Abstract
Marine coastal areas are highly productive due to the presence of various inputs of organic matter, including terrestrial material, which fuels food webs. However, the ecological mechanisms underlying the productivity of benthic and demersal fish species in estuarine areas are poorly understood. By means of C and N stable isotope analysis and Bayesian mixing models, we investigated the trophic niches of three common fish species: Citharus linguatula, Pegusa lascaris (flatfish) and Liza ramada (mullet) in the Gulf of Gaeta (Italy). Fish were collected from the north-western area and the south-eastern area of the Gulf of Gaeta, the latter affected by organic inputs from the Garigliano River. The results highlighted the riverine terrestrial origin of the organic matter at the base of the food web in the south-eastern area and marine autochthonous input in the north-western area. All fish species increased their trophic specialisation in proximity to the river mouth. L. ramada specialised on seston of terrestrial origin, reducing its niche overlap with C. linguatula and P. lascaris. Away from the river mouth, all species were characterised by longer individuals, increased intraspecific diet variability and higher interspecific similarity in resource use. Organic input from the river represented a complementary trophic niche axis that enabled lower interspecific niche overlap in the south-eastern area, where fish populations were found at higher densities. In conclusion, this study provided information about the effects of the flow of material from the basal compartment up to abundant fish species in areas enriched by organic matter of varying origin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine coastal ecosystems are among the most productive ecosystems in the world, supporting great density and diversity of life. However, despite their value to humans, these ecosystems are becoming increasingly vulnerable, since they are exposed to strong pressures from anthropic activities including coastal urbanisation, tourism and fish farming (Halpern et al. 2008), as well as the consequent rise in organic and inorganic inputs (Careddu et al. 2015; Calizza et al. 2015; Jona-Lasinio et al. 2015; Rossi et al. 2018). In marine ecosystems, benthic predators such as decapods and fish, including important commercial species, are influenced by sediment and vegetation structure, faunal composition, energy fluxes and nutrient cycles (Fazi and Rossi 2000; Carrozzo et al. 2014; Careddu et al. 2017). In turn, fish are important consumers, influencing aquatic food web structure and functioning (Frederiksen et al. 2006; Mancinelli et al. 2007). Their foraging behaviour and feeding ecology are generally characterised by high trophic plasticity. Specifically, their dynamics seem to be closely related to the dynamics of organic matter pools (Darnaude et al. 2004; Buchheister and Latour 2011; Kostecki et al. 2012; di Lascio et al. 2013; Selleslagh et al. 2015; Bentivoglio et al. 2016), in many cases promoting exportation of organic matter via migration from highly productive to less productive areas (Le Loc’h et al. 2015).
In this context, the study of trophic niches represents a useful tool for describing the trophic pathways and foraging behaviour of species in coastal areas (Kostecki et al. 2012; Careddu et al. 2015). Stable Isotope Analysis (SIA) is increasingly being used as an efficient method for studying trophic niches and fish ecology (Buchheister and Latour 2011; Layman and Allgeier 2012; Le Loc’h et al. 2015; Parzanini et al. 2017; Adams et al. 2017; Costantini et al. 2018). Indeed, the isotopic signatures of carbon (δ13C) and nitrogen (δ15N) give information about the resources exploited by a consumer and its trophic position in the food web (Post 2002; Inger and Bearhop 2008; Kowskowsi and Trembaczowski 2015; Gallagher et al., 2017). Moreover, the δ13C and δ15N values of consumers within a given environment can be plotted on an isotopic biplot, allowing the quantification of isotopic niche metrics (Layman et al. 2007; Jackson et al. 2011; Calizza et al. 2017), which can provide useful insight concerning the trophic structure of a population and interspecific interactions. In addition, the recent improvement of multivariate Bayesian models and bootstrapped procedures has enabled the incorporation of isotopic signature uncertainty, providing more robust information about the diet and trophic niches of the studied species (Parnell et al. 2010; Jackson et al. 2011; Phillips 2012; Rossi et al. 2015).
As well as enabling reconstruction of trophic niche and diet, δ13C also provides information on the various origins of consumer resources (Post 2002; Inger and Bearhop 2008; Kostecki et al. 2012; Faria et al. 2018), since it varies widely among primary producers (e.g. terrestrial C3 plants, marine producers) (Marshall et al. 2007; Rossi et al. 2010; Careddu et al. 2015). This makes it possible to determine the importance of terrestrial inputs to food webs in estuarine habitats (Kostecki et al. 2012; Careddu et al. 2015).
Many studies in the Mediterranean Sea have focused on the trophic ecology of pelagic fish species (Stergiou and Karpouzi 2002; Pinnegar et al. 2003; Zeug et al. 2017). However, less is known about benthic and demersal fish species in productive coastal areas. For these categories, our understanding of the influence of nutrient inputs and environmental factors on their diet and their role in the food web is relatively limited (Darnaude et al. 2004; Rumolo et al. 2016). Indeed, although estuarine areas are generally considered to be highly productive, the ecological mechanisms underlying such productivity in benthic and demersal fish communities have not been extensively investigated. Among others, flatfish are valuable as a product for human consumption, mullets are important low-cost sources of protein, and both are commercially exploited worldwide. Being bottom-feeders, they are able to use both autochthonous and allochthonous inputs, such as terrestrial-derived food inputs transported by river run-off in coastal areas, thereby linking grazing and detritus chains and thus playing a key ecological role in coastal and estuarine habitats (Darnaude et al. 2004; Buchheister and Latour 2011; Kostecki et al. 2012). Understanding the species’ feeding behaviour and the main trophic sources supporting fish populations is fundamental to the management of areas crucial for fisheries. From this perspective, the aim of this study was to investigate, by stable isotope analysis, the trophic niches and overlap of flatfish and mullet in the Gulf of Gaeta. This is an important recruitment zone in the Mediterranean Sea (Colloca et al. 2015; Criscoli et al. 2017), crucial for fisheries and fish farming (Guerriero et al. 2017; Mazzola and Sarà 2001), characterised by a gradient of influence exerted by a large river mouth (Orlandi et al. 2014; Careddu et al. 2015, 2017; Rossi et al. 2018). We assessed the spatial variation of (i) trophic niches and (ii) the strength of interspecific interaction between the flatfish Pegusa lascaris (Risso 1810) and Citharus linguatula (Linnaeus 1758) and the mullet Liza ramada (Risso 1826), which are among the most common fish in the Gulf, in association with riverine input of terrestrial-derived organic matter.
