Abstract
To date, studies of food overlap in Antarctic fish have been performed on a mixture of late juvenile and adult stages, leaving the young immature specimens (TL ≤ 10 cm) practically unexplored. We studied diet overlap and potential competition among early juvenile individuals in a coastal notothenioid community at Potter Cove, by analysing the stomach contents of 225 fish of 5 species collected in the summer of 2009–2010. We used frequency of occurrence (F %) and the coefficient “Q” for diet evaluation and the method of Tyler and the similarity index “S” for food overlap. Amphipods of the suborder Gammaridea were the main (Q > 2.900) and most frequent (% F) prey for all species, although Notothenia coriiceps also consumed gastropods of the family Littorinidae, mostly Laevilitorina antarctica. Secondary prey were algae for Notothenia rossii and N. coriiceps, calanoid (pelagic) and harpacticoid (benthic) copepods for Trematomus newnesi and the latter copepods and isopods of the family Munnidae for Lepidonotothen nudifrons. The reoccurrence of prey among fish species was 39.6 % and food overlap between 90 % of species pairs was under 58 %. Because similarly low values of diet overlap were reported for intermediate/advanced juveniles and adults of the same species at the same site, we conclude that there is no difference in the degree of interspecific food overlap and therefore potential competition between the immature and mature fraction of the fish community. Food competition is avoided by resource partitioning along a depth gradient or by different prey species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As both predators and prey, fish occupy the intermediate trophic level in the food webs of the Southern Ocean (Kock et al. 2012). The dominant and endemic coastal demersal group, the Antarctic Notothenioidei, are the main predators of benthos, feeding on virtually all the organisms present below their own trophic level from algae to fish, as well as on zooplankton in the water column (Barrera-Oro 2002). They have developed a wide range of feeding strategies, which allow them to utilise food resources in a variety of habitats (Gröhsler 1994), thus reducing dietary overlap. As food overlap may be reflected in competition under conditions of limited resource availability (Odum 1971), the utilisation of such strategies may help to diminish interspecific competition.
Studies of food overlap in Antarctic fish are limited. A few studies are focused on pairs of species in the Ross Sea (Vacchi et al. 1994; La Mesa et al. 1997) and the South Shetland Islands (Moreno and Bahamonde 1975), while others analyse food overlap between multiple species in fish assemblages of the western Antarctic Peninsula and the South Shetlands (Rakusa-Suszczewski and Piasek 1973; Daniels 1982; Barrera-Oro 2003), the South Orkney Islands (Targett 1981) and the Weddell Sea (Schwarzbach 1988). However, all of these investigations focus on advanced juveniles and adult specimens of notothenioid species, thus, leaving the early juvenile stage, e.g. smaller than 10 cm in total length, practically unexplored. The feeding habits/dietary composition of a fish species may vary during ontogeny. Thus, in a fish assemblage, the degree of interspecific food overlap and therefore potential competition at young stages may differ from that at maturity.
The ecosystem of Potter Cove, in the South Shetland Islands, has been well studied in the framework of multidisciplinary scientific programs (Wiencke et al. 1998, 2008). The waters of the cove are usually calm and ice-free most of the year, and the fauna associated with the algal beds is rich and diverse (Quartino and Boraso de Zaixso 2008). Fish, particularly early stages of notothenioids, live permanently around macroalgae substrates, where they obtain food and are protected from potential predators (Barrera-Oro and Piacentino 2007; Barrera-Oro and Winter 2008).
Our collection of immature specimens of several notothenioid species at Potter Cove allowed us to evaluate food overlap and potential competition among early juveniles of the fish community. These data are compared with those of intermediate/late juvenile and adult stages of the same species from the same site and from other areas.
