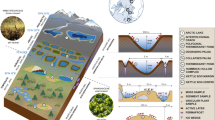
Abstract
As part of the reconstruction of the Brazilian Antarctic Station on King George Island, three areas of moss carpet were transplanted to minimize the impact of the new facilities on the local biodiversity. A total of 650 m2 of moss carpet was transplanted to neighboring but previously uncolonized locations and has subsequently survived for the last 3 years. Antarctic moss carpets typically comprise low moss species diversity and are often monospecific. We investigated the cryptic biodiversity that was transplanted along with the carpets using a metabarcoding approach through high throughput sequencing. We targeted 16S rRNA for Bacteria and Archaea, ITS for Fungi and Viridiplantae and Cox1 for Metazoa. We detected DNA representing 263 taxa from five Kingdoms (Chromista, Fungi, Metazoa, Protista and Viridiplantae), two Domains (Archaea and Bacteria) and 33 Phyla associated with the carpet. This diversity included one Archaea, 189 Bacteria, 24 Chromista, 19 Fungi, eight Metazoa, seven Protista and 16 Viridiplantae. Bacteria was the most abundant, rich and diverse group, with Chromista second in diversity and richness. Metazoa was less diverse but second highest in dominance. This is the first study to attempt transplanting a significant area of moss carpet to minimize anthropogenic environmental damage in Antarctica and to use metabarcoding as a proxy to assess diversity associated with Antarctic moss carpets, further highlighting the importance of such habitats for other organisms and their importance for conservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antarctica is well known for its extreme environmental conditions. Its terrestrial vegetation comprises only two native angiosperms and about 116 species of Bryophyta (Ochyra et al. 2008; Ellis et al. 2013a, b, 2013a; Sollman et al. 2015; Câmara et al. 2019). However, Antarctic vegetation is still poorly characterized in terms of genetic diversity and few studies using molecular tools have yet been carried out (Biersma et al. 2018a, b, 2020; Câmara et al. 2018, 2019). In particular, although the dominant bryophyte vegetation of the maritime Antarctic provides the habitats occupied by multiple other groups of organisms (e.g. Block et al. 2009), molecular tools are yet to be widely applied in assessing the overall biodiversity potentially occupying and relying on the bryosphere.
Antarctica is governed under the Antarctic Treaty, an international treaty that was negotiated in 1959 and came into force in 1961. Antarctic Treaty governance applies to all areas south of the 60° latitude parallel. The Treaty is dedicated to peace and science, with a primary purpose of protecting the Antarctic environment. Brazil became a Consultative Party to the Antarctic Treaty in 1984 and, promoting its interests in scientific research in Antarctica, its research station “Comandante Ferraz" was built in 1983 on the Keller Peninsula in Admiralty Bay on King George Island (South Shetland Islands). Tragically, in February 2012, the station was destroyed by fire. Subsequently, Brazil set the goal of rebuilding the station at the original location, considerably modernizing its facilities and increasing the area of its footprint. As is the required procedure under the Protocol on Environmental Protection to the Antarctic Treaty (hereafter referred to as the Protocol), the rebuild plans underwent detailed international scrutiny through the Protocol’s Environmental Impact Assessment (EIA) process. The expanded ground area where the new station and its associated facilities were to be built was partially occupied by established moss carpets that would inevitably have been destroyed during the construction of new structures such as fuel tanks and the new helipad. Therefore, to minimize the environmental impact of the rebuild and attempt to actively preserve these moss carpets, the Brazilian Ministry of Environmental Affairs and its Environmental Agency (MMA/IBAMA) made the decision to relocate the moss carpets to a geomorphologically similar but uncolonised location close to the station, the first such translocation attempt anywhere in Antarctica. Globally, there have been very few significant transplant attempts for bryophytes, especially when compared with other groups of plants (Brooker et al. 2011; Flagmeier et al. 2016), and particularly in remote and extreme regions, with most conservation actions being in a context of habitat restoration rather than the preservation of existing bryophyte habitat. The use of transplant approaches, either locally as we describe here or at a larger scale, has been highly debated as a conservation tool (Brooker et al. 2011; Hewitt et al. 2011).
Moss carpets are a typical feature in the maritime Antarctic (Smith 1984; Ochyra et al. 2008), although it is important also to recognize that the overall area of vegetation development is itself only a small fraction of the already very small area of ice-free ground (Hughes et al. 2016). The most commonly encountered wet moss carpets typically consist of extensive and almost completely monospecific stands of mosses of the genus Sanionia. Pleurocarpous mosses such as Sanionia are characterised by their low genetic diversity (Shaw et al. 2003), as has been reported in other pleurocarps in Antarctica (Biersma et al. 2017). Studies of Antarctic Sanionia populations have also confirmed low levels of genetic diversity (Hedenas 2012; Hebel et al. 2018), further promoted by the lack of sexual reproduction in this genus in Antarctica (Smith and Convey 2002; Ochyra et al. 2008; Hebel et al. 2018).
Moss carpets also harbor a potentially diverse community of organisms including various microbial groups (prokaryotes, algae, fungi, protists), micro-invertebrates (nematodes, rotifers, tardigrades) and micro-arthropods (mites, springtails and the native fly Belgica antarctica where it occurs) that depend on the habitats provided by the moss. However, while these groups are reasonably well known at a regional scale, detailed community characterization studies have been carried out at very few specific locations (e.g., Usher and Booth 1986; Richard et al. 1994; Convey and Quintana 1997; Convey and Smith 1997). Recent and rapid advances in molecular biology, such as DNA metabarcoding through high throughput sequencing (HTS), have provided important new tools for the detection of species by their DNA, with particular value in identifying rare elements of the biodiversity present (Rippin et al. 2018; Ruppert et al. 2019). For instance, in a study on Livingston Island (South Shetland Islands; Rippin et al. 2018) a molecular approach yielded richness estimates 11 times higher than did traditional morphological approaches. A range of recent metabarcoding studies of microbial groups in different habitats in the South Shetland Islands and northern Antarctic Peninsula have detected the presence of DNA representing a much higher number of taxa than those previously reported using traditional methods (e.g. Câmara et al. 2020; Garrido-Benavent et al. 2020; Rosa et al. 2020a,b; Ogaki et al. 2021), although it should also be noted that metabarcoding approaches alone do not confirm the presence of living organisms or viable propagules. In this study, we describe what we believe to be the largest moss transplant attempted in Antarctica and investigate the cryptic and previously uncharacterized biodiversity associated with the transplanted moss carpet with the use of metabarcoding.