Materials and methods
Study area and sampling activities
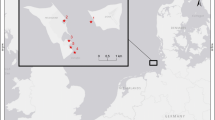
Sampling was carried out in October and November 2012 in the marine coastal area of the Gulf of Gaeta (Italy, central Tyrrhenian Sea), extending from the town of Gaeta (41°12′51″N, 13°34′16″E) to the Garigliano River mouth (41°13′23.36″N, 13°45′40.66″E; average discharge 120 m3/s) (Fig. 1). Currents mostly flow from south-east to north-west following the cyclonic circulation of the Tyrrhenian Sea (La Rosa et al. 2002). However, there is another current flowing from offshore towards the coast near the village of Scauri (Aguzzi et al. 2012). These currents cause organic matter and nutrients to accumulate off the Garigliano River mouth in the south-eastern portion of the Gulf of Gaeta (Rossi et al. 2018). Two areas were selected for the sampling activities: the first in the north-western part of the Gulf (hereafter called NW) between the town of Gaeta and the promontory of Gianola-Monte Scauri, and the second between the promontory and the Garigliano River mouth to the south-east (hereafter called SE) (Fig. 1).
Within each area, benthic macroinvertebrates were collected in October and November 2012. In order to collect the diverse invertebrate taxa potentially included in the fish’s diets, invertebrates were sampled by multiple methods at four sampling sites at depths ranging from 5 to 12 m in each area (Fig. 1). Three replicate samples per site were collected, using a Van Veen grab (0.035 m2), leaf litter bags containing autochthonous leaf detritus (30 g dry weight) (Van Dokkum et al. 2002; Costantini et al. 2009; Calizza et al. 2013a) and fish traps for larger decapods and gastropods (Careddu et al. 2015). Given the different methods of collection, the abundance of invertebrates is indicated as the number of individuals per sampling site. Sediment collected with the grab was sieved through a 500-µm mesh to isolate the infauna specimens. Additional sediment replicates were collected in order to determine the isotopic composition of organic matter in the sediments (hereafter SOM) and to isolate samples of coarse particulate organic plant matter (hereafter detritus) from the sediment bulk (Calizza et al. 2013b). The organic matter component (dead and living) present in the water column (hereafter seston) was sampled by a 20-µm WP2 plankton net (Rolff 2000). The same procedure was used to obtain seston samples from the Garigliano River, 1 km upstream from the river mouth, which made it possible to measure the δ13C and δ15N values of organic matter transported by the river. Water samples were stored in 5 L refrigerated dark bottles and transported to the laboratory. Here, samples were prefiltered in order to collect particles within the range of 100–500 µm, which enabled us to exclude both small phytoplankton and coarse detritus particles.
In each area, adult specimens of L. ramada, C. linguatula and P. lascaris were captured with the help of professional fishermen at the end of November. In both areas, using an 8-m fishing boat, a 400-linear metre gillnet (mesh size 10 mm) was lowered to a depth of 10 m for 12 h.
Laboratory procedures and stable isotope analysis
Samples of seston were concentrated by centrifugation (2000 rpm for 20 min). Water from the layer containing seston was then collected and passed through pre-combusted Whatman GF/F filters to obtain a sufficient amount of biomass, which was analysed as a whole for its isotopic signature (Kostecki et al. 2012; di Lascio et al. 2013). Samples of detritus and fresh seagrass were isolated and rinsed in distilled water. SOM was collected from the sediment surface (Mancinelli and Rossi 2002). All the collected macroinvertebrates were transferred to the laboratory in refrigerated plastic bags, sorted, identified using binocular microscopes and enumerated. Fish were measured for standard length (SL) to the nearest mm and weighed (W, wet weight) to the nearest g. For the stable isotope analysis, one sample of dorsal muscle was taken from each fish (Darnaude et al. 2004; Bentivoglio et al. 2016; Wolters et al. 2018). All the samples were stored at − 80 °C and then freeze-dried. Detritus samples were further observed under a binocular microscope to remove any inorganic residual impurities. Seagrass fragments were identified, gently scraped to remove epibionts and washed with distilled water. To remove carbonates, which could interfere with δ13C signatures, subsamples of sediment and seston were acidified (HCl 1M) drop by drop and re-dried (60 °C, 72 h) before being analysed for δ13C (Kennedy et al. 2005; Kostecki et al. 2012). In this case, the δ15N signatures were obtained from unacidified subsamples. For analysis of macroinvertebrates, exoskeletal parts, valves and shells were removed under a dissection microscope, and only very small organisms were acidified drop by drop to remove inorganic carbon and then re-dried (60 °C, 72 h) before analysis. All samples were ground to a fine powder using a ball mill (Fritsch Mini-Mill Pulverisette 23). Subsamples of 0.20 ± 0.05 mg (animal tissues) and 3.0 ± 0.5 mg (basal resources) were placed in tin capsules (Post 2002; Rossi et al. 2007; Costantini et al. 2014). The samples were analysed using an Elementar Vario Micro-Cube elemental analyser (Elementar Analysensysteme GmbH, Germany) coupled with an IsoPrime100 isotope ratio mass spectrometer (Isoprime Ltd., Cheadle Hulme, UK). Stable carbon and nitrogen ratios were expressed in δ units in per mil (‰) as the difference with respect to international standards (Vienna Pee Dee Belemnite—PDB for carbon and atmospheric N2 for nitrogen) in accordance with the equation: δX (δ13C or δ15N) = [(Rsample/Rstandard)/Rstandard] × 103 (Ponsard and Arditi 2000), where R is either the 13C/12C or the 15N/14N ratio. The internal laboratory standard was IAEA-600 Caffeine. Measurement error was found to be typically smaller than 0.05‰. In accordance with Post et al. (2007), for fish with C/N ratios > 3.5, the δ13C signatures were corrected for lipid content using the equation: δ13Ccorrected = δ13Cmeasured − 3.32 + 0.99 × C/N (Post et al. 2007). As in Abrantes et al. (2013), the δ13C signatures of invertebrates were not corrected for lipid content because shifts in δ13C associated with lipid removal can be highly variable, small and taxon-specific (Logan et al. 2008; Mateo et al. 2008).