Materials and methods
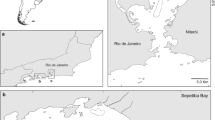
Two hundred and twenty-five notothenioid specimens were collected at Potter Cove, King George/25 de Mayo Island, close to the Argentine scientific station “Carlini” (formerly known as “Jubany”, 62°14′S and 58°40′W) from December 2009 to February 2010 (Fig. 1). Detailed information of the biota and abiotic features of this site is given in Casaux et al. (1990). For sampling, two different types of gear were operated from rubber boats preferably where the seabed was a uniform rocky bottom covered mainly with red and brown macroalgae: (1) A bottom trawl net (mouth 1 m2, length 2 m and mesh 4 mm) was deployed at day and night for 15–30 min at depths of 4–30 m (average, 12 m); (2) Trammel nets (length 15 m, width 1.5 m, inner mesh 2.5 cm, outer mesh 12 cm) were fixed to rocks and set on the bottom at depths of 5–40 m (average, 14 m) from 16 to 24 h (Fig. 1). Further data on the fish species examined are summarised in Table 1. Fish nomenclature follows Gon and Heemstra (1990).
Total and standard length to the nearest 0.1 cm below, weight in g and sex of fish were recorded. The gonad stage was determined according to the scale in Kock and Kellermann (1991). The stomachs were removed and frozen at −20 °C for subsequent analysis at the laboratory in the Museum of Natural Sciences “Bernardino Rivadavia”.
The diet analysis was conducted using the frequency of occurrence of each prey item, expressed as a percentage of all stomachs containing food (F %) and the dietary coefficient “Q”, which is the product of the percentage by number and the percentage by weight of each prey type (Hureau 1970). This reduces biases due to the use of numeric or gravimetric methods. The Q index arbitrarily separates prey into three categories, defined main (Q > 200), secondary (200 > Q > 20) or occasional food (Q < 20). To estimate the percentage by number of algae, the number of algae species present in each stomach content was considered as the number of specimens represented in the sample.
The stomach fullness was estimated using a 5-point scale: 0 (empty), 1 (1/4 full), 2 (1/2 full), 3 (3/4 full), 4 (full). Likewise, the degree of digestion of prey was evaluated as 0 (undigested), 1 (partially digested), and 2 (fully digested).
Dietary overlap between species was analysed following Tyler (1972), where the reoccurrence of prey or percentage overlap among predator species is the number of reoccurrences of prey among predators divided by the number of possible reoccurrences (Table 2). Thus, one reoccurrence means that a prey taxon occurs in two predator species. The total number of potential reoccurrences is the number of predators minus one, multiplied by the number of prey.
Prey overlap between fish species pairs was described by the dietary similarity index “S” (Linton et al. 1981):
where P xi and P yi are the proportions of prey i in the diets of fish species x and y, respectively. S ranges from 0, if no prey is shared, to 100, if the diet of two fish species is identical. S was estimated by considering the contribution of the different prey to the diet by weight.
Finally, dietary diversity was express by the number of taxa P present in the stomach contents and feeding niche breadth of the species was calculating using dietary diversity Index H′:
where pi is the percentage by number of the ith prey (Shanon and Weaver 1949).
Results
Size and sex composition
Size and sex proportions of the species investigated are reported in Table 1. All the fish were sexually immature, at stages I (immature) and II (maturing virgin) of gonad development. Notothenia coriiceps and Notohenia rossii had the greatest size range (6.3–19.9 cm) and Lepidonotothen nudifrons and Harpagifer antarcticus the smallest ones (3.4–8.7 cm), whereas Trematomus newnesi were of intermediate sizes (6.7–13.5 cm).
Diet composition
In general, the degree of prey digestion and stomach fullness were similar for the five species studied. On average, 83 % of the stomachs examined contained food in good condition (stages 0 and 1), thus not preventing identification of the contents, while identification was not possible in the remaining 17 % of the samples (stage 2).
