Abstract
The trophic ecology of several cryonotothenioid species has been well studied, but scarcely on the juvenile fraction of their populations. Particularly, till date the inter-annual variation of the diet composition and feeding habits of these young notothenioid stages has not been explored. Herein, we analysed the trophic ecology of 755 specimens of the species Notothenia rossii, Notothenia coriiceps, Nototheniops nudifrons, Trematomus newnesi and Harpagifer antarcticus, collected in the inshore waters of Potter Cove, South Shetlands Islands, during four consecutive sampling periods from 2008 to 2012. The stomach-content analysis using the IRI % and the Amundsen et al. (1996) methods provided data on feeding habits and trophic niche breadth. Our results indicate that the species are demersal and benthophagous, have a generalized type of feeding strategy and prey chiefly on demersal–benthic amphipods (mainly genus Gondogeneia and Oradarea), and on other epibenthic invertebrates associated with macroalgal beds. Significant differences were observed in the diet of all species between sampling periods, and ontogenetically, only in N. rossii and T. newnesi. According to the estimated trophic levels, the juvenile cryonotothenioids were identified as secondary consumers. Ambush feeding was the predominant feeding behaviour in all species, and in N. rossii and N. coriiceps, also grazing. Likewise, these two fish species exhibited a wider diet diversity than the other notothenioids. Using literature information on the trophic ecology of the late juvenile–adult stages of the same species at Potter Cove, we identified differences and likenesses with our results on the early juvenile–juvenile fraction of the fish community. This study highlights the key role of nearshore areas in the cryonotothenioids life cycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The diet of a fish species is affected by a combination of factors such as the differential mechanical capacity to ingest prey during ontogeny (predator size), seasonal availability of prey (presence and size of prey) and their geographical origin (Kock 1992). In the Southern Ocean, the Antarctic krill Euphausia superba occupies a central role in the marine food web being the main food of vertebrates, from fish to marine mammals and also of squids (e.g. Kock et al. 2012; Caccavo et al. 2021). Antarctic fish occupy an intermediate trophic level playing the role as both predators and prey (e.g. Kock 1992; La Mesa et al. 2004; Ciaputa and Sicinski 2006). Nevertheless, at inshore coastal areas krill importance decreases since its occurrence is restricted to juvenile stages and sporadically mainly in summer (Siegel 1988; Miller and Hampton 1989; Perry et al. 2019; Meyer et al. 2020). There, the dominant coastal demersal group of fishes, the Suborder Notothenioidei (Eastman 2005; Eastman and Eakin 2021) takes a key role in the food web as main predators of benthos feeding on all organisms below their own trophic level from algae to fish, as well as of zooplankton in the water column (e.g. Hureau 1994; Barrera-Oro 2002; Kock et al. 2012). In this way, energy flows from benthos to land through the higher fish predators in the form of fish remains, pellets (birds), regurgitated and faeces (birds and seals) (Barrera-Oro 2002).
Potter Cove (PC) is an inshore locality at King George Island/Isla 25 de Mayo, South Shetland Islands (SSI), where the Argentinean Station “Carlini” is located. At this ecosystem, several aspects of the trophic ecology from young juveniles to adult stages of cryonotothenioid species have been studied in the last 3 decades (summarized in Barrera-Oro and Casaux 2008; Moreira et al. 2014, 2020, 2021; Barrera-Oro et al. 2019), including the diet seasonal variation in 1 year (Casaux et al. 1990). Only recently, the complexity, structure and function of the food web in PC have been analysed (Marina et al. 2018). An integral comprehension of the food web mechanisms is essential for a correct understanding of communities’ structure, not only to know the trophic ecology of each actor but also to predict possible changes in the web architecture.
Due to the lack of swim bladder, the Antarctic ichthyofauna has developed a wide range of feeding strategies. In this sense, late juveniles and adult stages of notothenioid species have been categorized into feeders on benthos (e.g. Notothenia coriiceps, Gobionotothen gibberifrons, Nototheniops nudifrons, Harpagifer antarcticus), zooplankton/nekton (Chaenocephalus aceratus, Parachaenichthys charcoti) or both (Notothenia rossii, Trematomus newnesi, Trematomus bernacchii) (Daniels 1982; Barrera-Oro 2002). The use of such strategies, the predator spatial distribution and the resource partitioning along a depth gradient of different prey taxa help to reduce dietary overlap and therefore interspecific competition (Gröhsler 1994; Iken et al. 1997; Barrera-Oro 2003). The feeding behaviour of these fish stages includes ambush feeding, bottom slurping, grazing and water column feeding (Daniels 1982). Notothenia rossii displays ambush, grazing and water column feeding; N. coriiceps combines ambush feeding and grazing; both N. nudifrons and H. antarcticus are ambush feeders, whereas T. newnesi feeds in the water column (compiled in Barrera-Oro 2002).