Trophic niche analysis and mixing models
Isotopic niche metrics were applied to each species in accordance with Layman et al. (2007). The range between the lowest and highest δ15N values (NR) indicates the maximum distance between the basal resource and the top consumer in the food web. The range between the lowest and highest δ13C values (CR) gives information about the range of resources used by organisms. The mean nearest neighbour distance (MNND) is the mean distance between nearest values in the isotopic biplot space, providing information about the trophic redundancy of individuals (lower values indicate higher trophic redundancy), and its standard deviation (SDNND) gives information about the evenness of isotopic data distribution. The mean distance to centroid (CD) is the average Euclidean distance of each individual to the centroid, indicating the average trophic diversity (Layman et al. 2007). In addition to the Layman et al. (2007) metrics, standard ellipse areas were calculated (as SEAc; SIBER analysis, Jackson et al. 2011). The standard ellipse area is comparable to standard deviation for univariate cases applied to bivariate data. It provides robust information about the bidimensional isotopic niche area and allows comparisons for small population sizes (i.e. for n ≥ 5, Perkins et al. 2018; Bašic et al. 2018) because it is largely insensitive to sample size (Jackson et al. 2011). Bayesian Stable Isotope Mixing Models were run in order to evaluate the contributions of macroinvertebrates, SOM, detritus and seston to the fish diets in each area. Given (i) the relatively shallow sampling depth (10 m), and (ii) previous evidence demonstrating that seston contributes to the diet of flatfish in estuarine areas (Kostecki et al. 2012), seston was considered a potential trophic source for both the mullet and the two flatfish. The seagrass samples, mainly Posidonia oceanica (Delile 1813), were not included as input in the mixing models because they were only occasionally found in the northern area. In the northern area, the mullet L. ramada presented two separate isotopic δ13C groups (see Results). Thus, specimens of these two groups were considered separately for the determination of their diet by means of Bayesian mixing models. The SIAR model requires the isotopic signatures of target consumers and potential resources (mean and standard deviation), as well as the Trophic Enrichment Factor (TEF), which is the difference between the isotopic signatures of consumers and their potential diets. We chose a TEF of 2.9 ± 0.3‰ for δ15N and 1.3 ± 0.3‰ for δ13C, as measured in muscle tissue, on the basis of literature data (McCutchan et al. 2003). The output of the model is a density function distribution of plausible proportion values, whose central tendency (mode, mean, median) and upper and lower limits of credibility intervals (CI: 50%, 75%, 95%) reveal the range of feasible contributions to fish diets of each resource (Parnell et al. 2010).
Following Calizza et al. (2017), the potential limiting effect between species pairs (αij, i.e. the effect of species j on species (i) in each area was calculated from the ratio between the intra- and interspecific isotopic distance between specimens. Briefly, the mean isotopic distances of each specimen from (i) all its conspecifics (MNDintra), and (ii) all specimens of each remaining species (MNDinter) were calculated and compared. At the local scale, the ratio of MNDintra to MNDinter has been shown to be a robust measure of the strength of interspecific interaction between populations sharing trophic resources (Calizza et al. 2017; Britton et al. 2019). It is not affected by sample size and is directly dependent on the similarity in diet between specimens (Calizza et al. 2013b, 2017, 2018).
Data analysis
Data were tested for normality and transformed if necessary before performing statistical analyses. To test for potential differences between the isotopic distributions (δ13C and δ15N) of the three fish species, a Permanova test based on Euclidean distances was performed (Calizza et al. 2017).
The parameters a and b of the length–weight relationship were estimated by logarithmic transformation of the equation W = a*Lb, in which W is the body weight (g); L is the standard body length (mm); a is the intercept; and b is the slope indicating the condition of fish (Ricker 1973; Jones 2009). ANCOVA was performed at 0.05 significance in order to compare the condition of each fish species between areas, with significantly higher b values indicating better conditions (Jones 2009).
Results
Food sources
δ13C and δ15N values in seston collected in the Garigliano River were − 29.9 ± 1.0‰ and 7.2 ± 0.8‰ respectively. The δ13C values of seston from marine water, detritus and SOM were lower in SE than in NW (t test: t(seston) = 2.28, t(detritus) = 8.13 and t(SOM) = 4.02, all p < 0.05), as were the δ15N values of detritus and SOM (t test: t(detritus) = 2.12, t(SOM) = 2.23, both p < 0.05), whereas no differences were found in the δ15N values of seston (Table 1). The abundance of macroinvertebrates did not differ significantly between the two areas: 693.26 ± 84.04 individuals/site were sampled in NW and 517.53 ± 72.59 individuals/site in SE (t test, t = 1.58, p > 0.05). In addition, average macroinvertebrate δ13C and δ15N values were similar (t test, δ13C: t = 1.1, p > 0.05; δ15N: t = 0.04, p > 0.05), and several taxa had overlapping isotopic values (Table 2). The most 13C-enriched taxa were Cumacea and Tanaidacea in NW and SE respectively, while the most 13C-depleted taxon was the sea urchin (Spatangoida) in both areas (Table 2).
Fish species
Overall, 70 specimens of L. ramada (8 in NW and 62 in SE), 18 specimens of C. linguatula (11 in NW and 7 in SE) and 45 specimens of P. lascaris (7 in NW and 38 in SE) were collected (Table 3). Individuals of L. ramada, C. linguatula and P. lascaris were longer in NW than in SE (Table 3; Student t test, t(L. ramada) = 4.20, t(C. linguatula) = 3.97 and t(P. lascaris) = 11.50, p always < 0.001). The slopes (b) of the log-transformed length–weight relationships (W = a*Lb) of L. ramada (bNW = 2.30, bSE = 3.12; ANCOVA, F = 5.5, p < 0.05), P. lascaris (bNW = 1.92, bSE = 2.67; ANCOVA, F = 4.7, p < 0.05) and C. linguatula (bNW = 3.11, bSE = 2.05; ANCOVA, F = 5.7, p < 0.05) differed significantly between areas.
In each area, the isotopic distribution of δ13C and δ15N values differed between fish species (Fig. 2; NW: PERMANOVA, F = 2.98, p < 0.05; SE: PERMANOVA, F = 113.5, p < 0.05). In the NW area, C. linguatula had the highest δ15N values (one-way ANOVA, F = 14.89, p < 0.05, Tukey test, p < 0.05). In contrast, the δ13C values of the three species overlapped substantially, lying within the δ13C range of marine resources. In SE, L. ramada had significantly lower carbon signatures (PERMANOVA, F = 26.55, p < 0.05), while the isotopic signatures of C. linguatula and P. lascaris were similar to what was observed in NW (PERMANOVA, F = 1.98 and F = 2.37 respectively, p > 0.05 for both species), and the three species overlapped on the δ15N axis (Fig. 2). Overall, δ13C values increased with body length in C. linguatula and L. ramada (r = 0.78, p < 0.001 and r = 0.50, p < 0.001 respectively), while δ15N values increased with body length in C. linguatula only (r = 0.50, p < 0.05).