Methods
Moss carpet transplantation
Three distinct areas of moss carpet required transplanting to avoid destruction as a result of the station rebuild. The three transplanted patches were documented using ImageJ software (Schneider et al. 2012), and are located as follows: Area 1: located between the station helipad and meteorology laboratory (62.086986ºS 58.394754ºW); with an area of 530 m2, this is the largest patch and contains moss carpet originally located at the new helipad site; area 2, located near the new fuel tanks (62.086964ºS 58.394197ºW), with an area of 100 m2, contains moss carpet originally located at the site of the new fuel tanks; area 3, located near to the station’s wind generators (62.083501ºS 58.392200ºW), with an area of 20 m2, contains moss carpet originally located on the sites now occupied by the wind turbines. The transplanted carpets comprised mainly the moss Sanionia uncinata (Hedw.) Loeske, with small proportions of Schistidium antarctici (Cardot) L.I. Savicz and Smirnova, Polytrichastrum alpinum (Hedw.) G.L.Sm. and a few clumps of Antarctic hairgrass (Deschampsia antarctica E. Desv.). The following methodology was applied to transport the transplants: the moss carpets were divided into manageable units (about 400 cm2) and, with the help of a knife or chisel, were manually lifted from the underlying surface and immediately relocated to a previously selected area on slightly higher ground, a few meters from their original location. The new area was selected to be as similar as possible to the original location in terms of its slope and orientation, water availability and type of underlying substrate, but free of vegetation. The surface layer of soil was also translocated to attempt to maintain possibly vital fungal connections and to minimize the change in nutrient availability and pH relative to the original site and thereby assist in the moss re-establishment (Glime 2017a, b). According to Glime (2017a, b), signs of death can occur rapidly in moss transplants, but they become stabilized within a few weeks. The transplants were carried out in February 2017. The site was visited again after one year and the condition of the transplanted patches assessed. The patches were then photographed two seasons later using a UAV (Phantom5® drone) during the austral summer of 2019/20.
Metabarcoding analysis
A single sample from the largest moss carpet was collected in the austral summer of 2019/20. One single shoot of Sanionia uncinata was removed using gloves and previously sterilized forceps and placed in a sterilized WhirlPak bag (Sigma-Aldric, USA). The sample was taken immediately to the microbiology laboratory at Comandante Ferraz Station where DNA extraction was completed in a laminar flow cabinet. Extraction used the QIAGEN Power Soil Kit (QIAGEN, Carlsbad, USA), following the manufacturer’s instructions. DNA quality was analyzed by agarose gel electrophoresis (1% agarose in 1 × Trisborate-EDTA) and then quantified using Quanti-iT™ Pico Green dsDNA Assay (Invitrogen). We aimed to target DNA from six groups of organisms: Bacteria, Chromista, Fungi, Metazoa, Protista and Viridiplantae.
The internal transcribed spacer 2 (ITS2) of the nuclear ribosomal DNA was used as a DNA barcode for molecular species identification of Chromista, Protista, Viridiplantae and Fungi (Chen et al. 2010) using the universal primers ITS3 and ITS4 (White et al. 1990). For Bacteria and Archaea, we used the 16S rRNA gene V3-V4 region (Herlemann et al. 2001; Klindworth et al. 2013) and for Metazoa, Cox1 (Folmer et al. 1994). Library construction and DNA amplification were performed using the Library kit Herculase II Fusion DNA Polymerase Nextera XT Index Kit V2, following Illumina 16S Metagenomic Sequencing Library Preparation Part #15,044,223 Rev. B protocol. Paired-end sequencing (2 × 300 bp) was performed on a MiSeq System (Illumina) by Macrogen Inc. (South Korea).
Raw fastq files were filtered using BBDuk version 38.34 (BBMap – Bushnell B. –sourceforge.net/projects/bbmap/) to remove Illumina adapters, known Illumina artefacts, and the PhiX Control v3 Library. Quality read filtering was carried out using Sickle version 1.33 -q 30 -l 50 (Joshi et al. 2011), to trim 3’ or 5’ ends with low Phred quality score, and sequences shorter than 50 bp were discarded. The remaining sequences were imported to QIIME2 version 2019.10 (https://qiime2.org/) for bioinformatics analyses (Bolyen et al. 2019). The qiime2-dada2 plugin is a complete pipeline that was used for filtering, dereplication, turn paired-end fastq files into merged, and remove chimeras (Callahan et al. 2016). Taxonomic assignments were determined for amplicon sequence variants (ASVs) using the qiime2-feature-classifier (Bokulich et al. 2018) classify-sklearn against SILVA 138 Ref NR 99 (Quast et al. 2013) for the 16S rRNA gene, UNITE Eukaryotes ITS database version 8.2 (Abarenkov et al. 2020) for Eukaryota, and MIDORI (Leray et al. 2018) for COX1, trained with Naïve Bayes classifier. We aimed to maximize resolution by obtaining data from specific and curated databases for the specific target groups.
Rarefaction calculations were carried out using the rarefaction analysis command in the platform MOTHUR (Schloss 2009), where we clustered sequences into OTUs by setting a 0.03 distance limit. Many factors, including extraction, PCR and primer bias, can affect the number of reads obtained (Medinger et al. 2010), and thus lead to misinterpretation of absolute abundance (Weber and Pawlowski 2013). However, Giner et al. (2016) concluded that such biases did not affect the proportionality between reads and cell abundance, implying that more reads are linked with higher abundance (Deiner et al. 2017; Hering, 2018). Therefore, for comparative purposes, we used the number of reads as a proxy for relative abundance. Ecological indices were calculated using PAST 1.90 (Hammer et al. 2001).
Sequences from the Phyla Ochrophyta and Oomycota were obtained from Cox1 and the Midori database while Ciliophora used ITS and the UNITE database.
The classification systems used were: Garrity et al. (2012) and Yilmaz et al. (2014) for Bacteria; Leliaert et al. (2012) for Viridiplantae; Cavalier-Smith (2007) for Chromista, Protista and Metazoa; Kirk, (2011), Tedersoo et al. (2011), MycoBank (http://www.mycobank.org) and the Index Fungorum (http://www.indexfungorum.org) for Fungi.
Results
Moss carpet transplantation
The first assessment of the transplanted moss carpet condition, a year after the transplant, confirmed that the plants not only survived the transplant but were thriving, based on direct observation and comparison with non-transplanted areas. Pictures, obtained three years after the transplant, using the UAV (Figs. 1, 2, 3) showed no differences in color or gross morphology.
Metabarcoding analysis
The calculated rarefaction curves for all taxa investigated approached a plateau, indicating that the reads gave an accurate representation of the local sequence diversity (Suppl. Figs. 1, 2). For 16S, a total of 275,658 DNA reads was generated and 20,111 reads remained after quality filtering. For ITS a total of 160,430 reads was generated and 29,454 reads remained after quality filtering and for Cox1 a total of 423,264 reads was generated and 48,543 reads remained after quality filtering. Sequences from two prokaryotic Domains (Bacteria and Archaea) were detected, representing 263 taxa in 18 Phyla. Sequences of representatives of five Eukaryota Kingdoms were detected: 24 representing three Phyla of Chromista, 19 representing four Phyla of Fungi, eight representing four Phyla of Metazoa, seven representing two Phyla of Protista and 16 representing two Phyla of Viridiplantae (Fig. 4). Krona charts (Suppl. Figs. 3–5) illustrate the relative abundances of the different groups.