On the other hand, the early juvenile and juvenile fraction of the notothenioid community live in a demersal stratum associated with the macroalgae beds, where they feed on invertebrates and find protection from higher predators (Barrera-Oro and Piacentino 2007; Moreira et al. 2014). In the SSI area, research on trophic ecology in a community of notothenioid species focussed on juvenile stages is limited to a single study done also at Potter Cove (Moreira et al. 2014). That work concludes that there is no difference in the degree of interspecific food overlap and therefore no potential competition between the immature and mature fraction of the fish can be assumed. Nevertheless, as the samples were collected in a single summer season, how inter-annual changes in food availability would impact on these young fish diet remains unexplored.
Herein, we study the trophic ecology in juvenile stages of five notothenioid species collected during four consecutive years at the inshore ecosystem of Potter Cove. Our work aims to (1) evaluate the inter-annual diet variation; (2) provide information on feeding habits, trophic niche breadth and trophic position of the species, and (3) using literature information from the same site, identify differences and likenesses with the late juvenile–adult stages of the same species in the inshore fish community of the area.
Materials and methods
Sampling and initial measurements
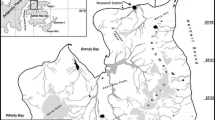
Juvenile notothenioid specimens were collected at Potter Cove, close to the Argentine scientific station “Carlini” (62° 14′ S and 58° 40′ W) (Fig. 1). The abiotic features and biotic components of this area are described in Barrera-Oro et al. (2019). The samples were obtained during the austral spring–summer (September to March) over a period of four consecutive Antarctic campaigns, from years 2008–2009 to 2011–2012. The most abundant species in the area were analysed: the nototheniids N. rossii and N. coriiceps (TL ≤ 21 cm), N. nudifrons and T. newnesi (TL ≤ 17 cm) and the harpagiferid H. antarcticus (TL < 10 cm). No samples of N. nudifrons and H. antarcticus were obtained in sampling periods 2011–2012 and 2008–2009, respectively. For sampling, a bottom trawl net (mouth 1 m2, length 2 m and mesh 4 mm) was trawled mostly at daylight during 15–30 min at depths of 4–30 m (average, 12 m) from Zodiac boats preferably where the seabed is a uniform rocky bottom covered mainly with red and brown macroalgae. Total and standard length to the nearest 0.1 cm below, weight in g and sex of fish were recorded. The macroscopic gonadal stage was determined according to the scale in Kock and Kellermann (1991). The stomach contents were weighted to 0.0001 g precision and frozen at − 20 °C for the posterior diet analysis. The proportion of specimens with empty stomachs was estimated and the degree of food digestion was evaluated as 0 (undigested), 1 (partially digested) and 2 (fully digested). Scales and sagittal otoliths were extracted for age determination. Following the method of counting annual rings in scales, similar to that described in Barrera-Oro and Casaux (1996), the ages of the species N. rossii, N. coriiceps, N. nudifrons and T. newnesi were estimated. Based on the age estimations the size groups were defined for each species.
General view of the South Shetland Islands and Antarctic Peninsula (a) with enlargements of King George Island/Isla 25 de Mayo (b) and Potter Cove (c). The maps were generated using the Quantarctica package (Matsuoka et al. 2021)
Diet composition
Fish diets at each season were analysed using the index of relative importance (IRI) of each prey type (Pinkas et al. 1971) according to the equation: IRI = F % (N % + W %), where F% (frequency of occurrence) is the percentage of stomachs containing a specific prey item; N% (numerical composition) is the number of items of a specific prey category expressed as a percentage of the total number of prey items of all stomachs examined; and W% (weight composition) is the weight of a specific prey item expressed as a percentage of the total weight of ingested prey. The IRI values were standardized to 100% by calculating the percentage of the total IRI contributed by each prey type (IRI%), which ranged from 0 (absent from diet) to 100 (the only prey item consumed) (Cortés 1997).
Diet variation
For each notothenioid species, differences in diets among sampling periods and size classes were estimated applying multivariate methods. Specimens with empty stomachs or only with occasional (= rare) food were not included in the analysis. A Bray–Curtis coefficient similarity matrix was obtained from the full-standardized data set of the stomach-content components [Logarithmic transformation (Ln X + 1)]. Subsequently, a non-metric multidimensional scaling (nMDS) was applied to the similarity matrix to display fish in a two-dimensional plane according to their relevant diet similarity. Analysis of Similarity (ANOSIM) was performed on diet data to test for significant differences in the diet among season and fish size groups, for each species, taking as significance a p value less than 5%. Finally, a Similarity Percentage analysis (SIMPER routine) was carried out to determine the contribution of each prey species to differences in fish diets. Diet similarity analyses were performed using the PRIMER software package (v. 6, Plymouth Routines in Multivariate Ecological Research, Clarke and Warwick 2001) and the open-source statistical package “R” (R Core Team 2022).