Trophochemical graphs of δ13C and δ15N values with standard ellipses for the three fish species (1: Citharus linguatula, 2: Pegusa lascaris and 3: Liza ramada) sampled in two areas of the Gulf of Gaeta: north-west, south-east. Dashed polygons indicate the variation in isotopic signals (mean ± standard deviation) of the potential resources: seston (SES), sediment organic matter (SOM), Detritus (DET) and Macroinvertebrates (MACRO)
Isotopic and trophic niches
In both areas, the trophic niche area, calculated as the SEAc, was largest for L. ramada and smallest for C. linguatula (Table 3 and Fig. 2). Despite the higher number of individuals of L. ramada and P. lascaris sampled, niche area was smaller and intraspecific similarity greater for all species in SE than in NW. L. ramada had the largest CR and NR. In NW, this species was characterised by two separate isotopic groups differing in their δ13C values (− 23.72‰ ± 0.55 vs. − 15.72‰ ± 0.37, t test, t = 12.03, p < 0.0001) but with similar δ15N values (9.32‰ ± 1.15 vs. 10.65‰ ± 0.48, t test, t = 1.1, p > 0.05) (Fig. 2). As a consequence, when considering all the specimens sampled in NW, L. ramada had the lowest trophic redundancy among individuals, as denoted by the highest CD, MNND and SDNND values (Table 3 and Fig. 2). In contrast, the flatfish C. linguatula had the lowest CD, MNND and SDNND values, indicating the highest degree of trophic similarity between individuals (Table 3 and Fig. 2). The SEAc (R2 = 0.17), CD (R2 = 0.08), MNND (R2 = 0.02) and SDNND (R2 = 0.02) values were all independent of the number of individuals sampled (p > 0.05 in all cases).
The trophic overlap (αij) between species was significantly higher in NW, and varied between species pairs (Fig. 3, Two-way ANOVA, effect of area: F = 41.6, p < 0.0001, effect of species pair: F = 25.1, p < 0.0001, interaction: F = 10.9, p < 0.0001). The only exception was the effect of C. linguatula on P. lascaris, which was higher in SE (Two-way ANOVA and post hoc comparisons, p < 0.0001). In NW, all species pair interactions were found to be asymmetric (i.e. αij ≠ αji, one-way ANOVA and post hoc comparisons, p < 0.05), whereas in SE, asymmetry in interaction strength was observed only between C. linguatula and P. lascaris (Fig. 3). For each species, the mean αij measured at each location was not dependent on the number of individuals sampled (R2 = 0.05, p > 0.05).
Strength of the potential interspecific limiting effect between Liza ramada, Citharus linguatula and Pegusa lascaris in two areas of the Gulf of Gaeta (Italy) (black symbols: north-west; empty symbols: south-east). The interspecific limiting effect (αij) is measured with reference to intra- and interspecific isotopic similarity between specimens, and represents the potential effect of species j (upper row) on species i (lower row)
While the diet of the two flatfish species relied on both macroinvertebrates and seston in NW (Fig. 4a), they showed a marked preference for macroinvertebrates in SE (Fig. 4b). In contrast, the diet of L. ramada included all potential resources in NW while it specialised on seston in SE (Fig. 4). However, two groups of individuals were evident in NW: one characterised by more negative δ13C values, consuming mainly SOM and seston, and one characterised by less negative δ13C values, consuming mainly macroinvertebrates and detritus (Fig. 4a).
Proportional contribution of food sources (Proportion) to the diet of Citharus linguatula, Pegusa lascaris and Liza ramada in the north-west (a) and south-east (b) areas of the Gulf of Gaeta (Italy). In the north-west, the diet of L. ramada is presented separately for two subsets of specimens, which showed significantly different δ13C values. Macro: macroinvertebrates; SOM: sediment organic matter; Detritus: coarse organic detritus; Seston: suspended particles in the water column, including plankton and dead organic matter. Grey shades, from dark to light, represent 50%, 75% and 95% credible intervals around the modal contribution of each food source. Data obtained through isotopic Bayesian mixing models (SIAR package, R software)
Discussion
Trophic niche of fish populations
This study investigated the trophic ecology of the flatfish C. linguatula, P. lascaris and the mullet L. ramada in a Mediterranean coastal sea, obtaining novel information on their feeding strategies and interspecific interactions in an important recruitment area influenced by run-off from a large river (the average discharge of the Garigliano is 120 m3/s). For these and other more commercially important fish species, the Gulf of Gaeta is considered a crucial area in the central Mediterranean (Mazzola and Sarà 2001; Colloca et al. 2015; Criscoli et al. 2017; Guerriero et al. 2017). Here, fish species benefit from suitable conditions for growth, such as high food availability, favourable water temperature and potential refuge against predators (França et al. 2011).
Our results highlighted differences in the trophic behaviour of three species in the Gulf in response to changing environmental conditions. Specifically, mullets were found to vary their diet significantly across and within areas whereas flatfish exhibited a more consistent and homogeneous feeding pattern. Indeed, flatfish relied for most of their diet on macroinvertebrates, displaying very narrow trophic niches with limited variation among individuals. Interindividual diet variability increased in all three species in NW, far from the river mouth, where populations were characterised by greater average length of individuals and terrestrial-derived organic inputs were limited. Of the three species, only L. ramada shifted its food preferences towards terrestrial-derived food near the river mouth, which reduced its niche overlap and strength of interaction with the other two species. The independence of SEAc, CD, MNND, SDNND and αij from population densities makes it possible to state confidently that niche breath, intraspecific trophic similarity and the strength of interspecific interactions were not biased by the lower number of specimens of L. ramada and P. lascaris sampled in NW (Calizza et al. 2017).
