671 female patients with invasive breast tumours comprised our study population. The mean interval between the times of 18 F-FDG PET/CT and surgery was 13.5 days (range 1–34 days).
18 F-FDG PET/CT
18 F-FDG PET/CT images were obtained using a Biograph instrument (Siemens Medical Solutions, Hoffman Estates, IL, USA) from the skull base to the upper thigh 1 h after intravenous administration of 18 F-FDG. All patients fasted for at least 8 h and had serum glucose levels <120 mg/dL at the time of 18 F-FDG injection (5.2 MBq/kg of body weight). First, a CT was acquired for attenuation correction (tube voltage 120 kVp, current intensity 80-170 mAs, and slice thickness 3 mm) without the use of a contrast agent, and a PET was then acquired in three-dimensional mode for 3 min per bed position. PET images were reconstructed to 168 × 168 image matrices using an iterative algorithm (two iterations, 21 subsets). The CT, PET, and fused PET/CT images were displayed in axial, coronal, and sagittal planes and were reviewed on a workstation.
The PET/CT images were interpreted by two experienced nuclear medicine physicians with consensus. Regions of interest were placed manually over the most intense areas on primary breast tumour slices on attenuation-corrected images. The SUVmax within the regions of interest was obtained and calculated as follows: SUVmax = maximum activity concentration in region of interest (MBq/g) / [injected dose (MBq)/body weight (g)].
Histological examination and immunohistochemistry
All patients underwent surgical resection for breast cancer with sentinel lymph node biopsy and/or axillary lymph node dissection. The histological type of invasive breast cancer, tumour size, histological grade, and lymphovascular invasion (LVI) status were determined from the surgically excised specimens. TNM staging was performed according to the American Joint Committee on Cancer (AJCC) sixth edition [18]. The tumours were graded histologically using the Nottingham combined histological grade system [19].
The formalin-fixed, paraffin-embedded tissue blocks were used for IHC. The expression status of ER, PR, HER2, p53, and Ki-67 was determined based on the surgical specimens by the avidin-biotin complex immunohistochemical technique. The ER and PR status was assessed using the Allred score, which was expressed as the sum of the proportion score and the intensity score of positively stained tumour cells [20]. Tumours with an Allred score of at least 3 were regarded as positive. The intensity of HER2 staining was scored as 0, 1+, 2+, or 3+ [21]. Tumours with a 3+ score were considered HER2-positive, and those with a 0 or 1+ score were considered HER2-negative. Tumours with a 2+ score were subjected to fluorescence in situ hybridization (FISH) analysis to determine the HER2 status. If the ratio of the HER2 gene signal to chromosome 17 probe signal was > 2.2, the tumour was deemed HER2-positive. p53 was considered positive if there was ≥ 10 % positive nuclear staining regardless of the intensity [22]. For Ki-67, nuclear staining ≥ 14 % was considered high-level expression [23].
In the present study, breast cancers were classified into three molecular subtypes based on IHC and FISH analyses of ER, PR, and HER2 statuses as follows: ER-positive/HER2-negative (ER-positive and/or PR-positive, HER2-negative), HER2-positive (ER-negative, PR-negative and HER2-positive), and triple-negative (ER-negative, PR-negative and HER2-negative).
Statistical analysis
The clinicopathological features were compared between patients with and without ALN metastasis using the chi-square test or Fisher’s exact test for the categorical variables and the independent sample t-test for the continuous variables. The relationships between the tumour SUVmax and clinicopathological parameters were calculated using the Mann-Whitney U-test and the Kruskal-Wallis test. A receiver operating characteristic (ROC) curve analysis was used to determine the optimal cut-off value of the SUVmax. Multivariate logistic regression analysis was performed to determine the variables independently associated with ALN metastasis by including all of the significant factors (P < 0.05) from univariate analysis.
SPSS software (version 18.0, SPSS, Chicago, IL, USA) was used for all data analyses except ROC curve analysis, which was performed using the MedCalc software program (version 10.3.0.0, MedCalc Software, Mariakerke, Belgium). A P value of less than 0.05 was considered to indicate statistical significance.