Feeding strategy
Each species feeding strategy (i.e. generalist or specialist), the importance of the ingested prey (i.e. dominant or rare) and the prey contribution to niche width were analysed following the graphical method proposed by Amundsen et al. (1996). The dietary composition is graphically represented by a two-dimensional diagram, by plotting the prey-specific abundance (Pi%) against the frequency of occurrence (F%). Pi was calculated as the percentage of the weight of prey i divided by the total weight of prey in the stomachs that contained prey i.
Trophic level
Trophic level for each species were estimated as TLj = 1 + \(\sum\nolimits_{{i = 1}}^{s} {TLi}\)* pij, where TLi is the trophic level of prey i, pij is the proportion of prey i in the diet of the predator j and S is the total number of trophic species. Fish trophic levels were calculated from the identification of prey items in each stomach to the lowest possible taxonomic level in each sampling season. The trophic level value of each prey was obtained from that previously reported for the Potter Cove network (Marina et al. 2018).
Feeding category and behaviour
The feeding categories or types of the notothenioids from Potter Cove, according to their predominant prey, have been adapted from the description in Kock (1992). The feeding behaviours of the species analysed were defined according to Daniels (1982).
Trophic niche and diet biodiversity
Niche breadth was evaluated by the Diet Diversity Index H′, H = ∑i pi (log pi), where pi is the proportion of the item prey i in the diet. To assess fish diet biodiversity, we used the number of prey taxa (P) present in the stomach contents. We estimated the Margalef Index of diversity (DMG), according to the equation: DMG = S-1/ln N, where S is the number of species and N is the total number of individuals with stomach content.
Results
Sample composition
Sampling details are shown in Table 1. A total of 755 individuals were examined, all were sexually immature, at stages I (immature) and II (maturing virgin) of development. A low proportion of empty stomachs was found in the five fish species: 0–16.67% and in general, the stomach contents in all of them were not or slightly digested (degree of food digestion: 0–1 > 80%), which allowed a good prey identification.
Diet variation
The variation of the species diet composition along the sampling periods are summarized in Table 2.
Notothenia rossii
We identified 42 prey taxa in the diet of N. rossii. The nMDS revealed (1) a clear difference among sampling periods (2D stress: 0.14, ANOSIM: Global R = 0.123, p = 0.001, Online Resource 1), and (2) no clear separation between the two size groups (2D stress: 0.14, ANOSIM: Global R = 0.155, p = 0.001) (Fig. 2).
In both plots in season 2010–2011, there are isolated specimens from the main set of points: one (middle lower-left sector, TL = 12.3 cm), whose diet consisted almost exclusively of hyperiid amphipods and bivalves; two (lower right sector, TL < 12 cm), which only consumed harpacticoids; and another one (upper sector, TL = 6.9 cm), which ingested only algae.
The ANOSIM routine revealed that the diet composition observed in 2010–2011 differs from sampling periods 2008–2009 and 2009–2010 (Online Resource 1). The SIMPER routine showed that in sampling period 2008–2009, the specimens consumed a greater amount of both algae and amphipods (explaining almost 80% of difference among sampling periods), while in 2010–2011 krill was primarily consumed and the ingestion of algae was insignificant. The decrease of algae and amphipods consumption and the importance of krill as main prey during 2010–2011 explain the differences between 2009–2010 and 2010–2011 (Online Resource 2).
Regarding the differences registered in the diet between size groups, a higher incidence of amphipods and a higher consumption of algae and krill was observed in specimens smaller and larger than 12 cm of TL, respectively (Online Resource 2).
Notothenia coriiceps
We identified 38 prey taxa in the diet of N. coriiceps. The nMDS revealed (1) differences among sampling periods (2D stress: 0.16, ANOSIM: Global R = 0.128, p = 0.002, Online Resource 1), and (2) no clear separation between the two size groups (2D stress: 0.16, ANOSIM: Global R = 0.013, p = 0.435) (Fig. 3).
The ANOSIM routine revealed that the diet composition observed in 2008–2009 differs from the other sampling periods (Online Resource 1). The SIMPER routine showed that both, algae and amphipods, were the main contributors to the dissimilarities in seasons with significant differences (Online Resource 2). A lower consumption of algae and gastropods and a higher consumption of amphipods were observed in sampling periods 2009–2010 and 2011–2012 compared to the sampling period 2008–2009. In the sampling period 2010–2011, a lower consumption of algae, amphipods and gastropods, but a higher consumption of isopods were registered in comparison with the sampling period 2009–2010.