The slopes between the length and weight of the three species differed between areas. When comparing conspecific populations or subpopulations, these slopes can be used as a proxy of fish condition, with a higher slope generally being considered indicative of a better condition (Jones 2009). Specifically, for L. ramada and P. lascaris, the slope was significantly higher in SE than in NW. This suggests that additional resource inputs from riverine transport, coupled with lower interspecific limitation associated with reduced niche overlap, may translate into better conditions for fish specimens observed in the estuarine area than in the NW sector of the Gulf. On the other hand, the greater incorporation of marine-derived organic matter through the benthic food chain can explain the correlation between δ13C values and length in individuals sampled in the northern area of the Gulf. Indeed, such intraspecific changes in isotopic carbon signatures with body length suggest migration of larger specimens from the river mouth-influenced area to the north-western one. The lower δ13C values measured in marine seston sampled in SE strongly resembled those measured 1 km upstream from the river mouth, further confirming that more negative δ13C values observed in L. ramada in SE were dependent on the assimilation of river-transported organic matter (Careddu et al. 2015).
Of the two flatfish, P. lascaris was more generalist than C. linguatula. However, like C. linguatula, its diet was based on macroinvertebrates, in accordance with Marinaro and Bouabid (1983) and what is generally reported for flatfish (Link et al. 2002; Darnaude et al. 2004). Specifically, P. lascaris juveniles are known to feed preferentially on macroinvertebrates, such as Cumacea, Bivalvia, Decapoda and Amphipoda (Cabral et al. 2002). In addition, Le Pape and Cognez (2016) observed that flatfish species used estuaries mainly as nursery areas or temporary habitats as an alternative to coastal areas, and that the range of movement increased with body length (Kramer and Chapman 1999; Le Pape and Cognez 2016). These observations support our hypothesis that bigger flatfish specimens migrate from SE to NW, which may thus explain the lower body size and higher abundance of specimens sampled in the area directly affected by river run-off.
The mullet L. ramada was the most generalist and omnivorous of the three species and showed wide trophic plasticity and intraspecific variability, particularly evident in the NW area. Here, mullets relied on all the basal resource compartments, although the two distinct δ13C isotopic groups reflected different contributions to the diets, showing that the marked trophic generalism observed at the population level was mainly dependent on intraspecific diet differentiation between individuals. In contrast, seston dominated the diet of mullets near the mouth of the Garigliano River. This result is consistent with Rumolo et al. (2016), who dealt with pelagic fish, and Careddu et al. (2015, 2017), who demonstrated the uptake of terrestrial organic matter supplied by run-off from the Garigliano River in benthic food webs in the Gulf of Gaeta, thereby indicating the presence of distinct ecological conditions. A clear spatial isotopic carbon gradient from riverine terrigenous to marine input has been already observed in estuarine areas by other authors (Vizzini et al. 2005; Kopp et al. 2013; Selleslagh et al. 2015). Various studies also show that land-derived C3 carbon reaches higher food web trophic levels via consumption by predators of detritivores and filter feeders, which rely in turn on the terrestrial detrital pathway (Darnaude et al. 2004; Calizza et al. 2012; Costantini et al. 2012; Careddu et al. 2015; Leal et al. 2017). Here, the shift from higher to lower terrestrial input was accompanied by falling fish abundance and body condition for L. ramada and P. lascaris, the two most abundant species, coupled with stronger interspecific limitation due to stronger overlap of food sources between species pairs.
Mullets are highly abundant in coastal areas (Carpentier et al. 2014) and this success may be due to opportunistic feeding behaviour, combined with their high tolerance to variation in salinity, which enables them to enter rivers. Indeed, they are able to feed on both live and dead organic matter from primary producers (Almeida 2003), thanks to a highly adapted digestive tract (e.g. a protractile and thin mouth with developed lips, specialised gill rakers and a gizzard) (Carpentier et al. 2014). In terms of trophic role, they have therefore been variously described as herbivorous, omnivorous, plankton feeders, or invertebrate predators (Carpentier et al. 2014). Phytoplankton have been found to be a food item for L. ramada especially in the upper reaches of estuaries (Cardona and Castelló 1994; Almeida 2003), and this particular trophic behaviour has encouraged many studies of their foraging strategy (Lebreton et al. 2011; Carpentier et al. 2014; Le Loc’h et al. 2015). In our study, the δ13C signatures of mullets near the river mouth were congruent with the depleted δ13C values typical of inland carbon sources (the average δ13C values of C-3 plants is about − 27‰; Marshall et al. 2007), which was also consistent with the δ13C values measured in seston in this area and upstream from the river mouth. Lastly, the observed shift from depleted to enriched δ13C signatures typical of marine carbon sources in larger mullet specimens in NW indicates migration from the river mouth to the northern area of the Gulf during specimen growth. This is acknowledged as a common feeding strategy in this opportunistic species (Lebreton et al. 2011) and was also observed for the two flatfish species. Larger specimens are expected to enlarge their home range due to higher resource requirements and the lower energetic costs of habitat exploration in comparison to smaller ones (Kramer and Chapman 1999). Migration may also reduce their overlap in resource use with smaller conspecifics and thus can limit intraspecific competition and promote differences in habitat use between size classes (Svanbäck and Bolnick 2006; Chapman et al. 2012).
In conclusion, this study helps to clarify the diet and feeding behaviour of C. linguatula, P. lascaris and L. ramada in an important breeding and fishing area of the central Mediterranean Sea. The study showed that the presence of the Garigliano River mouth affects the trophic habits of these fish species in different ways, as previously described for invertebrate consumers and predators in the area (Careddu et al. 2015, 2017). Far from the river, all species populations were characterised by longer individuals and increased interindividual variability in diet. However, while the flatfish species did not substantially change their trophism near the river mouth, the river provided basal organic inputs of terrestrial origin, which made up a large part of the diet of the mullets. Thus, our results suggest that food input from river run-off represents a key factor promoting stable coexistence between the three studied species in the Gulf at the high densities observed in the south-eastern area. Indeed, representing an abundant supplementary food source, seston from the river allows specimens of L. ramada to specialise on it, reducing niche overlap with other fish species, thereby lowering potential interspecific limitations on population density (Calizza et al. 2017; Duarte et al. 2017).
Finally, this study confirms stable isotope analysis as a useful tool for tracing organic matter flow, supplying information about resources and foraging areas exploited by fish species during successive life stages. This technique can thus help to evaluate ad hoc management strategies for ecosystems that are fundamental foraging habitats for marine species and are characterised by dynamic environmental conditions, such as coastal and estuarine Mediterranean ecosystems. In our specific study case focusing on the Gulf of Gaeta, the results suggest that both fish populations and catches will benefit from fishing in the river mouth area where, in spite of higher fish density, interspecific competition seems to be less limiting for the fish populations due to high resource availability. Fishing costs will increase away from the mouth of the river, since fishing efforts will concentrate on less dense fish assemblages with larger specimens, which are expected to contribute more than the smaller ones to population recruitment, due to their higher egg production, with consequent damage for fish populations.