A large number of sequences that could not be assigned to any rank was also found (47% for 16S; 62% for COX1 and 10% for ITS), suggesting that they represent taxa that are absent from the consulted databases, or new and as yet undescribed taxa (or both).
Domain bacteria
A total of 20,111 sequences were obtained representing this domain, distributed in 189 ASVs. Chloroplast DNA was the source of 8,551 of these sequences, and mitochondrial DNA contributed a further 1,515 sequences; both were removed from the analysis. The remaining sequences represented 18 bacterial phyla, and no archaeal 16S rRNA gene sequence was detected (Suppl. Table 1). The largest proportion of sequences represented the phylum Actinobacteria (25%), followed by Proteobacteria (20%) and Bacteroidetes (18%). The detected sequences represented 141 bacterial families, but only 23 families contributed > 1% of relative abundance. Intrasporangiaceae (Actinobacteria) and Chitinophagaceae (Bacteroidetes) were the most abundant families, contributing 9.2% and 6.7%, respectively (Fig. 5). The highest relative abundance at the generic level was 5% for “BIrii41” (Myxococcota), Kineosporia (Actinobacteria) and Ferruginibacter (Bacteroidetes).
Kingdom Chromista
A total of 8,682 sequences were assigned to Chromista, distributed in 24 ASVs (Suppl. Table 2). Taxa were divided in three Phyla, Ciliophora, Ochrophyta and Oomycota, with the Oomycota being the most diverse and abundant phylum (Fig. 6).
Kingdom Fungi
A total of 7,984 reads were assigned to 19 fungal ASVs. These represented 11 genera from the phyla Ascomycota, Basidiomycota, Chytridiomycota and Monoblepharomycota, in rank order of abundance (Suppl. Table 3). However, 20,492 (71.9%) of the total of 28,474 fungal reads detected could only be classified as Fungal sp., again likely to represent as yet undescribed taxa or taxa not included in the UNITE database. Relative abundances of fungal phyla are presented in Fig. 7
Kingdom Metazoa
A total of 39,439 metazoan sequences was obtained, representing eight ASVs (Suppl. Table 4). These were divided across four phyla: Arthropoda, Nematoda, Rotifera and Tardigrada (Fig. 8). Arthropoda had by far the greatest relative abundance while Arthropoda and Rotifera had the greatest diversity, although absolute taxon numbers in each phylum were low.
Kingdom Protista
A total of 880 sequences were assigned to Protista, representing seven ASVs (Suppl. Table 5) in two phyla, Discosea and Excavata (Fig. 9). Sequences representing Discosea were the most diverse and had the greatest relative abundance.
Kingdom Viridiplantae
A total of 29,244 Viridiplantae sequences were obtained, representing 16 ASVs (Suppl. Table 6) from two Phyla: Chlorophyta (green algae) and Bryophyta (mosses). Sequences representing Bryophyta had the greatest relative abundance while Chlorophyta contained the greatest sequence diversity (Fig. 10).
Molecular Sequence Diversity and Ecological Indices
The overall sequence diversity of each of the targeted groups and the calculated ecological indices are shown in Table 1. Bacteria were the most diverse, rich and abundant group present in terms of sequence (ASV) diversity detected. Chromista was the second most diverse and rich group found. Metazoa sequences, while indicating low diversity and richness, were present at very high abundance when compared with all other groups except Bacteria.
Discussion
Environmental sensitivity and protection in Antarctica
Antarctic terrestrial ecosystems are fragile and vulnerable to physical damage. The dominant bryophyte vegetation, lacking the roots of higher plants and with weak or no connection to the underlying substrate, is vulnerable to being damaged or dislodged. This fragility has become a conservation issue and challenge in Antarctica, owing to the combination of the small overall amount of ice-free ground available, the small proportion of that area actually colonized by vegetation, and the inevitable competition this leads to with the demand for locations where human activity is concentrated (Tin et al. 2009; Terauds et al. 2012; Hughes et al. 2016; Brookes et al. 2019). Guidelines prepared by the Antarctic Treaty System’s Committee for Environmental Protection and the Scientific Committee on Antarctic Research clearly enunciate the vulnerability of these ecosystems and the need for careful management of human behavior to avoid damage. Nevertheless, multiple and continuing instances of such damage, which can remain apparent for many decades after the event, have been reported and many more are not formally reported (Tin et al. 2009; Braun et al. 2012, 2018; Convey 2020). The ‘footprint’ of human activities is particularly great in the South Shetland Islands, where multiple nations operate research stations and field facilities and tourism operators use regular visitor sites, to the extent that virtually all ice-free areas or significant headlands in the archipelago host research stations, campsites, field instrumentation or sampling sites, refuges or other visitor sites.
Despite the scale of human interaction with and damage to terrestrial ecosystems, other than the guidelines mentioned above and the need for preparation of environmental impact assessments, one of whose roles is to minimize environmental impacts of activities such as station construction, we are not aware of any instances of pre-emptive conservation measures, such as transplantation, taken to avoid loss of vegetation that inevitably lies within construction footprints. Similarly, no restoration approaches have been applied aiming to assist the recovery of areas of vegetation known to have been damaged. Further, no monitoring effort has been applied to known damaged areas to assess the possibility or speed of natural recovery. We are aware of a single study that assessed vegetation recovery after the removal of a small refuge hut in the South Shetland Islands (Putzke et al. 2019), concluding certain elements of moss vegetation could potentially recover quite rapidly. However, Smith (2003) concluded that vehicle damage to wet moss carpets (including Sanionia) on the Fildes Peninsula remained apparent after several decades and may never fully recover (Tin et al. 2009).
With this background, to our knowledge no pre-emptive moss transplants aiming to conserve extensive moss carpets and their contained biota that would otherwise inevitably be lost have been proposed or attempted previously in Antarctica. Moss transplant studies have been reported elsewhere, generally involving only small areas and often in a domestic and gardening-oriented context (Glime 2017a, b). In Northern Hemisphere temperate forests, Parker et al. (1997) used small-scale transplants to investigate the role of hair-cap mosses in the regeneration of spruce forests, Cobb et al. (2001) investigated moss recolonization processes on maple branches and Frego (1996) investigated the regeneration of boreal bryophytes.