Nototheniops nudifrons
We identified 19 prey taxa in the diet of N. nudifrons. The nMDS revealed (1) a clear difference among sampling periods (2D stress: 0.01, ANOSIM: Global R = 0.432, p = 0.001, Online Resource 1), and (2) no clear separation between the two size groups (2D stress: 0.01, ANOSIM: Global R = 0.025, p = 0.37) (Fig. 4). In the upper left angle of both plots there are a number of overlapping specimens (N = 6, LT < 10 cm) whose diet consisted exclusively of harpacticoid copepods.
The ANOSIM routine revealed that the diet composition observed in 2010–2011 differs from the other two sampling periods (Online Resource 1). The SIMPER routine showed that the consumption of amphipods was lower in season 2010–2011 compared to the other sampling periods. In the sampling period 2010–2011, there was a substantial occurrence of harpacticoid copepods in the diet (Online Resource 2).
Trematomus newnesi
We identified 26 prey taxa in the diet of T. newnesi. The nMDS revealed (1) clear differences among sampling periods (2D stress: 0.14, ANOSIM: Global R = 0.272, p = 0.001, Online Resource 1), and (2) a clear separation between the two size groups (2D stress: 0.14, ANOSIM: Global R = 0.153, p = 0.001) (Fig. 5). The diet of the isolated specimen represented in the lower-left angle of the plot consisted only of algae.
The ANOSIM routine revealed that the diet composition observed in 2008–2009 differs from the sampling periods 2009–2010 and 2010–2011, but showed no differences with the sampling period 2011–2012 (Online Resource 1). The diet composition registered in 2009–2010 differs from that observed in 2010–2011. The SIMPER routine showed that in the sampling period 2010–2011, the specimens consumed great amounts of krill, whereas in the sampling period 2009–2010 the amphipod consumption was higher. The diet analysis showed that while krill was equally consumed by both size groups, a higher intake of amphipods and calanoid copepods was observed in specimens larger than 9.00 cm TL (Online Resource 2).
Harpagifer antarcticus
We identified 17 prey taxa in the diet of H. antarcticus. The nMDS revealed (1) clear differences among sampling periods (2D stress: 0.01, ANOSIM: Global R = 0.136, p = 0.001, Online Resource 1), and (2) no clear separation between the two size groups (2D stress: 0.01, ANOSIM: Global R = 0.013, p = 0.363) (Fig. 6).
The ANOSIM routine revealed that the diet composition observed in 2009–2010 was different in comparison to the other sampling periods (Online Resource 1). The SIMPER routine showed that in the mentioned season the specimens consumed greater amounts of amphipods (Online Resource 2).
Feeding strategy
The feeding-strategy axis splits the diagram into an upper part (specialization) and a lower part (generalization) (Fig. 7). In general, as most taxa were grouped in the lower-left corner meaning that all prey taxa have been occasionally eaten, the feeding strategy of all the species juvenile stages tends towards a generalization in all the sampling periods. Only few prey categories positioned in the upper part, such as amphipods and euphausiids, were eaten in large amounts (high prey-specific abundance) in some sampling periods. While the presence of amphipods was important for all the species in almost every season, the importance of euphausiids was restricted to N. rossii and T. newnesi in the sampling period 2010–2011.
Graphic representation of dietary composition of juvenile stages of notothenioid species from Potter Cove for each sampling periods analysed. a Diagram showing the food items. NOR: Notothenia rossii; NOC: Notothenia coriiceps; NOD: Nototheniops nudifrons, TRN: Trematomus newnesi; HPF: Harpagifer antarcticus. Sampling periods 08–09: 2008–2009, 09–10: 2009–2010, 10–11: 2010–2011, 11–12: 2011–2012. b Explanatory diagram for interpretation of feeding strategy, niche width contribution and prey importance (Amundsen et al. 1996)
Trophic levels
The trophic levels calculated for the five fish species along the sampling periods indicate that all of them are secondary consumers (Table 3).
Feeding category and behaviour
The feeding category of the juvenile stages analysed was defined as benthos feeders. Likewise, they share an ambush feeding behaviour. Notothenia rossii and N. coriiceps are also grazers. When krill was available, both N. rossii and T. newnesi preyed on this euphausiid but in very low frequency.
Trophic niche and diet diversity
The H diversity index indicated a wider trophic niche in N. rossii and N. coriiceps in comparison to a constricted trophic niche in the other fish species (Table 4). In line with this, the mean DMG and P values show a higher diet diversity in N. rossii and N. coriiceps (Table 5).