Results
Patient characteristics
The median age of the 671 patients was 52 years (range, 23–88 years). Mastectomy (n = 227) or breast-conserving surgery (n = 444) was performed for all breast cancers. A complete axillary lymph node dissection was performed in 557 (83.0 %) patients, whereas an axillary sentinel lymph node biopsy without axillary dissection was performed in 114 (17.0 %) patients. Of 671 tumours, 279 (41.6 %) breast cancers were diagnosed as having ALN metastasis.
Patients’ characteristics and the association with axillary nodal status are presented in Table 1. The mean tumour size was 2.5 ± 1.5 cm (range, 1.0–11.7 cm). The tumour subtype in most cases was ER-positive/HER2-negative (n = 461, 68.7 %) followed by triple-negative (n = 113, 16.8 %) and HER2-positive (n = 97, 14.5 %) tumours. The histological types of invasive cancer included the following: invasive ductal carcinoma (n = 610, 90.9 %), invasive lobular carcinoma (n = 23, 3.4 %), and other specified cancers (n = 38, 5.7 %; 17 mucinous carcinomas, ten metaplastic carcinomas, seven papillary carcinomas, three tubular carcinomas, and one medullary carcinoma). ALN status correlated with the mean tumour size, pathological tumour stage, histological grade, Ki-67 status, LVI, type of surgery, and tumour subtype (Table 1).
FDG PET/CT and clinicopathologic parameters of the primary tumour
Table 2 shows the tumour SUVmax in relation to clinicopathologic parameters. The primary tumour SUVmax was significantly higher in patients with ALN metastasis than without ALN metastasis (8.6 ± 4.9 vs. 6.2 ± 4.9, P < 0.001). With regard to tumour subtypes, the mean SUVmax of the triple-negative and HER2-positive tumours was 9.6 ± 6.0 and 8.9 ± 4.6, respectively, which was higher than that of ER-positive/HER2-negative tumours (6.7 ± 4.6) ( P < 0.001 and P < 0.001, respectively). The primary tumour SUVmax was also significantly higher in the tumours of younger patients (<45 years), those of a larger size (>2 cm) or higher histological grade (grade 3), those that were ER-negative, PR-negative, HER2-positive, or p53-positive, and those with higher expression of Ki 67, lymphovascular invasion, or ductal histology (Table 2).
Table 3 shows the tumour SUVmax values according to nodal status and tumour subtype. ALN positivity was present in 40.6 % (187 of 461) of ER-positive/HER2-negative tumours, 55.7 % (54 of 97) of HER2-positive tumours, and 33.6 % (38 of 113) of triple-negative tumours. In the triple-negative phenotype, there was no significant difference between the tumour SUVmax with and without ALN metastasis (9.4 ± 7.5 vs. 10.6 ± 5.3, P = 0.092) (Table 3) (Fig. 1).
Univariate and multivariate analysis
Based on ROC curve analysis, the SUVmax of the primary tumours significantly discriminated the ALN involvement in breast cancer (area under the curve = 0.704, P < 0.001). In addition, the optimal cut-off value of the tumour SUVmax was identified as 4.25. On univariate analysis, a high SUVmax (≥4.25) was associated significantly with ALN metastasis (P < 0.001). Large tumour size (>2 cm) (P < 0.001), higher histological grade (grade 3) (P = 0.001), higher expression of Ki 67 (≥14 %) (P = 0.005), and presence of LVI (P < 0.001) were also associated with a higher probability of ALN metastasis. Those variables that showed statistical significance (P < 0.05) on univariate analysis were included in the multivariate analysis. In the multivariate analysis, a high SUVmax (≥4.25) (adjusted odds ratio (OR): 3.497, 95 % CI: 2.245, 5.446, P < 0.001), large tumour size (>2 cm) (adjusted OR: 1.794, 95 % CI: 1.223, 2.632, P = 0.003), and presence of LVI (adjusted OR: 5.301, 95 % CI: 3.670, 7.657, P < 0.001) were associated independently with ALN metastasis (Table 4).