Complete data on the trophic spectrum of juvenile specimens of the fish assemblage and on dietary overlap using Tyler’s method are shown in Table 2. The diet diversity was higher in N. coriiceps (H′ = 1.09, P = 29) and N. rossii (H′ = 0.85, P = 27) and more limited in the remaining species. Diversity values calculated for each species are given at the bottom of Table 2. From the eleven taxonomic groups represented in the diet, only a few represented important food items. The main and most frequent prey for all fish species investigated were demersal-benthic amphipods, predominantly of the suborder Gammaridea (Q > 2,900, F % > 36 %) and gastropods (mostly Laevilitorina antarctica) for N. coriiceps. Secondary preys were algae for N. rossii and N. coriiceps, calanoid (pelagic) and harpacticoid (benthic) copepods for T. newnesi and harpacticoid copepods and isopods of the family Munnidae for L. nudifrons. Other taxa such as polychaetes, tanaidaceans, ostracods, chitons, bivalves and krill (Euphausia superba), were occasional food items and many of them were of negligible importance (Q < 0.1, F % < 8). Among the gammarideans, Gammarellidae, Eusiridae and Iphimediidae were the best represented families, specifically Gondogeneia sp. Oradarea sp. and Gondogeneia antarctica, the dominant species. Among the algae, Desmarestia sp. and Palmaria sp. were the most important food items.
A comparison of prey consumed by the fish species at Potter Cove between early juveniles from this study and intermediate/advanced juveniles and adults reported elsewhere (Casaux et al. 1990; Casaux 1998) is summarised in Fig. 2. Gammaridean amphipods constituted the main food in all fish sizes. Small prey, such as copepods and gastropods, were main and secondary food in smaller fish of some species, whereas they were not consumed by the larger sizes of fish. Conversely, the larger prey, such as krill (~5 cm TL) and fish, were absent or insignificant in the stomachs of smaller fish (Fig. 2a) but were secondary or occasional food for intermediate and larger sizes of some species (Fig. 2b). No ontogenetic shift in diet was found in H. antarcticus, a small species where the difference in the size range between juveniles (3.40–8.60 cm, Table 1) and mature specimens (6.5–11 cm, Casaux 1998) was negligible.
Comparative analysis of the main taxonomic groups represented in the diets of a notothenioid fish assemblage throughout ontogeny at Potter Cove. a small/young juveniles (this study); b advanced juveniles–adults (Casaux et al. 1990). The gap in the Y -axis denotes a change of scale in the values of the prey “amphipods”
Feeding intensity
Stomach fullness indicates high feeding intensity in all species investigated, 69–88 % of stomachs being full or nearly full (degrees 3–4), whereas only 0–4 % of stomachs were completely empty (Table 1; Fig. 3).
Dietary overlap
The reoccurrence of prey among fish species was 39.6 % (Table 2). The gammarideans Gondogeneia sp. and Oradarea sp. and harpacticoid copepods were the most important (main and secondary food) reoccurring prey. Occasional but full reoccurrent food items were gammarideans of family Stenothoidae and algae of genus Desmarestia sp.
The food overlap between pairs of fish based on the index “S” is showed in Table 3. The diet similarity between 90 % of species is lower than 58 %. The highest value of prey overlap was recorded between H. antarcticus and L. nudifrons (67 %), whereas the lowest were between N. coriiceps and the species L. nudifrons (28 %) and T. newnesi (34 %).
Discussion
The early juvenile size ranges of fish species of this study are complementary to those of intermediate/advanced juveniles and adults caught with trammel nets (Casaux et al. 1990) and by hand in tide pools (only H. antarcticus, Casaux 1998) in Potter Cove.