Elsewhere, moss transplants appear to be relatively successful (Lewis and Smith 1977; Longton 1981; Kallio and Saarnio 1986). In the Mojave Desert, up to 50% of transplanted small patches of Syntrichia caninervis Mitt. survived for 27 months (Cole et al. 2008). There are a number of instances of transplants being used as a conservation tool (e.g. Flagmeier et al. 2016). Gunnarsson and Söderström (2007) successfully transplanted Sphagnum angermanicum Melin to new sites in Sweden and Kooijman et al. (1994) re-introduced Scorpidium scorpioides (Hedw.) Limpr. from Ireland into the Netherlands, from where it had become extinct. Gauthier et al. (2018) and Hugron et al. (2013) have successfully demonstrated the potential for transplants to be used in the restoration of both mining-related and glacially-influenced habitats. Graf and Rochefort (2010) and Aradottir (2012) also successfully used moss transplants in restoration experiments. Transplants have also been used in studies focused on microenvironmental or ecophysiological aspects of moss success or distribution (e.g. Merinero et al. 2020; Dahlberg, 2014; Graf and Rochefort 2010; Frego 1996)). However, such studies are not directly comparable with the current conservation-oriented study and all involved much smaller transplanted areas.
Taxonomic Diversity
The relatively high number of DNA reads obtained from a single moss gametophyte shoot revealed a cryptic (hidden) diversity inhabiting moss carpets that is usually ignored, as is the ecological role of such carpets and their contained diversity, as we discuss below.
Bacteria
Bacterial communities associated with mosses are commonly dominated by the phyla Proteobacteria and Bacteroides (Wang et al. 2018; Holland-Moritz et al. 2018). This was the case for the transplanted Sanionia uncinata in the present study. However, in contrast with a study of boreal S. uncinata (Holland-Moritz 2018), the transplanted sample analyzed here generated the highest relative proportion of sequences belonging to the phylum Actinobacteria, as also reported in another study on Antarctic S. uncinata (Park et al. 2013). Other bacterial families commonly present in boreal S. uncinata, such as the Proteobacteria families Comamonadaceae and Sphingomonadaceae, as well as the Nostocaceae (Cyanobacteria) and Chitinophagageae (Bacteroidetes), were detected in the bacterial community associated with the transplanted samples, but they were not dominant. Park et al.’s (2013) study of Antarctic S. uncinata reported the presence of 56 identified bacterial genera, considerably fewer than the 196 genera identified here. This difference could represent a true difference between the two samples, or be a result of differences in the experimental design. For instance, Park et al. (2013) analyzed endophytic bacteria from multiple samples and used pyrosequencing to obtain the data. In the present study, both epiphytic and endophytic bacterial DNA were analyzed by Illumina MiSeq platform, and the database was considerably updated since 2013 with many sequences previously considered unclassified now having a taxonomic assignment.
Chromista
The Oomycota, a group formerly considered to be fungi, was the most diverse group found with 12 taxa. Most members of this group are obligate parasites and cause plant and animal diseases. Among the Peronosporales sequences assigned, Peronospora is an obligate parasite of angiosperms (Göker et al. 2009), causing severe diseases such as downy mildew, and Phytophthora (meaning ‘plant destroyer’) with ca. 313 species described worldwide (www.mycobank.org) is also responsible for multiple plant diseases (Cavalier-Smith and Chao 2006). However, while the genera are well-known worldwide, it is not clear how many representatives are present in Antarctica. Among the Pythiales, Pythium with ca. 355 described species (www.mycobank.org) is one of the few oomycete genera that has been widely reported in Antarctica (Bridge et al. 2008), and is also known to cause plant disease. The species P. tenue has been reported in continental Antarctica (Knox and Paterson 1973), and Pythium sp. have been reported from several sites in both continental and maritime Antarctica (e.g. Paterson and Knox 1971; Ellis-Evans 1985; Hughes et al. 2003; Fell et al. 2006; Bridge and Denton 2007). Bridge et al. (2008) isolated representatives of this genus from visibly unhealthy liverworts from Signy Island (South Orkney Islands) and confirmed their potential pathogenicity to local vascular plants. The genus Aphanomyces (known as ‘water moulds’) includes about 30 species (Johnson et al. 2002) mostly known from Europe and North America, some of which are plant or animal pathogens (Grünwald et al. 2003). Among the Order Lagenidiales, Lagenidium giganteum Couch ex Redhead is associated with infections in dogs, cats and humans (Grooters et al. 2004; Mendoza and Vilela 2013; Reinprayoon et al. 2013) and has been reported from the South Orkney Islands (Willoughby 1969). At present, there is no means of assessing whether these pathogens are native to Antarctica or have been introduced in association with human activity. It is perhaps appropriate to note that in the early decades of operation of research stations (generally late 1940s onwards in the South Orkney and South Shetland Islands) and the preceding whaling industry (1930s), exploring expeditions (1930s) and sealing industry (Nineteenth Century) the presence of cats and other domestic animals on stations and ships and, subsequently, sledge dogs (until the mid-1990s) was commonplace, which may be consistent with the detection of some animal parasites not known to be associated with native marine mammals and birds.
Ciliophora
Known for feeding on bacteria, the sequences assigned to some ciliates reported here represent taxa that are often common and widespread and may well have been previously overlooked. Some of the taxa identified are predominantly marine (e.g. Anteholosticha, Cyrtohymena) but the study site is very close to the shore and subject to marine spray. Species of Cyrtohymena have been found in soil and/or moss/litter in tropical areas, such as C. australis in the Amazonian rain forest (Foissner 1995) and C. candens, C. citrine and C. quadrinucleata in Kenya (Foissner 1999). Two species of Anteholosticha (A. rectangular and A. sigmoidea) and two species of Cyrtohymena (C. candens and C. citrina) have been reported from Antarctica (Thompson et al. 2019). The genus Halteria, whose members are known as ‘jumping oligothichs’, is widely distributed globally and H. grandinella has been reported from Antarctica (Thompson et al. 2019). The genus Hemicycliostyla is poorly known, and consists of only four species (Paiva et al. 2012), none of which has previously been reported from Antarctica. The genus Homalogastra has one species record (H. setosa) from Antarctica, while the genus Kahliella has not previously been recorded in Antarctica.
Some taxa were only identified at higher rank (Class Oligohymenophorea, Order Urostylida and family Oxytrichidae), making it more difficult to access their geographical range. According to the Register of Antarctic Marine Species (De Broyer et al. 2020), ciliates in the classes Spirotrichea and Oligohymenophorea can occur in both marine and terrestrial environments in Antarctica. Ciliate communities present in marine, freshwater and soil ecosystems are known to be important bacterial consumers and nutrient cyclers (Foissner 1999; Grossmann et al. 2015). In Antarctica, they may play an important role making scarce resources available to plants.
Ochrophyta
Five species of Spumella are known worldwide (Europe, South America and New Zealand; Guiry and Guiry 2020), all from freshwater habitats. The only species recorded from Antarctica is S. vulgaris (Findenig et al. 2010). Boenigk et al. (2006) isolated several cold-tolerant strains of Spumella-like ciliates from samples of fresh water and soil from both maritime and continental Antarctic sites (Signy Island, Alexander Island and Davis Valley). These strains, although morphologically similar to already reported Antarctic morphotypes, are clearly distinct in molecular analyses (Tong et al. 1997; Butler 1999), and may represent an interesting case of microbial geographic isolation (cf. Vyverman et al. 2010; Verleyen et al. 2021). According to Guiry and Guiry (2020), the genus Segregastopumella only includes a single species (S. dracosaxi) considered to be endemic to Europe, which would make this the first report for Antarctica.