Considering the correlation of the tumour SUVmax with the tumour subtype (Table 2), a sub-analysis was performed to investigate potential differences in the results obtained among the various tumour molecular subtypes. In the ER-positive/HER2-negative and the HER2-positive tumours, the tumour SUVmax maintained independent significance on multivariate analysis for predicting ALN involvement, after controlling for tumour size, LVI, histological grade and Ki-67 status (adjusted OR: 3.277, 95 % CI: 1.980, 5.421, P < 0.001 and adjusted OR:14.637, 95 % CI: 2.386, 89.784, P = 0.004, respectively) (Figs. 2 and 3). In the triple-negative tumours, the SUVmax was not associated with ALN metastasis (P = 0.161) (Table 5) (Fig. 4).
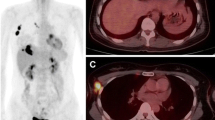
ER-positive/HER2-negaitve breast cancer in a 46-year-old woman. 18 F-FDG PET/CT showed a hypermetabolic mass in the upper inner quadrant of the left breast with a SUVmax of 7.8 (a). There was no abnormal metabolism in the left axillary area (b). Surgical histopathology revealed a 3.0-cm invasive ductal carcinoma with a histological grade of 2 that was ER-positive, PR-positive, and HER2-negative. The Ki 67 index was 50 % and p53 was negative. Axillary lymph node metastasis was found in one out of 4 resected nodes
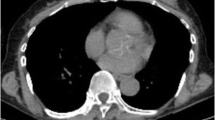
HER2-positive breast cancer in a 57-year-old woman. 18 F-FDG PET/CT showed a hypermetabolic mass in the lower outer quadrant of the left breast with a SUVmax of 9.5 (a), and one enlarged lymph node with focal FDG uptake (SUVmax 3.9) in the left axillary area (arrow) (b). Surgical histopathology revealed a 2.1-cm invasive ductal carcinoma with a histological grade of 3 that was ER-negative, PR-negative, and HER2-positive. The Ki 67 index was 20 % and p53 was negative. Axillary lymph node metastasis was found in one out of 13 resected nodes
Triple-negative breast cancer in a 43-year-old woman. 18 F-FDG PET/CT showed a hypermetabolic mass in the upper outer quadrant of the right breast with a SUVmax of 10.4 (a). There was no abnormal metabolism in the right axillary area (b). Surgical histopathology revealed a 3.5-cm invasive ductal carcinoma with a histological grade of 3 that was ER-negative, PR-negative, and HER2-negative. The Ki 67 and p53 indices were 40 % and 85 %, respectively. No axillary lymph node metastasis was found
Discussion
Consistent with the results of previous studies, we found that ALN metastasis was associated with LVI [5, 24]. In the present study, the presence of LVI was the strongest predictor of ALN metastasis (adjusted OR: 5.301, 95 % CI: 3.670, 7.657, P < 0.001) regardless of the tumour subtype. In particular, for triple-negative tumours, LVI was the only variable independently associated with ALN status (adjusted OR: 10.423, 95 % CI: 3.761, 28.889, P < 0.001). Despite its strong predictive value, LVI can be assessed histologically only after surgery using haematoxylin and eosin staining. On the other hand, a great advantage of the tumour SUVmax as a biomarker for predicting ALN metastasis is that it can be easily assessed preoperatively. In the present study, the tumour SUVmax was the second strongest prognostic factor in 671 patients with invasive breast cancer (adjusted OR: 3.497, 95 % CI: 2.245, 5.446, P < 0.001).
However, in the subgroup analysis, the tumour SUVmax was found to have prognostic value for predicting ALN metastasis that differed depending on the breast cancer subtype. The tumour SUVmax on preoperative PET/CT correlated well with ALN metastasis for ER-positive/HER-negative and HER2-positive tumours (P < 0.001, P = 0.004, respectively), but not for triple-negative tumours (P = 0.161). This observation may be due to the aggressive biology of triple-negative breast cancers. The majority of triple-negative tumours had high histological grade (grade 3, 73.5 %) and higher expression of Ki 67 (≥14 %, 82.3 %), so that they were more likely to show a higher SUVmax (mean, 9.6) regardless of axillary nodal status, which could explain the lack of prognostic significance of SUVmax in this group of breast cancers. In addition, for triple-negative tumours, the earlier reported correlation between tumour size and node positivity was not observed. Although the reason is not clear, one possible explanation is that the mechanism of spread of tumour may differ between triple-negative and other subtypes. It has been suggested that triple-negative breast cancers tend to spread haematogenously rather than via lymphatics [25]. We consider that the triple-negative subtype is a separate biological phenotype that necessitates preoperative prognostication to tailor individual treatment plans.