References
Abrantes KG, Barnett A, Marwick TR, Bouillon S (2013) Importance of terrestrial subsidies for estuarine food webs in contrasting East African catchments. Ecosphere 4:1–33
Adams JN, Brodeur RD, Daly EA, Miller TW (2017) Prei availability and feeding ecology of juvenile Chinook (Oncorhynchus tshawytscha) and coho (O. kisutch) salmon in the northern California Current ecosystem, based on stomach content and stable isotopes analyses. Mar Biol 164:98
Aguzzi L, Bianco I, Cortese M, Monfrinotti M, Perna V, Sangiorgi V (2012) Stato dell’ambiente marino costiero del Golfo di Gaeta (LT) dieci anni di monitoraggio 2001–2011. Reporto/Acqua_01.Report_2012_SLT.SRS.RI_01 ARPALAZIO
Almeida PR (2003) Feeding ecology of Liza ramada (Risso, 1810) (Pisces, Mugilidae) in a south-western estuary of Portugal. Estuar Coast Shelf Sci 57:313–323
Bašić T, Copp GH, Edmonds-Brown VR, Keskin E, Davison PI, Britton JR (2018) Trophic consequences of an invasive, small-bodied non-native fish, sunbleak Leucaspius delineatus, for native pond fishes. Biol Invasions 21:261–275
Bentivoglio F, Calizza E, Rossi D, Carlino P, Careddu G, Rossi L, Costantini ML (2016) Site-scale isotopic variations along a river course help localize drainage basin influence on river food webs. Hydrobiologia 770:257–272
Britton JR, Gutmann Roberts C, Amat Trigo F, Nolan ET, De Santis V (2019) Predicting the ecological impacts of an alien invader: experimental approaches reveal the trophic consequences of competition. J Anim Ecol. https://doi.org/10.1111/1365-2656.12996
Buchheister A, Latour RJ (2011) Trophic ecology of summer flounder in lower Chesapeake Bay inferred from stomach content and stable isotope analyses. Trans Am Fish Soc 140:1240–1254
Cabral HN, Lopes M, Loeper R (2002) Trophic niche overlap between flatfishes in a nursery area on the Portuguese coast. Sci Mar 66:293–300
Calizza E, Costantini ML, Rossi D, Carlino P, Rossi L (2012) Effect of disturbance on an urban river food web. Freshw Biol 57:2613–2628
Calizza E, Rossi L, Costantini ML (2013a) Predators and resources influence phosphorus transfer along an invertebrate food web through changes in prey behaviour. PLoS ONE 8:e65186
Calizza E, Costantini ML, Carlino P, Bentivoglio F, Orlandi L, Rossi L (2013b) Posidonia oceanica habitat loss and changes in litter-associated biodiversity organization: a stable isotope-based preliminary study. Estuar Coast Shelf Sci 135:137–145
Calizza E, Aktan Y, Costantini ML, Rossi L (2015) Stable isotope variations in benthic primary producers along the Bosphorus (Turkey): a preliminary study. Mar Pollut Bull 97:535–538
Calizza E, Costantini ML, Careddu G, Rossi L (2017) Effect of habitat degradation on competition, carrying capacity, and species assemblage stability. Ecol Evol 7:5784–5796
Calizza E, Careddu G, Caputi SS, Rossi L, Costantini ML (2018) Time-and depth-wise trophic niche shifts in Antarctic benthos. PLoS ONE 1(3):e0194796
Cardona L, Castelló F (1994) Relative importance of plankton and benthos as food for Mugil cephalus and Liza ramada in Israeli semi-intensive fish ponds. Isr J Aquac 46:197–202
Careddu G, Costantini ML, Calizza E, Carlino P, Bentivoglio F, Orlandi L, Rossi L (2015) Effects of terrestrial input on macrobenthic food webs of coastal sea are detected by stable isotope analysis in Gaeta Gulf. Estuar Coast Shelf Sci 154:158–168
Careddu G, Calizza E, Costantini ML, Rossi L (2017) Isotopic determination of the trophic ecology of a ubiquitous key species—the crab Liocarcinus depurator (Brachyura: Portunidae). Estuar Coast Shelf Sci 191:106–114
Carpentier A, Como S, Dupuy C, Lefrançois C, Feunteun E (2014) Feeding ecology of Liza spp. in a tidal flat: evidence of the importance of primary production (biofilm) and associated meiofauna. J Sea Res 92:86–91
Carrozzo L, Potenza L, Carlino P, Costantini ML, Rossi L, Mancinelli G (2014) Seasonal abundance and trophic position of the Atlantic blue crab Callinectes sapidus Rathbun 1896 in a Mediterranean coastal habitat. Rend Fis Acc Lincei 25:201–208
Chapman BB, Hulthén K, Brodersen J, Nilsson PA, Skov C, Hansson LA, Brönmark C (2012) Partial migration in fishes: causes and consequences. J Fish Biol 81:456–478
Colloca F, Garofalo G, Bitetto I, Facchini MT, Grati F et al (2015) The seascape of demersal fish nursery areas in the North Mediterranean Sea, a first step towards the implementation of spatial planning for trawl fisheries. PLoS ONE 10:e0119590
Costantini ML, Rossi L, Fazi S, Rossi D (2009) Detritus accumulation and decomposition in a coastal lake (Acquatina–southern Italy). Aquat Conserv 19:566–574
Costantini ML, Zaccarelli N, Mandrone S, Rossi D, Calizza E, Rossi L (2012) NDVI spatial pattern and the potential fragility of mixed forested areas in volcanic lake watersheds. For Ecol Manag 285:133–141
Costantini ML, Calizza E, Rossi L (2014) Stable isotope variation during fungal colonisation of leaf detritus in aquatic environments. Fungal Ecol 11:154–163
Costantini ML, Carlino P, Calizza E, Careddu G, Cicala D, Caputi SS, Rossi L (2018) The role of alien fish (the centrarchid Micropterus salmoides) in lake food webs highlighted by stable isotope analysis. Freshw Biol 63(9):1130–1142
Criscoli A, Carpentieri P, Colloca F, Belluscio A, Ardizzone G (2017) Identification and characterization of nursery areas of Red Mullet Mullus barbatus in the Central Tyrrhenian Sea. Mar Coast Fish 9:203–215
Darnaude AM, Salen-Picard C, Polunin NVC, Harmelin-Vivien ML (2004) Trophodynamic linkage between river runoff and coastal fishery yield elucidated by stable isotope data in the Gulf of Lions (NW Mediterranean). Oecologia 138:325–332