The young specimens of the notothenioid assemblage in Potter Cove are demersal, preying chiefly on gammaridean amphipods and other invertebrates of the benthic community associated with macroalgae beds. These findings partially agree with previous observations on the diet of juvenile N. rossii and T. newnesi from the same site (Barrera- Oro and Piacentino 2007; Barrera-Oro and Winter 2008). High algae densities not only offer food but also protection from higher predators such as penguins and mammals. Unlike late juveniles and adults (Casaux et al. 1990), in the early stages of the fish species analysed in this work, no evidence of vertical migration in the water column for feeding on pelagic organisms was found in the early stages of species analysed in this work, except in T. newnesi, which preyed on calanoid copepods secondarily and on krill and ostracods in negligible amounts. However, the vertical distribution of krill in inshore shallow waters such as Potter Cove can reach the bottom in summer, making it accessible also too demersal-benthic species, without the implicitness of significant movements of krill predators along the water column (Everson 1977; Takahashi and Iwami 1997; Eastman and Barrera-Oro 2010; among others).
It has been demonstrated that algae are consumed deliberately (Barrera-Oro and Casaux 1990) and actively selected by fish (Iken et al. 1997). The dietary coefficient Q underestimates algae as they are counted as single individual in each stomach, despite they often occupy a large volume (Casaux et al. 1990). Nevertheless, some algae species (e.g. Desmarestia sp. and Palmaria sp.) may become main food items for N. rossii and N. coriiceps, as evidenced by frequency of occurrence (F % > 20, Table 2).
A diet comparison for some notothenioid species from Potter Cove between small sizes of early juvenile specimens from this study and larger sizes of intermediate/advanced juveniles and adults reported in previous investigations shows that, as it is common in Antarctic fish, variation of diet during ontogeny mainly concerns different sizes of the same taxa of prey (Fig. 2). This contributes to intraspecific trophic segregation.
During the sampling period, there was a high availability of food (Fig. 3), in agreement with previous studies on trophic ecology of fish from the same area also carried out in summer (reviewed in Barrera-Oro and Casaux 2008). This stage of “unrestricted access to prey” may contribute to reduction in the interspecific competition for food. Unlike krill availability, which is mainly restricted to summer, gammarideans are available in Potter Cove all year round (Casaux et al. 1990). Therefore, it is to be expected that food overlapping and potential competition among the early stages of notothenioids did not change substantially throughout the year. In this regard, it will be mandatory to extend sampling beyond the summer months.
The proportional food overlap (according to Tyler’s method) among young specimens in Potter Cove was low (<40 %) with some gammaridean and harpacticoid copepods as the most important (higher Q) reocurrent prey (Table 2). Likewise, the S index value from the comparative dietary analysis at the lowest possible taxonomic level was low between most of species pairs (<58 %) and slightly higher between H. antarcticus and L. nudifrons (67 %). The two latter species showed a narrower diet, based chiefly on gammarideans. They also share a specific common habitat in Potter Cove: H. antarticus occurs under rocks on muddy bottoms at 3–6 m depth, and occasionally in tide pools and also, as does L. nudifrons, at the entrance of the cove at least down to 25 m depth. These two factors could contribute to the higher diet overlap found between the two species.
Comparison of present results with those obtained previously at the same site shows no different in the degree of interspecific food overlap and potential competition between early juveniles (this work) and advanced juveniles/mature specimens (Barrera-Oro 2003) in an inshore fish community such as Potter Cove. In the previous study, values of prey reocurrences among fish predators and S index between most species pairs were also low, 33 % and <50 % respectively. The low dietary overlap was explained by resource partitioning among generalised and specialised feeders along a depth gradient or by different prey species, with no evidence of food competition, as similarly found for young notothenioids in this study. Similar findings on the absence of substantial food competition and its possible causes in other fish assemblages, although not specific to the early juvenile community, have been reported for other Antarctic (Targett 1981; Daniels 1982; Schwarzbach 1988; Gon and Mostert 1992; Vacchi et al. 1994; La Mesa et al. 1997) and Arctic (Tyler 1972; Arntz 1980; Atkinson and Percy 1992; Klemetsen 1993; Murie 1995; Hoines and Bergstad 2002; among others) ecosystems.