Discussion
In recent years, biomarkers and amino acids analyses have been used as complementary methodologies to assess the trophic ecology of animal species, including Antarctic fish (Chikaraishi et al. 2009; Moreira et al. 2021). Nevertheless, in fish the conventional direct observation of stomach contents has provided accurate information on the prey items that constitute the trophic spectrum of notothenioid species at Potter Cove (summarized in Barrera-Oro and Casaux 2008; Moreira et al. 2014, 2020, 2021; Barrera-Oro et al. 2019). Results of our present work with the same traditional method, but for first time inter-annually on juvenile stages of the same fish community, indicate that the five species are demersal and benthophagous, have a generalized type of feeding strategy and prey chiefly on demersal–benthic amphipods and on other epibenthic invertebrates associated with macroalgae beds. Significant diet differences were observed for each fish species among sampling periods, where gammaridean amphipods, mainly the genus Gondogeneia and Oradarea, were in general the most important and frequent prey, including ontogenetically, in the two nototheniid species—N. rossii and T. newnesi—(Online Resource 1).
Fish diet depends on a number of factors such as the depth gradient of prey and their seasonal availability, as well as the fish species differential mechanical capacity for prey ingestion throughout their ontogeny. Since the juvenile fish we sampled at Potter Cove share a common habitat of 3–36 m depth, where the substrate consists of rocky bottom with macroalgal beds that holds similar benthic fauna (Barrera-Oro et al. 2019), it is likely that the depth of fish distribution was not a relevant factor in their diet variation.
Regarding the seasonality effects, previous studies at Potter Cove have described that the fauna associated to the macroalgal bed is available throughout the year, whereas pelagic prey such as krill occur only in the spring–summer (Casaux et al. 1990).
Considering the mechanical capacity of prey ingestion, the juvenile fish stages analysed are limited to consume only juvenile krill (< to 35 mm, according to the standardized CCAMLR protocol). Therefore, krill availability would likely bring differences in diet composition between sampling periods. In particular, analyses of Adelie penguin Pygoscelis adeliae stomach contents obtained at the nearby Potter Peninsula showed an increase of juvenile krill abundance in 2010–2011 and 2011–2012 sampling periods (Juáres 2013).
The diet compositions in N. rossii and T. newnesi were influenced by their size, which reflect ontogenetic variations. While in N. rossii, algae and krill consumption was more important in the larger group, in T. newnesi krill was consumed in equal proportion by both size groups. As in N. rossii the fish size was homogeneously represented in most sampling periods, the differences observed in the diets of 2010–2011 would be explained by a higher juvenile krill availability during that sampling period. Similarly, in T. newnesi, diet differences were due to an increased presence of juvenile krill in the stomachs during the same sampling period. In the remaining fish species, differences throughout the ontogeny were not registered, so prey availability was the main source of diet variation. These variations in diet, particularly between sampling periods, arise from the observed dissimilar frequencies of occurrence of certain prey taxa.
Although this study does not include integrative monthly sampling throughout entire year periods, it is the first to compare the diet among juvenile stages of notothenioids in consecutive spring-summers. Factors such as seasonality and photoperiod, at least in those sampling periods, seem not to be determinants factors for the juvenile stages, which depend mainly on marine benthos, where pelagic prey are practically absent in their diets.
The use of the modified Costello method (Amundsen et al. 1996) on the inter-annual diet data set of juvenile notothenioid species from Potter Cove allowed identifying the main characteristics of their feeding strategies.
The diet of N. rossii showed a high frequency of occurrence of amphipods; but in general these organisms contributed moderately in terms of weight. Algae generally occurred less frequently than amphipods in the stomachs, but in some sampling periods the incidence in weight of both food items was very similar. The importance of other taxa as prey was comparatively low and it remained relatively constant throughout the sampling period. Krill was practically absent in the stomachs of N. rossii except in 2010–2011, where it contributed moderately to the total weight of the stomach contents.
In N. coriiceps, amphipods and algae were the most important food items in terms of frequency of occurrence and weight in all sampling. Several taxa were represented in the diet revealing the widest trophic spectrum among the fish species, some of which presented inter-annual variations in both, frequency of occurrence and in different percentages of specific abundance.
Regarding N. nudifrons, amphipods were the main prey in frequency of occurrence and weight. Harpacticoid copepods appeared at a high frequency, but due to their low weight, their relative specific abundance was generally low. The rare prey observed in the diet presented a significant year-on-year variation which enhanced in the sampling period 2009–2010.
For T. newnesi, amphipods and harpacticoid copepods were important prey. While both food items occurred at a high frequency, amphipods exhibited a higher percentage of specific abundance. Other organisms were eaten at low frequency and contributed little to the weight of the stomach contents. Inter-annual variations were observed in the incidence of these other prey; however, in season 2010–2011 the consumption of krill was important in terms of both, frequency of occurrence and specific abundance, thus contributing significantly to the total weight.