Knowledge of axillary nodal status before surgery is important because it influences the decision making of clinicians regarding the management of patients with breast cancer. Although sentinel lymph node biopsy is considered an alternative to ALN dissection in clinically node-negative patients due to its high sensitivity and accuracy [26, 27], this procedure is still invasive and carries a risk of surgical complications such as lymphoedema, axillary paraesthesia, seroma, and wound infection [28]. Additionally, sentinel lymph node biopsy is performed at the time of primary tumour excision, resulting in a longer surgical duration. Multiple non-invasive imaging methods such as mammography, ultrasound, and magnetic resonance imaging have been used for preoperative axillary staging, but accurate prediction remains a challenge. In a recent study, 14 % of the patients who had no suspicious lymph node findings on physical examination, mammography, ultrasound, and magnetic resonance imaging were found to have positive lymph nodes confirmed histologically following surgery [29]. Furthermore, in breast cancer patients, 18 F-FDG PET/CT is effective for detection of distant metastasis, but its value for axillary staging is not established. In the previous studies, the diagnostic accuracy of 18 F-FDG PET/CT for ALN metastasis showed a low sensitivity, ranging from 37 % to 84.5 %, whereas the specificity was higher than 96 % [12, 30–32]. Therefore, it is suggested that 18 F-FDG PET/CT could play a possible role in preselecting candidate for axillary dissection for patients with positive axillary nodes on PET/CT, which could reduce unnecessary sentinel lymph node biopsy [30]. New techniques for improving spatial resolution of PET/CT are needed to increase detectability of small lymph nodes.
18 F-FDG PET is not currently recommended for routine use in breast cancer patients [33], but it has been used increasingly in many hospitals not only for staging work-up, but also for predicting the therapeutic response to neoadjuvant chemotherapy [14]. A significant correlation was found among 18 F-FDG uptake in breast cancer, histobiological factors, and tumour angiogenesis [15, 16, 34]. Ueda et al. [16] demonstrated that tumours with a high SUVmax were associated with increased 10-year relapse and 10-year mortality rates in patients with breast cancer. Furthermore, Song et al. [35] found that the SUVmax of metastatic ALN was predictive of disease recurrence. Therefore, we believe that tumour 18 F-FDG uptake on preoperative PET/CT could be an emerging prognostic biomarker for breast cancer that may improve preoperative prediction of ALN status in breast cancer patients with particular tumour subtypes.
Histologically similar tumour phenotypes may behave differently clinically, which is believed to be due to molecular differences in these tumours. Previous studies have shown that different molecular types of breast cancers have different treatment response and survival outcomes [9, 36]. Triple-negative breast cancers have more aggressive biological features and are associated with poorer clinical outcomes compared with other subtypes [37, 38]. Recently, the association of gene expression with tumour metabolism was investigated by 18 F-FDG PET/CT. As expected, clinically aggressive tumours such as triple-negative and HER2-positive tumours showed greater FDG uptake than did luminal tumours [17, 39]. We also confirmed metabolic differences, represented by the SUVmax on 18 F-FDG PET/CT, in tumours by molecular subtype. A better understanding of molecular biology could improve our understanding of breast cancer biology, and accurate prognostic prediction based on molecular biomarkers may facilitate improved prevention and individualized management of breast cancer patients.
Our study had several limitations. First, this was a retrospective study from a single centre, which inevitably introduces considerable selection bias. Second, we categorized breast cancer subtypes by IHC instead of gene expression profiles, which could have affected our results. However, previous studies suggested that the IHC profile can be a useful surrogate for microarray-based gene expression profiling [23, 40]. Finally, we did not evaluate the correlation between tumour FDG uptake and long-term clinical outcomes because the follow-up duration was short. ALN status has been shown to be associated with mortality in breast cancer patients [41]. Further prospective studies are needed to validate our results.