di Lascio A, Rossi L, Carlino P, Calizza E, Rossi D, Costantini ML (2013) Stable isotope variation in macroinvertebrates indicates anthropogenic disturbance along an urban stretch of the river Tiber (Rome, Italy). Ecol Indic 28:107–114
Duarte RC, Flores AA, Vinagre C, Leal MG (2017) Habitat-dependent niche portioning between colour morphs of the algal-dwelling shrimp Hippolyte obliquimanus. Mar Biol 164:215
Faria FA, Albertoni EF, Bugoni L (2018) Trophic niche and feeding relationships of shorebirds in southern Brazil. Aquat Ecol 52:281–296
Fazi S, Rossi L (2000) Effects of macro-detritivores density on leaf detritus processing rate: a macrocosm experiment. Hydrobiologia 435:127–134
França S, Costa MJ, Cabral HN (2011) Inter- and intra-estuarine fish assemblage variability patterns along the Portuguese coast. Estuar Coast Shelf Sci 91:262–271
Frederiksen M, Edwards M, Richardson AJ, Halliday NC, Wanless S (2006) From plankton to top predators: bottom-up control of a marine food web across four trophic levels. J Anim Ecol 75:1259–1268
Gallagher AJ, Shiffman DS, Byrnes EE, Hammerschlag-peyer CM, Hammerschlag N (2017) Patterns of resources use and isotopic niche overlap among three species of sharks occurring within a protected subtropical estuary. Aquat Ecol 51:435–448
Guerriero G, Rabbito D, Alwany MA, Madonna A, Temraz TA et al (2017) Fisheries and biodiversity along Mediterranean sea: Italian and Egyptian coast overview. Mediterr J Environ Integr 2:16
Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, D’agrosa C, Bruno JF, Casey KS, Ebert C, Fox HE, Fujita R, Heinemann D, Lenihan HS, Madin EMP, Perry MT, Selig ER, Spalding M, Steneck R, Watson R (2008) A global map of human impact on marine ecosystems. Science 319:948–952
Inger R, Bearhop S (2008) Applications of stable isotope analyses to avian ecology. Ibis 150:447–461
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER—stable isotope bayesian ellipses in R. J Anim Ecol 80:595–602
Jona-Lasinio G, Costantini ML, Calizza E, Pollice A, Bentivoglio F, Orlandi L, Rossi L (2015) Stable isotope-based statistical tools as ecological indicator of pollution sources in Mediterranean transitional water ecosystems. Ecol Indic 55:23–31
Jones HG (2009) The ecology of snow-covered systems: a brief overview of nutrient cycling and life in the cold. Hydrol Process 13:2135–2147
Kennedy P, Kennedy H, Papadimitriou S (2005) The effect of acidification on the determination of organic carbon, total nitrogen and their stable isotopic composition in algae and marine sediment. Rapid Commun Mass Sp 19:1063–1068
Kopp D, Le Bris H, Grimaud L, Nérot C, Brind’Amour A (2013) Spatial analysis of the trophic interactions between two juvenile fish species and their preys along a coastal–estuarine gradient. J Sea Res 81:40–48
Kostecki C, Roussel JM, Desroy N, Roussel G, Lanshere J, Le Bris H, Le Pape O (2012) Trophic ecology of juvenile flatfish in a coastal nursery ground: contributions of intertidal primary production and freshwater particulate organic matter. Mar Ecol Prog Ser 449:221–232
Kowskowsi J, Trembaczowski A (2015) Fish reduce habitat coupling by a waterbird: evidence from combined stable isotope and conventional dietary approach. Aquat Ecol 49:21–31
Kramer DL, Chapman MR (1999) Implications of fish home range size and relocation for marine reserve function. Environ Biol Fish 55:65–79
La Rosa T, Mirto S, Favaloro E, Savona B, Sarà G, Danovaro R, Mazzola A (2002) Impact on the water column biogeochemistry of a Mediterranean mussel and fish farm. Water Res 36:713–721
Layman CA, Allgeier JE (2012) Characterizing trophic ecology of generalist consumers: a case study of the invasive lionfish in the Bahamas. Mar Ecol Prog Ser 448:131–141
Layman CA, Arrington DA, Montaña CG, Post DM (2007) Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 88:42–48
Le Loc’h F, Durand JD, Diop K, Panfili J (2015) Spatio-temporal isotopic signatures (δ13C and δ15N) reveal that two sympatric West African mullet species do not feed on the same basal production sources. J Fish Biol 86:1444–1453
Le Pape O, Cognez N (2016) The range of juvenile movements of estuarine and coastal nursery dependent flatfishes: estimation from a meta-analytical approach. J Sea Res 107:43–55
Leal RL, Furness RW, McGill RAR, Santos RA, Bugoni L (2017) Feeding and foraging ecology of Trinidade petrels Pterodroma armininjoniana during the breeding period in the South Atlantic Ocean. Mar Biol 164:211
Lebreton B, Richard P, Parlier EP, Guillou G, Blanchard GF (2011) Trophic ecology of mullets during their spring migration in a European salt marsh: a stable isotope study. Estuar Coast Shelf Sci 91:502–510
Link JS, Garrison LP, Almeida FP (2002) Ecological Interactions between elasmobranchs and groundfish species on the northeastern U.S. continental shelf. I. Evaluating predation. N Am J Fish Manag 22:550–562
Logan JM, Jardine TD, Miller TJ, Bunn SE, Cunjak RA, Lutcavage ME (2008) Lipid corrections in carbon and nitrogen stable isotope analysis: comparison of chemical extraction and modelling methods. J Anim Ecol 77:838–846
Mancinelli G, Rossi L (2002) The influence of allochthonous leaf detritus on the ccurrence of crustacean detritivores in the soft-bottom macrobenthos of the Po River Delta Area (northwestern Adriatic Sea). Estuar Coast Shelf Sci 54:849–861
Mancinelli G, Costantini ML, Rossi L (2007) Top-down control of reed detritus processing in a lake littoral zone: experimental evidence of a seasonal compensation between fish and invertebrate predation. Int Rev Hydrobiol 92:117–134
Marinaro JY, Bouabid M (1983) Ecologie alimentaire de deux soles d’Algérie, Pegusa nasuta (Pallas) et P. lascaris (Risso) (Téléostéens soléidés). Rapp Commun Int Mer Médit 28:73–75