Finally, for H. antarcticus, only amphipods were important prey. In the sampling periods 2010–2011 and 2011–2012 five food categories appeared as rare prey. Only two of these food items, algae and harpacticoid copepods were also represented as rare in season 2009–2010.
The results of the present study with data obtained throughout four consecutive sampling periods and those from the literature, all of them obtained at the nearshore ecosystem of Potter Cove, allow to highlight some differences and likenesses on aspects of the trophic ecology, between the juvenile and the adult stages (pre-mature in the case of N. rossii) of the notothenioid community:
Comparison of diets of notothenioid species from Potter Cove between the juvenile stages obtained in this work and late juveniles and adult stages previously reported (Barrera-Oro and Casaux 1990, 1998; Casaux et al. 1990) confirm that there are certain differences in terms of the taxa consumed. While gammaridean amphipods are the main food item in all the fish species and ontogenetic stages, prey such as copepods and gastropods are the main and secondary food of juvenile stages but are not consumed by late juvenile and adult stages. Moreover, larger prey such as krill (TL ~ 5 cm) and fish are main prey items in the diet of late juveniles and adult stages of many of the fish species, while are scarce or absent in the stomachs of the juvenile fish. Noteworthy, H. antarcticus showed no substantial changes in the diet composition throughout their ontogeny probably because its mouth morphological features and therefore mechanical capacity of prey ingestion are restricted to small prey items (Casaux 1998).
Observations from the trawled bottom sampling (this study; Moreira et al. 2014), ROV and scuba diving (Cristian Lagger, personal communication), confirm that the juvenile fish—mainly the early stages—live in a vertical range of 1–2 m over the algal bed. The occurrence of any pelagic prey in their stomachs was scarce or null, with the exception of N. rossii and T. newnesi, that showed signs of possible vertical movements within the demersal stratum. While T. newnesi preyed secondarily on pelagic calanoid copepods in sampling periods 2009–2010, 2010–2011 and 2011–2012, N. rossii preyed on this item in all sampling periods. Moreover, krill was the main food for both species in season 2010–2011, while in the rest of the sampling periods this prey was negligible or even null. Differentially, it is known that adult stages of some nototheniid species such as N. rossii, T. newnesi and also sporadically, N. coriiceps, are capable of making vertical migrations of several metres from the bottom into the water column to feed on pelagic prey such as krill (> 5 cm), hyperiid amphipods and even fish (Casaux et al. 1990; Barrera-Oro and Casaux 1990).
Considering the trophic levels, the juvenile fish of this work were identified as secondary consumers, whereas the late juveniles–adult stages of N. rossii, N. coriiceps, L. nudifrons, T. newnesi and H. antarcticus are tertiary consumers (Casaux et al.1990; Marina et al. 2018).
In all the juvenile fish species the predominant feeding behaviour was ambush feeding, while in N. rossii and N. coriiceps the grazing behaviour was also detected. In the adult stages ambush feeding is also predominant and some species are also water column feeders (e.g. N. rossii and T. newnesi), while the herbivory behaviour remains throughout the ontogeny in N. rossii and N. coriiceps (Barrera-Oro 2002; Moreira et al. 2021).
For both, juvenile and adult stages, the diet diversity and the feeding niche breadth in N. rossii and N. coriiceps were wider than in the other notothenioid species (this study; Casaux et al. 1990). Mean values of the diet diversity expressed in terms of number of taxa (P) and Margalef Diversity Index (MDI) showed the same trend, indicating a greater diversity in the diet of N. coriiceps and N. rossii.