Fungi
Our data indicated the presence of 18 fungal ASVs representing 11 genera from the phyla Ascomycota, Basidiomycota, Chytridiomycota and Monoblepharomycota. The most common fungal taxa reported in Antarctic studies are generally representatives of Ascomycota, followed by Basidiomycota (Rosa et al. 2019). In the current study, we also detected representatives of Chytridiomycota and Monoblepharomycota, which are less frequently reported in Antarctic studies. Notably, the majority of DNA sequences detected could only be as assigned at the level of Fungal sp., most likely highlighting that currently available databases covering this group are very incomplete and also suggesting that moss carpets may contain currently unknown fungal taxa.
Metazoa
Being a very diverse group, we consider each phylum in this kingdom separately here.
Arthropoda: The high number of reads associated with Insecta is perhaps surprising as there are only two species of native insect in Antarctica, the midges Belgica antarctica and Parochlus steinenii. There is also some possibility that Collembola DNA could match as insects sensu lato in databases as it is only relatively recently that studies (e.g. Gao et al. 2008) removed Collembola from the Insecta sensu stricto and placed them basally in the Hexapoda.
A possible confounding factor is the potential presence of exotic insects or, more likely, their remains. According to Chwedorzewska, (2013), 359 invertebrates and their remains were found in cargo transported to the Polish Arctowski Station (also in Admiralty Bay), the majority of which were insects (23 families of insects including food pests, wood − destroying pests and domestic insects). The close proximity to the Brazilian Ferraz station combined with seven years of construction activity may increase the likelihood that insect remains could have been transported with the large quantities of cargo involved. The assigned sequence with a species-level match in the database consulted was a Collembola, Cryptopygus antarcticus Willem. This collembolan is a very common native species throughout the Antarctic Peninsula region that reaches some of its highest population densities in Sanionia moss carpets (e.g. Convey and Smith 1997). Vegetation cover mitigates a number of the environmental challenges faced by insects and other organisms in Antarctica, making it a crucial environment for the survival of these groups (Grantz et al. 2018). According to Hogg and Stevens (2002), 14 Collembola and 28 Acari species are known from the South Shetland Islands.
Nematoda: approximately 68 nematode species are currently reported from Antarctica (Andrássy 1998; Kagoshima et al. 2019). Our sequence data identified only one species, Plectus frigophilus, which is a freshwater species endemic to Antarctica and one of the few that has been provisionally reported from both continental and maritime Antarctica (Yeates 1979; Maslen and Convey 2006; Kagoshima et al; 2019). However, traditional morphological taxonomy remains inadequate to confirm these identifications (Maslen and Convey 2006) and molecular studies of Antarctic nematodes are in their infancy (Kagoshima et al. 2019), although it is clear that virtually no species occur across both maritime and continental Antarctica. It is important to note that our collection did not include soil samples where most species (habitat specialized or not) are expected to occur (Tomasel et al. 2013). Free-living nematodes are among the groups of invertebrates that are commonly found in bryophytes (Glime 2017a, b).
Rotifera: These microscopic animals have had a long evolutionary relationship with bryophytes (Waggoner and Poinar 1993). Rotifers have very well-developed cryptobiotic adaptations, especially in the Bdelloidea, and are able to withstand long periods of drought or freezing. They are easily dispersed along with fragments of the moss they inhabit (Glime 2017a). Representatives of both genera whose sequences were identified in this study are common in maritime Antarctica. Members of Macrotrachela are found in terrestrial and freshwater habitats, with 16 species known in Antarctica. Members of Philodina are found in marine, freshwater and terrestrial habitats, with eight species reported from Antarctica (RAS 2020). In general, the most abundant and dominant rotifers in mosses are bdelloids, with the typically damp carpets of Saniona providing an ideal habitat (Glime 2017a; Linhart et al. 2002; Vlčková et al. 2002). A much wider diversity of bdelloid rotifers is known from classical morphological studies in Antarctica than were detected in our study (cf. Priddle and Dartnall 1978; Dartnall 1980). Using an integrated phylogenetic approach, Iakovenko et al. (2015) reported 66 bdelloid morphospecies from various locations around the continent and 83–91 putative species were identified, with very high levels of Antarctic endemism (see also Cakil et al. 2021).
Tardigrada: Tardigrades are another important group of Antarctic terrestrial and freshwater meiofauna (Convey and McInnes 2005; Tsujimoto et al. 2014). Around 60 tardigrade species are currently recorded from Antarctica, with the greatest diversity being present in the maritime Antarctic (Guidetti et al. 2019). Molecular phylogenetic studies of Antarctic tardigrades are in their infancy, but it is already clear that considerable species-level cryptic diversity is present in the few species examined to date (Kihm et al. 2020; Guidetti et al. 2014). The single genus identified in this sequencing study, Dactylobiotus, is globally distributed in freshwater habitats. According to Kihm et al. (2020), until very recently only one species was reported from Antarctica (D. ambiguous), until a new species from King George Island was described (D. ovimutans).
Protista
Discosea: Members of the genus Cochliopodium, with about 23 described species worldwide, are amoeboid eukaryotes that inhabit mostly freshwater but also brackish and marine habitats (Tekle et al. 2013). Of the species assigned from sequences obtained here, C. pentatrifurcatum was described in 2013 as endemic to the United Kingdom and C. marri as endemic to Alabama, USA (Melton et al. 2019). The presence of the genus in Antarctica is noted in the SCAR Antarctic terrestrial biodiversity database (https://data.aad.gov.au/aadc/biodiversity/taxon_profile.cfm?taxon_id=114665). Only C. tentaculatus has been reported from Antarctica but as an incomplete record (Thompson et al. 2019). All the specific assignments obtained in the current study are new to Antarctica, suggesting that the diversity of the genus in the continent may be much higher than previously reported, but species identities cannot be confirmed in the absence of specimens. Vannella simplex is one of the best-documented and relatively easily recognizable species of amoebae. Representatives are known from both freshwater and brackish habitats globally (Smirnov et al. 2002), with three species including V. simplex reported from Antarctica (Thompson et al. 2019).
Excavata: Reclinomonas americana is the only described species in this genus. It has been reported from freshwater environments in New Zealand and North America (Flavin and Nerad 1993) but not from Antarctica. However, members of the Heterolobosea appear to have very wide distributions, including in extreme environments (Park and Simpson 2011) and are likely to have been overlooked in Antarctica.