In conclusion, the results of the present study suggest that tumour FDG uptake may be associated independently with ALN metastasis among patients with certain tumour subtypes, particularly ER-positive/HER-negative and HER2-positive tumours. The triple-negative tumours, however, failed to demonstrate this association. Further research is warranted to investigate tumour subtype-specific prognostic markers that enable individualized treatment plans for patients with invasive breast cancer.
References
Weiss RB, Woolf SH, Demakos E et al (2003) Natural history of more than 20 years of node-positive primary breast carcinoma treated with cyclophosphamide, methotrexate, and fluorouracil-based adjuvant chemotherapy: a study by the cancer and leukemia group B. J Clin Oncol 21:1825–1835
Arriagada R, Le MG, Dunant A, Tubiana M, Contesso G (2006) Twenty‐five years of follow‐up in patients with operable breast carcinoma. Cancer 106:743–750
Colleoni M, Rotmensz N, Maisonneuve P et al (2007) Prognostic role of the extent of peritumoral vascular invasion in operable breast cancer. Ann Oncol 18:1632–1640
Viale G, Zurrida S, Maiorano E et al (2005) Predicting the status of axillary sentinel lymph nodes in 4351 patients with invasive breast carcinoma treated in a single institution. Cancer 103:492–500
Yoshihara E, Smeets A, Laenen A et al (2013) Predictors of axillary lymph node metastases in early breast cancer and their applicability in clinical practice. Breast 22:357–361
Carey LA, Perou CM, Livasy CA et al (2006) Race, breast cancer subtypes, and survival in the carolina breast cancer study. JAMA 295:2492–2502
Van’t Veer LJ, Dai H, De Vijver V et al (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415:530–536
Nguyen PL, Taghian AG, Katz MS et al (2008) Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol 26:2373–2378
Desmedt C, Haibe-Kains B, Wirapati P et al (2008) Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res 14:5158–5165
Sánchez-Muñoz A, García-Tapiador AM, Martínez-Ortega E et al (2008) Tumour molecular subtyping according to hormone receptors and HER2 status defines different pathological complete response to neoadjuvant chemotherapy in patients with locally advanced breast cancer. Clin Transl Oncol 10:646–653
de Ronde JJ, Hannemann J, Halfwerk H et al (2010) Concordance of clinical and molecular breast cancer subtyping in the context of preoperative chemotherapy response. Breast Cancer Res Treat 119:119–126
Fuster D, Duch J, Paredes P et al (2008) Preoperative staging of large primary breast cancer with [18 F] fluorodeoxyglucose positron emission tomography/computed tomography compared with conventional imaging procedures. J Clin Oncol 26:4746–4751
Eubank WB, Mankoff D, Bhattacharya M et al (2004) Impact of FDG PET on defining the extent of disease and on the treatment of patients with recurrent or metastatic breast cancer. AJR Am J Roentgenol 183:479–486
Hatt M, Groheux D, Martineau A et al (2013) Comparison between 18 F-FDG PET image-derived indices for early prediction of response to neoadjuvant chemotherapy in breast cancer. J Nucl Med 54:341–349
Song B, Hong CM, Lee HJ et al (2011) Prognostic value of primary tumor uptake on F-18 FDG PET/CT in patients with invasive ductal breast cancer. Nucl Med Mol Imaging 45:117–124
Ueda S, Tsuda H, Asakawa H et al (2008) Clinicopathological and prognostic relevance of uptake level using 18 F-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging (18 F-FDG PET/CT) in primary breast cancer. Jpn J Clin Oncol 38:250–258
Koo HR, Park JS, Kang KW et al (2014) 18 F-FDG uptake in breast cancer correlates with immunohistochemically defined subtypes. Eur Radiol 24:610–618
Greene FL (2002) AJCC cancer staging manual. Springer
Elston C, Ellis I (1991) Pathological prognostic factors in breast cancer. I. the value of histological grade in breast cancer: experience from a large study with long‐term follow‐up. Histopathology 19:403–410
Allred DC, Harvey JM, Berardo M, Clark GM (1998) Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 11:155–168