Our study reinforces the key role of inshore coastal areas such as Potter Cove in the life cycle of the notothenioid community, including, as indicated in Novillo et al. (2021), the role as spawning and breeding grounds.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Amundsen PA, Gabler HM, Staldvik FJ (1996) A new approach to graphical analysis of feeding strategy from stomach contents data–modification of the Costello (1990) method. J Fish Biol 48:607–614
Barrera-Oro E (2002) The role of fish in the Antarctic marine food web: differences between inshore and offshore waters in the southern Scotia Arc and west Antarctic Peninsula. Antarct Sci 14:293–309
Barrera-Oro E (2003) Analysis of dietary overlap in Antarctic fish (Notothenioidei) from the South Shetland Islands: no evidence of food competition. Polar Biol 26:631–637
Barrera-Oro E, Casaux R (1990) Feeding selectivity in Notothenia neglecta, Nybelin, from Potter Cove, South Shetland Islands, Antarctica. Antarct Sci 2:207–213
Barrera-Oro E, Casaux R (1996) Validation of age determination in Notothenia coriiceps by means of a tag/recapture experiment at Potter Cove, South Shetland Islands. Arch Fish Mar Res 43:205–216
Barrera-Oro E, Casaux R (1998) Ecology of demersal fish species from Potter Cove. the Potter Cove Coastal Ecosystem, Antarctica 08/1998. Ber Polarforsch 299:156–167
Barrera-Oro E, Casaux R (2008) General ecology of coastal fish from the South Shetland Island and west Antarctic Peninsula areas. Beri Polar Und Meeresforsch 571:95–110
Barrera-Oro ER, Piacentino G (2007) Feeding habits of juvenile Trematomus newnesi (Pisces, Nototheniidae) at Potter Cove, South Shetland Islands, Antarctica. Polar Biol 30:789–796
Barrera-Oro E, Moreira E, Seefeldt M, Valli Francione M, Quartino ML (2019) The importance of macroalgae and associated amphipods in the selective benthic feeding of sister rockcod species Notothenia rossii and N. coriiceps (Nototheniidae) in West Antarctica. Polar Biol 42:317–334
Caccavo JA, Christiansen H, Constable AJ, Ghigliotti L, Trebilco R, Brooks CM, Cotte C, Desvignes T, Dornan T, Jones CD, Koubbi P, Saunders RA, Strobel A, Vacchi M, van de Putte AP, Walters A, Waluda CM, Woods BL, Xavier JC (2021) Productivity and change in fish and squid in the Southern Ocean. Front Ecol Evol 9:624918. https://doi.org/10.3389/fevo.2021.624918
Casaux R (1998) The contrasting diet of Harpagifer antarcticus (Notothenioidei, Harpagiferidae) at two localities of the South Shetland Islands, Antarctica. Polar Biol 19:283–285
Casaux R, Mazzotta A, Barrera-Oro E (1990) Seasonal aspects of the biology and diet of nearshore nototheniid fish at Potter Cove, South Shetland Islands, Antarctica. Polar Biol 11:63–72
Chikaraishi Y, Ogawa NO, Kashiyama Y, Takano Y, Suga H, Tomitani A, Miyashita H, Kitazato H, Ohkouchi N (2009) Determination of aquatic food-web structure based on compound-specific nitrogen isotopic composition of amino acids. Limnol Oceanogr Methods 7:740–750. https://doi.org/10.4319/lom.2009.7.740
Ciaputa P, Sicinski J (2006) Seasonal and annual changes in Antarctic fur seal (Arctocephalus gazella) diet in the area of Admiralty Bay, King George Island, South Shetland Islands. Pol Polar Res 27:171–184
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edn. PRIMER-E, Plymouth
Cortés E (1997) A critical review of methods of studying fish feeding based on analysis of stomach contents: application to elasmobranch fishes. Can J Fish Aquat Sci 54:726–738
Daniels RA (1982) Feeding ecology of some fishes of the Antarctic Peninsula. Fish Bull 80:575–588
Eastman J (2005) The nature of the diversity of Antarctic fishes. Polar Biol 28:93–107. https://doi.org/10.1007/s00300-004-0667-4
Eastman J, Eakin R (2021) Checklist of the species of notothenioid fishes. Antarct Sci 33:273–280. https://doi.org/10.1017/S0954102020000632
Gröhsler T (1994) Feeding habits as indicators of ecological niches: investigations of Antarctic Fish conducted near Elephant Island in late autumn/winter 1986. Arch Fish Mar Res 42:17–34
Hureau JC (1994) The significance of fish in the marine Antarctic ecosystems. Polar Biol 14:307–313
Iken K, Barrera-Oro E, Quartino ML, Casaux R, Brey T (1997) Grazing by the Antarctic fish Notothenia coriiceps: evidence for selective feeding on macroalgae. Antarct Sci 9:386–391
Juares MA (2013) Biología reproductiva y ecología trófica de dos especies simpátricas del género Pygoscelis en las Islas Shetland del Sur, Antártida. Tesis Doctoral. Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, La Plata, Buenos Aires, Argentina. http://hdl.handle.net/10915/27166
Kock KH (1992) Antarctic fish and fisheries. Cambridge University Press, Cambridge, p 359