Viridiplantae
The algal community identified by sequences obtained here is similar to that described by Câmara et al. (2020) from Deception island, including common snow algal taxa such as Chlamydomonas and some terrestrial taxa such as Chlorococcum. The presence of Chlorothrix, a marine genus is plausible as the study site is close to the coastline. Some taxa only assigned to higher ranks may again represent taxa not present in the consulted databases or new undescribed taxa. Among the mosses, the taxa assigned are most widely distributed in this region (Ochyra et al. 2008). Although Pohlia crudoides is not recorded from Antarctica other members of the genus are present, and this is likely to indicate a lack of resolution in the available sequence databases. The presence of multiple moss sequences may reflect the presence of either spores or propagules transported either by wind or water to the study site. The high number of reads of Bryum pseudotriquetrum is notable, being one of the most common moss species in Antarctica (including close to this study site) and one that is also commonly found with sporophytes.
Conclusions
In this study, DNA sequences assigned to 253 taxa representing 31 phyla were obtained from a single shoot of the moss Sanionia uncinata from the transplanted moss carpet. Our data are indicative that an entire community associated with the moss carpet are also transplanted, further emphasizing the Antarctic conservation importance of this transplant effort. This is the first study to use metabarcoding to assess diversity potentially associated with an Antarctic moss carpet, with the data obtained emphasizing that a moss carpet is far more than a monospecific group of shoots of a common moss species, but provides habitat for considerable and often overlooked, cryptic, biodiversity. We are aware that our sampling effort was low and that the detection and assignment of a DNA sequence do not confirm the presence or viability of a given organism. Further targeted studies are required to confirm the presence of a species. However, studies such as this provide important clues as to the diversity potentially present, particularly in ecosystems and taxonomic groups that have not been a focus of detailed research. Furthermore, Antarctic moss carpets and their associated diversity play important roles in nutrient cycling. They also have high aesthetic value bringing color and contrast to a white and grey continent and are a feature of many Antarctic protected areas.
References
Abarenkov K, Zirk A, Piirmann T, Pöhönen R, Ivanov F., Nilsson RH, Kõljalg U (2020) UNITE QIIME release for Fungi. Version 04.02.2020. UNITE Community. https://doi.org/10.15156/BIO/786385.
Andrássy I (1998) Nematodes in the sixth continent. J Nematode Morphol Syst 1: 107–186.
Aradottir AL (2012) Turf transplants for restoration of alpine vegetation: does size matter? J Appl Ecol 49(2):439–446. https://doi.org/10.1111/j.1365-2664.2012.02123.x
Biersma EM, Jackson JA, Bracegirdle TJ, Griffths H, Linse L, Convey P (2018a) Low genetic variation between South American and Antarctic populations of the bank-forming moss Chorisodontium aciphyllum (Dicranaceae). Polar Biol 41:599–610. https://doi.org/10.1007/s00300-017-2221-1
Biersma EM, Convey P, Wyber R, Robinson SA, Dowton M, van de Vijver B, Linse K, Griffiths H, Jackson JÁ (2020) Latitudinal biogeographic structuring in the globally distributed moss Ceratodon purpureus. Front Plant Sci 11:502359. https://doi.org/10.3389/fpls.2020.502359
Biersma EM, Jackson JA, Stech M, Griffiths H, Linse K, Convey P (2018b) Long-term in situ Antarctic persistence within Antarctica’s most speciose plant genus. Schistidium Front Ecol Evol 6:77. https://doi.org/10.3389/fevo.2018.00077
Block W, Lewis Smith RI, Kennedy AD (2009) Strategies of survival and resource exploitation in the Antarctic fellfield ecosystem. Biol Rev 84(3):449–484. https://doi.org/10.1111/j.1469-185x.2009.00084.x
Boenigk J, Pfandl K, Garstecki T, Harms H, Novarino G, Chatzinotas A (2006) Evidence for geographic isolation and signs of endemism within a protistan morphospecies. Appl Environ Microbiol 72(8):5159–5164. https://doi.org/10.1128/AEM.00601-06
Bokulich NA, Kaehler BD, Rideout JR, Dillon M, Boylern E, Knight R, Huttley GA, Caporaso JG (2018) Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6:90–107. https://doi.org/10.1186/s40168-018-0470-z
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith A, Alexander H, Alm EJ, Arumugan M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Chase J, Cope EK, Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzales A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, Koester I, Kosciolek T, Kreps J, Langille MGI, Lee J, Ley R, Liu Y, Loftfield LC, Maher M, Marotz C, Martin BD, McDonald D, McIver LJ, Melnik AV, Metcalf JL, Morgan SC, Morton JT, Naimey AT, Navas-Molina JA, Nothias LF, Orchanian SB, Pearson T, Peoples SL, Petras D, Preuss ML, Pruesse E, Rasmussen LB, Rivers A, Robeson MS II, Rosenthal P, Segata N, Shaffer M, Shiffer A, Sinha R, Song SJ, Spear JR, Swafford AD, Thompson LR, Torres PJ, Trinh P, Tripathi A, Turnbaugh PJ, Ul-Hasa S, van der Hoof JJJ, Vargas F, Vazques-Baeza Y, Vogtmann E, von Hippel M, Walters W, Wan Y, Wang M, Warren J, Weber KC, Williamson CHD, Willis AD, Xu ZZ, Zaneveld JR, Zhang Y, Zhu Q, Knight R, Caporaso JG (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. https://doi.org/10.1038/s41587-019-0209-9
Bridge PD, Denton G (2007) Isolation of diverse viable fungi from the larvae of the introduced chironomid Eretmoptera murphyi on Signy Island. Polar Biol 30:935–937
Bridge PD, Newsham KK, Denton GJ (2008) Snow mould caused by a Pythium sp.: a potential vascular plant pathogen in the maritime Antartic. Plant Pathol 57(6):1066–1072.
Brooker R, Britton A, Gimona A, Lennon J, Littlewood N (2011) Literature review: species translocations as a tool for biodiversity conservation during climate change. Scottish Natural Heritage Commissioned Report No.440.