Although there are no previous studies of this kind for comparison, our results from Potter Cove may be representative of the levels of interspecific dietary overlap and potential competition among early juvenile notothenioids in other Antarctic nearshore fish assemblages.
References
Arntz WE (1980) Predation by demersal fish and its impact on the dynamics of macrobenthos. In: Tenore KR, Coull BC (eds) Marine benthic dynamics. University of South Carolina Press, Columbia, pp 121–149
Atkinson EG, Percy JA (1992) Diet comparison among demersal marine fish from the Canadian Arctic. Polar Biol 11:567–573
Barrera-Oro ER (2002) The role of fish in the Antarctic marine food web: differences between inshore and offshore waters in the southern Scotia Arc and west Antarctic Peninsula. Antarct Sci 14:293–309
Barrera-Oro ER (2003) Analysis of dietary overlap in Antarctic fish (Notothenioidei) from the South Shetland Islands: no evidence of food competition. Polar Biol 26:631–637
Barrera-Oro ER, Casaux RJ (1990) Feeding selectivity in Notothenia neglecta, Nybelin, from Potter Cove, South Shetland Islands, Antarctica. Antarct Sci 2:207–213
Barrera-Oro ER, Casaux R (2008) General ecology of coastal fish from the South Shetland Island and west Antarctic Peninsula areas. Beri Polar und Meeresforsch 571:95–110
Barrera-Oro ER, Piacentino GLM (2007) Feeding habits of juvenile Trematomus newnesi (Pisces, Nototheniidae) at Potter Cove, South Shetland Islands, Antarctica. Polar Biol 30:789–796
Barrera-Oro ER, Winter D (2008) Age composition and feeding ecology of early juvenile Notothenia rossii (Pisces, Nototheniidae) at Potter Cove, South Shetland Islands, Antarctica. Antarct Sci 20:339–341
Casaux RJ (1998) The contrasting diet of Harpagifer antarcticus (Notothenioidei, Harpagiferidae) at two localities of the South Shetland Islands, Antarctica. Polar Biol 19:283–285
Casaux R, Mazzotta A, Barrera-Oro ER (1990) Seasonal aspects of the biology and diet of nearshore nototheniid fish at Potter Cove, South Shetland Islands, Antarctica. Polar Biol 11:63–72
Daniels RA (1982) Feeding ecology of some fishes of the Antarctic Peninsula. Fish Bull 80:575–588
Eastman JT, Barrera-Oro ER (2010) Buoyancy studies of three morphs of the Antarctic fish Trematomus newnesi (Nototheniidae) from the South Shetland Islands. Polar Biol 33:823–831
Everson I (1977) The living resources of the Southern Ocean. GLO/SO/77/1. FAO, Rome
Gon O, Heemstra PC (eds) (1990) Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahams Town
Gon O, Mostert D (1992) Aspects of the ecology of two nototheniid fish species in the inshore zone of the sub-Antarctic Marion Island. South Afr J Antarct Res 22:59–67
Gröhsler T (1994) Feeding habits as indicators of ecological niches: investigations of Antarctic Fish conducted near Elephant Island in late autumn/winter 1986. Arch Fish Mar Res 42:17–34
Hoeines AS, Bergstad OA (2002) Food partitioning by flatfish species on a herring spawning ground. Sarsia 87:19–34
Hureau JC (1970) Biologie comparée de quelques Poissons antarctiques (Nototheniidae). Bull Inst Oceanogr Monaco 68:1–244
Iken K, Barrera-Oro ER, Quartino ML, Casaux RJ, Brey T (1997) Grazing by the Antarctic fish Notothenia coriiceps: evidence for selective feeding on macroalgae. Antarct Sci 9:386–391
Klemetsen A (1993) The food of the long-rough dab (Hippoglossoides platessoides limandoides Bloch) in Balsfjorden, north Norway. Sarsia 78:17–24