Marshall JD, Brookes JR, Lajtha K (2007) Sources of variation in the stable isotopic composition of plants. Stab Isot Ecol Env Sci 2:22–60
Mateo MA, Serrano O, Serrano L, Michener RH (2008) Effects of sample preparation on stable isotope ratios of carbon and nitrogen in marine invertebrates: implications for food web studies using stable isotopes. Oecologia 157:105–115
Mazzola A, Sarà G (2001) The effect of fish farming organic waste on food availability for bivalve molluscs (Gaeta Gulf, Central Tyrrhenian, MED): stable carbon isotopic analysis. Aquaculture 192:361–379
McCutchan JH Jr, Lewis WM Jr, Kendall C, McGrath CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen and sulfur. Oikos 102:378–390
Orlandi L, Bentivoglio F, Carlino P, Calizza E, Rossi D, Costantini ML, Rossi L (2014) δ15N variation in Ulva lactuca as a proxy for anthropogenic nitrogen inputs in coastal areas of Gulf of Gaeta (Mediterranean Sea). Mar Pollut Bull 84:76–82
Parnell A, Inger R, Bearhop S, Jackson AL (2010) Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5:e9672
Parzanini C, Parrish CC, Heml JF, Mercier A (2017) Trophic ecology of a deep-sea fish assemblage in the Northwest atlantic. Mar Biol 164:206
Perkins MD, Durance I, Edwards FK, Grey J, Hildrew AG, Jackson M, Jones IJ, Lauridsen RB, Layer-Dobra K, Thompson MS, Woodward G (2018) Bending the rules: exploitation of allochtonous resource by a top-predator modifies size-abundance scaling in stram food webs. Ecol Let 21(12):1771–1780
Phillips LD (2012) Converting isotope values to diet composition: the use of mixing models. J Mammal 93:342–352
Pinnegar JK, Polunin NV, Badalamenti F (2003) Long-term changes in the trophic level of western Mediterranean fishery and aquaculture landings. Can J Fish Aquat Sci 60:222–235
Ponsard S, Arditi R (2000) What can stable isotopes δ15N and δ13C tell about the food web of soil macro-invertebrates? Ecology 81:852–864
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montanã CG (2007) Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152:179–189
Ricker WE (1973) Linear regressions in fisheries research. J Fish Res Board Can 30:409–434
Rolff C (2000) Seasonal variation in δ13C and δ15N of size fractionated plankton at a coastal station in the northern Baltic proper. Mar Ecol Prog Ser 203:47–65
Rossi L, Costantini ML, Brilli M (2007) Does stable isotope analysis separate transgenic and traditional corn (Zea mays L.) detritus and their consumers? Appl Soil Ecol 35:449–453
Rossi L, Costantini ML, Carlino P, di Lascio A, Rossi D (2010) Autochthonous and allochthonous plant contributions to coastal benthic detritus deposits: a dual- stable isotope study in a volcanic lake. Aquat Sci 72:227–236
Rossi L, di Lascio A, Carlino P, Calizza E, Costantini ML (2015) Predator and detritivore niche width helps to explain biocomplexity of experimental detritus-based food webs in four aquatic and terrestrial ecosystems. Ecol Complex 23:14–24
Rossi L, Calizza E, Careddu G, Rossi D, Orlandi L, Jona-lasinio G, Aguzzi L, Costantini ML (2018) Space-time monitoring of coastal pollution in the Gulf of Gaeta, Italy, using δ15N values of Ulva lactuca, landascape hydromorphology, and Bayesian Kriking modelling. Mar Pollut Bull 126:479–487
Rumolo L, Bonanno A, Barra M, Fanelli E, Calabro M, Genovese S, Ferreri R, Mazzola S, Basilone G (2016) Spatial variations in feeding habits and trophic levels of two small pelagic fish species in the central Mediterranean Sea. Mar Environ Res 115:65–77
Selleslagh J, Blanchet H, Bachelet G, Lobry J (2015) Feeding habitats, connectivity and origin of organic matter supporting fish populations in an estuary with a reduced intertidal area assessed by stable isotope analysis. Estuaries 38:1431–1447
Stergiou KI, Karpouzi VS (2002) Feeding habits and trophic levels of Mediterranean fish. Rev Fish Biol Fisher 11:217–254
Svanbäck R, Bolnick DI (2006) Intraspecific competition drives increased resource use diversity within a natural population. Proc R Soc B Biol Sci 274:839–844
Van Dokkum HP, Slijkerman DME, Rossi L, Costantini ML (2002) Variation in the decomposition of Phragmites australis litter in a monomictic lake: the role of gammarids. Hydrobiologia 482:69–77
Vizzini S, Savona B, Do Chi T, Mazzola A (2005) Spatial variability of stable carbon and nitrogen isotope ratios in a Mediterranean coastal lagoon. Hydrobiologia 550:73–82
Wolters JW, Verdonschot RCM, Schoelynck J, Brion N, Verdonschot PFM, Meire P (2018) Stable isotopes measurmements confirm consumption of submerged macrophytes by macroinvertebrates and fish taxa. Aquat Ecol 52:269–280
Zeug SC, Feyrer FV, Brodsky A, Melgo J (2017) Piscivore diet response to a collapse in pelagic prey populations. Environ Biol Fish 100:947–958
Acknowledgements
We thank two anonymous Reviewers for their comments, George Metcalf for revising the English text and Andrea for supporting field activities. The work complies with the legal requirements of the country in which it was carried out. Animal sampling was authorised by Latina Provincial Administration as part of the SAMOBIS project (PI: L. Rossi).
Funding
This research was supported by Latina Provincial Administration (Research Project: SAMOBIS, PI: L. Rossi), Sapienza University of Rome (Progetti di ricerca di Ateneo—L. Rossi and M.L. Costantini) and PNRA 2016 (L. Rossi).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Télesphore Sime-Ngando.
Rights and permissions
About this article
Cite this article
Cicala, D., Calizza, E., Careddu, G. et al. Spatial variation in the feeding strategies of Mediterranean fish: flatfish and mullet in the Gulf of Gaeta (Italy). Aquat Ecol 53, 529–541 (2019). https://doi.org/10.1007/s10452-019-09706-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-019-09706-3