Brooks ST, Jabour J, van den Hoff J, Bergstrom DM (2019) Our footprint on Antarctica competes with nature for rare ice-free land. Nat Sustain 2:185–190. https://doi.org/10.1038/s41893-019-0237-y
Butler HG (1999) Seasonal dynamics of the planktonic microbial community in a maritime Antarctic lake undergoing eutrophication. J Plankton Res 21(12):2393–2419
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583
Câmara PEAS, Carvalho-Silva M, Hernriques DK, Guerra J, Gallego MT, Poveda DR, Stech M (2018) Pylaisiaceae Schimp. (Bryophyta) revisited. J Bryol 40:251–264
Câmara PEAS, Carvalho-Silva M, Pinto OHB, Amorim ET, Henriques DK, Silva TH, Pellizzari F, Convey P, Rosa LH (2020) Diversity and Ecology of Chlorophyta (Viridiplantae) Assemblages in Protected and Non-protected Sites in Deception Island (Antarctica. Microb Ecol, South Shetland Islands) Assessed Using an NGS Approach. https://doi.org/10.1007/s00248-020-01584-9
Câmara PEAS, Soares AER, Hernriques DK, Peralta DF, Bordin J, Carvalho-Silva M, Stech M (2019) New insights into the species diversity of Bartramia Hedw. (Bryophyta) in Antarctica from a morpho-molecular approach. Antarct Sci 31:208–215
Cakil ZV, Garlasché G, Iakovenko N, Di Cesare A, Eckert EM, Guidetti R, Hamdan L, Janko K, Lukashanets D, Rebecchi L, Schiaparelli S, Sforzi T, Kašparová EŠ, Velasco-Castrillón A, Walsh EJ, Fontaneto D (2021) Comparative phylogeography reveals consistently shallow genetic diversity in a mitochondrial marker in Antarctic bdelloid rotifers. Journal of Biogeografy. https://doi.org/10.1111/jbi.14116
Cavalier-Smith T (2007) A revised six-kingdom system of life. Biol Rev 73(3):203–266. https://doi.org/10.1111/j.1469-185X.1998.tb00030.x
Cavalier-Smith T, Chao EEY (2006) Phylogeny and Megasystematics of Phagotrophic Heterokonts (Kingdom Chromista). J Mol Evol 62:388–420. https://doi.org/10.1007/s00239-004-0353-8
Chen S, Yao H, Han J, Liu C, Song J, Shi L, Zhu Y, Ma X, Gao T, Pang X, Luo K, Li Y, Li X, Jia X, Lin Y, Leon C (2010) Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS ONE 5(1):e8613. https://doi.org/10.1371/journal.pone.0008613
Chwedorzewska K, Korczak−Abshire M, Olech M, Lityńska−Zając M, Augustyniuk−Kram A, (2013) Alien invertebrates transported accidentally to the Polish Antarctic Station in cargo and on fresh foods. Pol Polar Res 34(1):55–66
Cobb A, Nadkarni N, Ramsey GA, Svoboda AJ (2001) Recolonization of bigleaf maple branches by epiphytic bryophytes following experimental disturbance. Can J Bot 79:1–8
Cole C, Stark LR, Bonine ML, Mcletchie, (2010) Transplant survivorship of bryophyte soil crusts in the mojave desert. Restor Ecol 18(2):198–205. https://doi.org/10.1111/j.1526-100X.2008.00445.x
Convey P, McInnes SJ (2005) Exceptional tardigrade-dominated ecosystems in Ellsworth Land, Antarctica. Ecology 86:519–527
Convey P, Quintana RD (1997) The terrestrial arthropod fauna of Cierva Point SSSI, Danco Coast, northern Antarctic Peninsula. Eur J Soil Biol 33:19–29
Convey P, Smith RIL (1997) The terrestrial arthropod fauna and its habitats in northern Marguerite Bay and Alexander Island, maritime Antarctic. Antarct Sci 9:12–26
Convey P (2020) The price of cumulative human activities in the Antarctic. Antarct Sci 32(6):425–425. https://doi.org/10.1017/S0954102020000577
Dahlberg CJ, Ehrle´n J, Hylander K, (2014) Performance of Forest Bryophytes with Different Geographical Distributions Transplanted across a Topographically Heterogeneous Landscape. PLoS ONE 9(11):e112943. https://doi.org/10.1371/journal.pone.0112943
Dartnall HJG (1980) Freshwater biology at Rothera Point, Adelaide Island: I. General description of the pools and the fauna. Brit Antarct Surv Bull 50:51–54
De Broyer C, Clarke A, Koubbi P, Pakhomov E, Scott F, Vanden Berghe E, Danis B (Eds.) (2020) Register of Antarctic Marine Species. Spirotrichea. Accessed at: http://www.marinespecies.org/RAMS/aphia.php?p=taxdetails&id=1348 on 2020–11–03
Deiner K, Bik HM, Mächler E, Seymour M, Lacoursière-Roussel A, Altermatt F, Creer S, Bista I, Lodge DM, Vere N, Pfrender ME, Bernatchez L (2017) Environmental DNA metabarcoding: transforming how we survey animal and plant communities. Mol Ecol 26:5872–5895
Ellis LT, Asthana AK, Gupta R, Nath V, Sahu V, Bednareck-Ochyra H, Ochyra R, Cykowska B, Calvo Aranda S, Fischer E, Gabriel R, Gosrski P, Gremmen N, Hespanhol H, Kurbatova LE, Lewsis-Smith RI, Long DG, Bell D, Mogro F, Sergio C, Garcia CA, Stow S, Martins A, Mith VR, Van J, Vanderpoorten A (2013a) New national and regional bryophyte records, 34. J Bryol 35:62–70
Ellis LT, Bednarek-Ochyra H, Ochyra R, Benjumea MJ, Saïs LV, Caparrós R, Lara Mazimpaka V, Dulin MV, Garilleti R, Gremmen N, Grundling P-L, Heras P, Infante M, Huttunens S, Ignatov MS, Korvenpää T, Lebouvier LS, MRI, Lin S-H, Yang J-D, Linström A, Novotný I, Plášek V, Rosselló JA, Sawicki J, Van Rooy J, Smith VR. (2013b) New national and regional bryophyte records, 35. J Bryol 34:129–139
Ellis-Evans JC (1985) Fungi from maritime Antarctic freshwater environments. British Antarctic Survey Bulletin 68:37–45
Fell JW, Scorzetti G, Connell L, Craig S (2006) Biodiversity of micro-eukaryotes in Antarctic dry valley soils with <5% soil moisture. Soil Biol Biochem 38:3107–3119
Findenig BM, Chatzinotas A, Boenigk J (2010) Taxonomic and ecological characterization of stomatocysts of Spumella-like flagellates (Chrysophyceae). J Phycol 46(5):868–881
Flagmeier M, Hollingsworth PM, Genney DR, Long DG, Muñoz J, Moreno-Jiménez E, Woodin SJ (2016) Transplanting the leafy liverwort Herbertus hutchinsiae: a suitable conservation tool to maintain oceanic-montane liverwort-rich heath? Plant Ecolog Divers 9(2):175–185. https://doi.org/10.1080/17550874.2016.1140845
Flavin M, Nerad TA (1993). Reclinomonas americana N. G., N. Sp., a New Freshwater Heterotrophic Flagellate. The Journal of Eukaryotic Microbiology, 40(2): 172–179. doi:https://doi.org/10.1111/j.1550-7408.1 993.tb04900.x
Foissner W (1995) Tropical Protozoan Diversity: 80 Ciliate Species (Protozoa, Ciliophora) in a Soil Sample from a Tropical Dry Forest of Costa Rica, with Descriptions of four New Genera and seven New Species. Arch Protistenk 145:37–79
Foissner W (1999) Notes on the soil ciliate biota (Protozoa, Ciliophora) from the Shimba Hills in Kenya (Africa): diversity and description of three new genera and ten new species. Biodivers Conserv 8:319–389
Folmer O, Black M, Hoeh W, Lutz R, Vrjenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan investrebrates. Mol Mar Biol Biotechnol 3:294–299
Frego K (1996) Regeneration of four boreal bryophytes: colonization of experimental gaps by naturally occurring propagules. Can J Bot 74:1937–1942
Gantz JD, Spatch DE, Lee RE (2018) A preliminary survey of the terrestrial arthropods of the Rosenthal Islands. Antarctica Polar Research. https://doi.org/10.1080/17518369.2018.1500266
Gao Y, Bu YL, Yun-Xia, (2008) Phylogenetic relationships of basal hexapods reconstructed from nearly complete 18S and 28S rRNA gene sequences. Zoolog Sci 25(11):1139–1145
Garrido-Benavent I, Pérez-Ortega S, Durán J, Ascaso C, Pointing SB, Rodríguez-Cielos R, Navarro F, de los Ríos A, (2020) Differential Colonization and Succession of Microbial Communities in Rock and Soil Substrates on a Maritime Antarctic Glacier Forefield. Front Microbiol. https://doi.org/10.3389/fmicb.2020.00126
Garrity et al. 2012. Bergey's Manual of Systematic Bacteriology (Whitman, W.B.; Goodfellow, M.; Kämpfer, P.; Busse, H.-J.; Trujillo, M.E.; Ludwig, W.; Suzuki, K.-i.; Parte, A. (January 29, 2012) [1984(Williams & Wilkins)]. George M. Garrity (ed.). The Actinobacteria. Bergey's Manual of Systematic Bacteriology. 4 (2nd ed.). New York: Springer. p. 1750. ISBN 978–0–387–95043–3. British Library no. GBA561951.)