Moeder CB, Giltnane JM, Harigopal M et al (2007) Quantitative justification of the change from 10 % to 30 % for human epidermal growth factor receptor 2 scoring in the american society of clinical oncology/college of american pathologists guidelines: tumor heterogeneity in breast cancer and its implications for tissue microarray based assessment of outcome. J Clin Oncol 25:5418–5425
Yamashita H, Toyama T, Nishio M et al (2006) p53 protein accumulation predicts resistance to endocrine therapy and decreased post-relapse survival in metastatic breast cancer. Breast Cancer Res 8:R48
Cheang MC, Chia SK, Voduc D et al (2009) Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 101:736–750
Bader AA, Tio J, Petru E et al (2002) T1 breast cancer: identification of patients at low risk of axillary lymph node metastases. Breast Cancer Res Treat 76:11–17
Ridriguez-Pinilla SM, Sarrio D, Honrado E et al (2006) Prognostic significance of basal-like phenotype and fascin expression in node-negative invasive breast cancers. Clin Cancer Res 12:1533–1539
Krag DN, Anderson SJ, Julian TB et al (2007) Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol 8:881–888
McMasters KM, Tuttle TM, Carlson DJ et al (2000) Sentinel lymph node biopsy for breast cancer: a suitable alternative to routine axillary dissection in multi-institutional practice when optimal technique is used. J Clin Oncol 18:2560–2566
Wilke LG, McCall LM, Posther KE et al (2006) Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol 13:491–500
Valente SA, Levine GM, Silverstein MJ et al (2012) Accuracy of predicting axillary lymph node positivity by physical examination, mammography, ultrasonography, and magnetic resonance imaging. Ann Surg Oncol 19:1825–1830
Kim J, Lee J, Chang E et al (2009) Selective sentinel node plus additional non-sentinel node biopsy based on an FDG-PET/CT scan in early breast cancer patients: single institutional experience. World J Surg 33:943–949
Veronesi U, De Cicco C, Galimberti VE et al (2007) A comparative study on the value of FDG-PET and sentinel node biopsy to identify occult axillary metastasis. Ann Oncol 18:473–478
Gil-Rendo A, Zornoza G, Garcia-Velloso MJ et al (2006) Fluorodeoxyglucose positron emission tomography with sentinel lymph node biopsy for evaluation of axillary involvement in breast cancer. Br J Surg 93:707–712
Fletcher JW, Djulbegovic B, Soares HP et al (2008) Recommendations on the use of 18 F-FDG PET in oncology. J Nucl Med 49:480–508
Groves AM, Shastry M, Rodriguez-Justo M et al (2011) 18 F-FDG PET and biomarkers for tumour angiogenesis in early breast cancer. Eur J Nucl Med Mol Imaging 38:46–52
Song BI, Lee SW, Jeong SY et al (2012) 18 F-FDG uptake by metastatic axillary lymph nodes on pretreatment PET/CT as a prognostic factor for recurrence in patients with invasive ductal breast cancer. J Nucl Med 53:1337–1344
Carey LA, Dees EC, Sawyer L et al (2007) The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res 13:2329–2334
Dent R, Trudeau M, Pritchard KI et al (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13:4429–4434
Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H (2010) Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol 28:1684–1691
Vicente AMG, Castrejón ÁS, Martín AL et al (2013) Molecular subtypes of breast cancer: metabolic correlation with 18 F-FDG PET/CT. Eur J Nucl Med Mol Imaging 40:1304–1311
Hugh J, Hanson J, Cheang MC et al (2009) Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol 27:1168–1176
Jatoi I, Hilsenbeck SG, Clark GM, Osborne CK (1999) Significance of axillary lymph node metastasis in primary breast cancer. J Clin Oncol 17:2334–2340
Acknowledgments
The scientific guarantor of this publication is Jin You Kim. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. No study subjects or cohorts have been previously reported. Methodology: retrospective, observational, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J.Y., Lee, S.H., Kim, S. et al. Tumour 18 F-FDG Uptake on preoperative PET/CT may predict axillary lymph node metastasis in ER-positive/HER2-negative and HER2-positive breast cancer subtypes. Eur Radiol 25, 1172–1181 (2015). https://doi.org/10.1007/s00330-014-3452-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3452-y