Kock K-H, Kellermann A (1991) Reproduction in Antarctic notothenioid fish (review). Antarct Sci 3:125–150
Kock K-H, Barrera-Oro E, Belchier M, Collins MA, Duhamel G, Hanchet S, Pshenichnov L, Welsford D, Williams R (2012) The role of fish as predators of krill (Euphausia superba) and other pelagic resources in the southern ocean. CCAMLR Sci 19:115–169
La Mesa M, Vacchi M, Castelli M, Diviacco G (1997) Feeding ecology of two nototheniid fishes, Trematomus hansoni and Trematomus loennbergii, from Terra Nova Bay, Ross Sea. Polar Biol 17:62–68
Linton LR, Davies RW, Wrona FJ (1981) Resource utilization indices: an assessment. J Anim Ecol 50:283–292
Moreno CA, Bahamonde N (1975) Nichos alimentarios y competencia por el alimento entre Notothenia coriiceps neglecta Nybelin y Notothenia rossii marmorata Fischer en Shetland del Sur, Antártica. Ser Cient Inst Antárt Chil 3:45–62
Murie DJ (1995) Comparative feeding ecology of two sympatric rockfish congeners, Sebastes caurinus (copper rockfish) and S. maliger (quillback rockfish). Mar Biol 124:341–353
Odum EP (1971) Fundamentals of Ecology. W.B Saunders Company, Piladelphia 574 pp
Quartino ML, Boraso de Zaixso AL (2008) Summer macroalgal biomass in Potter Cove, South Shetland Islands, Antarctica: its production and flux to the ecosystem. Polar Biol 31:281–294
Rakusa-Suszczewski S, Piasek A (1973) Size, feeding and action of proteolytic enzymes in the Antarctic fish of the Trematomus genus (Nototheniidae). Bull Pol Acad Sci Biol Sci Ser 21:139–144
Schwarzbach W (1988) The demersal fish fauna of the eastern and southern Weddell Sea: geographical distribution, feeding of fish and their trophic position in the food web. Ber Polarforsch 5:1–94
Takahashi M, Iwami T (1997) Summer diet of demersal fish at the South Shetland Islands. Antarct Sci 9:407–413
Targett TE (1981) Trophic ecology and structure of coastal Antarctic fish communities. Mar Ecol Prog Ser 4:243–263
Tyler AV (1972) Food resource division among northern, marine demersal fishes. J Fish Res Board Can 29:997–1003
Vacchi M, La Mesa M, Castelli A (1994) Diet of two coastal nototheniid fish from Terra Nova Bay, Ross Sea. Antarct Sci 6:61–65
Wiencke C, Ferreyra G, Arntz W, Rinaldi C (1998) The Potter Cove coastal ecosystem, Antarctica—Synopsis of research performed within the frame of the Argentinean–German Cooperation at the Dallmann Laboratory and Jubany Station (King George Island, Antarctica, 1991–1997). Ber Polarforsch 299:1–326
Wiencke C, Ferreyra GA, Abele D, Marenssi S (2008) The Antartic ecosystem of Potter Cove, King- George Island (Isla 25 de Mayo). Synopsis of research performed 1999-2006 at the Dallmann Laboratory and Jubany Station. Ber Polarforsch Meeresforsch 571:1–411
Acknowledgments
We thank Carlos Bellisio, Luis Vila and Oscar González, members of the Instituto Antártico Argentino for their field assistance. We are grateful to Drs. G. Alonso, J. Lopez Gappa, M. Landoni, and L. Quartino, and Lic. G. Campana and Lic. D. Deregibus helped with prey identification. We thank Dr. M. La Mesa, Prof. J. Eastman and an anonymous referee, whose critical comments improved the quality of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moreira, E., Juáres, M. & Barrera-Oro, E. Dietary overlap among early juvenile stages in an Antarctic notothenioid fish assemblage at Potter Cove, South Shetland Islands. Polar Biol 37, 1507–1515 (2014). https://doi.org/10.1007/s00300-014-1545-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-014-1545-3