Kock KH, Kellermann A (1991) Reproduction in Antarctic notothenioid fish (review). Antarct Sci 3:125–150
Kock KH, Barrera-Oro E, Belchier M, Collins MA, Duhamel G, Hanchet S, Pshenichnov L (2012) The role of fish as predators of krill (Euphausia superba) and other pelagic resources in the Southern Ocean. CCAMLR Sci 19:115–169
La Mesa M, Eastman JT, Vacchi M (2004) The role of notothenioid fish in the food web of the Ross Sea shelf waters: a review. Polar Biol 27:321–338
Marina TI, Salinas V, Cordone G, Campana G, Moreira E, Deregibus D, Torre L, Sahade R, Tatian M, Barrera-Oro E, De Troch M, Doyle S, Quartino ML, Saravia L, Momo FR (2018) The food web of Potter Cove (Antarctica): complexity, structure and function. Estuar Coast Shelf Sci 200:141–151. https://doi.org/10.1016/jecss.2017.10.015
Matsuoka K, Skoglund A, Roth G, de Pomereu J, Griffiths H, Headland R, Herried B, Katsumata K, Le Brocq A, Licht K, Morgan F, Neff PD, Ritz C, Scheinert M, Tamura T, Van de Putte A, Van den Broeke M, Von-Deschwanden A, Deschamps-Berger C, Van-Liefferinge B, Tronstad S, Melvær Y (2021) Quantarctica, an integrated mapping environment for Antarctica, the Southern Ocean, and sub-Antarctic islands. Environ Model Software 140:105015. https://doi.org/10.1016/j.envsoft.2021.105015
Meyer B, Atkinson A, Bernard KS, Brierley AS, Driscoll R, Hill SL, Marschoff E, Maschette D, Perry FA, Reiss CS, Rombolá E, Tarling GA, Thorpe SE, Trathan PN, Zhu G, Kawaguchi S (2020) Successful ecosystem-based management of Antarctic krill should address uncertainties in krill recruitment, behaviour and ecological adaptation. Commun Earth Environ 1:28. https://doi.org/10.1038/s43247-020-00026-1
Miller DGM, Hampton I (1989) Biology and ecology of the Antarctic krill. Biomass Sci Ser 9:1–166
Moreira E, Juáres M, Barrera-Oro E (2014) Dietary overlap among early juvenile stages in an Antarctic notothenioid fish assemblage at Potter Cove, South Shetland Islands. Polar Biol 37:1507–1515. https://doi.org/10.1007/s00300-014-1545-3
Moreira E, Novillo M, Eastman JT, Barrera-Oro E (2020) Degree of herbivory and intestinal morphology in nine notothenioid fishes from the western Antarctic Peninsula. Polar Biol 43:535–544. https://doi.org/10.1007/s00300-020-02655-w
Moreira E, Novillo M, Mintenbeck K, Alurralde G, Barrera-Oro E, De Troch M (2021) New insights into the autecology of the two sympatric fish species Notothenia coriiceps and N. rossii from western Antarctic Peninsula: a trophic biomarkers approach. Polar Biol 44:1591–1603. https://doi.org/10.1007/s00300-021-02903-7
Novillo M, Elisio M, Moreira E, Macchi G, Barrera-Oro E (2021) New insights into reproductive physiology in Antarctic fish: a trial in Lepidonotothen nudifrons. Polar Biol 44:1127–1139. https://doi.org/10.1007/s00300-021-02879-4
Perry FA, Atkinson A, Sailley SF, Tarling GA, Hill SL, Lucas CH et al (2019) Habitat partitioning in Antarctic krill: pawning hotspots and nursery areas. PLoS ONE 14:e0219325. https://doi.org/10.1371/journal.pone.0219325
Pinkas LM, Oliphant S, Iverson ILK (1971) Food habits of albacore, bluefin tuna and bonito in Californian waters. Calif Fish Game 152:1–105
R Development Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/
Siegel V (1988) A concept of seasonal variation of krill (Euphausia superba) distribution and abundance west of the Antarctic Peninsula. In: Sahrhage D (ed) Antarctic Ocean and resources variability. Springer, Berlin, pp 119–230
Acknowledgements
We are grateful to Carlos Bellisio for his help in field activities and laboratory tasks and to Gaia Gordon for her help in the manuscript preparation. We gratefully acknowledge two anonymous reviewers for helpful comments on the manuscript.
Funding
This study was supported by Grants from Dirección Nacional del Antártico, Instituto Antártico Argentino (PICTA 0100), Consejo Nacional de Investigaciones Científicas y Técnicas [PIP 2017–2019 GI 11220170100219CO, Resolution 2018-8-APN-DIR#CONICET] and Fondo para la Investigación Científica y Tecnológica [PICT 2018-03310 Res.401/19].
Author information
Authors and Affiliations
Contributions
EM and EBO conceived the project. EM and EBO conducted fieldwork and carried out laboratory work. EM, SGS and MN performed statistical analyses. EM, EBO and MN wrote the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moreira, E., Novillo, M., Gómez De Saravia, S. et al. Inter-annual dynamics in the trophic ecology of juveniles of five notothenioid fish species from the South Shetland Islands (Southern Ocean). Polar Biol 46, 993–1009 (2023). https://doi.org/10.1007/s00300-023-03179-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-023-03179-9