Gauthier ME, Rochefort L, Nadeau L, Hugron S, Xu B (2018) Testing the moss layer transfer technique on mineral well pads constructed in peatlands. Wetlands Ecol Manage 26(4):475–487
Giner CR, Forn I, Romac S, Logares RC, Massana R (2016) Environmental Sequencing Provides Reasonable Estimates of the Relative Abundance of Specific Picoeukaryotes. Appl Environ Microbiol 82(15):4757–4766. https://doi.org/10.1128/AEM.00560-16
Glime JM (2017) Bryophyte Ecology: Bryophyte Ecology Subchapters. 130.
Glime JM (2017a) Invertebrates: Nematodes. Chapt. 4–3. In: Glime, J. M. Bryophyte Ecology. Volume 2. Bryological Interaction. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 18 July 2020 and available at <https://digitalcommons.mtu.edu/bryophyte-ecology2/>.
Göker M, García-Blázquez G, Voglmayr H, Tellería MT, Martín MP (2009) Molecular taxonomy of phytopathogenic fungi: a case study in Peronospora. PLOS ONE. 4 (7): e6319.
Graf MD, Rochefort L (2010) Moss regeneration for fen Reforestation: Field and greenhouse experiments. Restor Ecol 18:121–130
Grooters AM, Proia LA, Sutton D, Hodgin EC (2004) Characterization of a previously undescribed Lagenidium pathogen associated with soft tissue infection: initial description of a new human oomycosis [abstract]. Focus on Fungal Infections 14. New Orleans, Louisiana, USA. p. 174.
Grossmann L, Bock C, Schweikert M, Boenigk J (2015) Small but Manifold – Hidden Diversity in “Spumella-like Flagellates.” J Eukaryot Microbiol. https://doi.org/10.1111/jeu.12287
Grünwald NJ, Coyne CJ, eds (2003) Proceedings of the Second International Aphanomyces Workshop. Pasco, Washington, USDA ARS.
Guidetti R, Rebecchi L, Cesari M, McInnes SJ (2014) Mopsechiniscus franciscae, a new species of a rare genus of Tardigrada from continental Antarctica. Polar Biol 37:1221–1233. https://doi.org/10.1007/s00300-014-1514-x
Guidetti R, Massa E, Bertolani R, Rebecchi L, Cesari M (2019) Increasing knowledge of Antarctic biodiversity: new endemic taxa of tardigrades (Eutardigrada; Ramazzottiae) and their evolutionary relationships. Syst Biodivers 17:573–593
Guiry MD in Guiry MD, Guiry GM (2020) AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on 26 September 2020.
Gunnarsson U, Söderström L (2007) Can artificial introductions of diaspore fragments work as a conservation tool for maintaining populations of the rare peatmoss Sphagnum angermanicum? Biol Cons 135(3):450–458
Hammer Ø, Harper DA, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9
Hebel I, Dacasa Rüdinger MC, Jaña RA, Bastias J (2018) Genetic structure and gene flow of moss Sanionia uncinata (Hedw.) Loeske in Maritime Antarctica and Southern Patagonia. Front. Ecol. Evol. 6:152. https://doi.org/10.3389/fevo.2018.00152
Hedenas L (2012) Global phylogeography in Sanionia uncinata (Amblystegiaceae: Bryophyta). Bot J Linn Soc 168:19–42
Hering D et al (2018) Implementation options for DNA-based identification into ecological status assessment under the European Water Framework Directive. Water Res 138:192–205
Herlemann DP, Labrenz M, Jürgens K, Bertilsson S, Waniek JJ, Andersson AF (2011) Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J 5(10):1571–1579
Hewitt N, Klenk N, Smith AL, Bazely DR, Yan N, Wood S, MacLellan JI, Lipsig-Mumme C, Henriques I (2011) Taking stock of the assisted migration debate. Biol Cons 144:2560–2572
Hogg ID, Stevens MI (2002) Soil Fauna of Antarctic Coastal Landscapes. – In: Beyer, L. & M. Bölter (eds): Geoecology of Antarctic Ice-Free Coastal Landscapes. Springer, Berlin Heidelberg New York: 265–280.
Holland-Moritz H, Stuart J, Lewis LR, Miller S, Mack MC, McDaniel SF, Fierer N (2018) Novel bacterial lineages associated with boreal moss species. Environ Microbiol 20(7):2625–2638
Hughes KA, Lawley B, Newsham KK (2003) Solar UV-B radiation inhibits the growth of Antarctic terrestrial fungi. Appl Environ Microbiol 69:1488–1491
Hughes KA, Ireland L, Convey P, Fleming AH (2016) Assessing the effectiveness of specially protected areas for conservation of Antarctica’s botanical diversity. Conserv Biol 30:113–120
Hugron S, Poulin M, Rochefort L (2013) Organic matter amendment enhances establishment of reintroduced bryophytes and lichens in borrow pits located in boreal forest highlands. Boreal Environ Res 18(3–4):317–328
Iakovenko NS, Smykla J, Convey P, Kasparová E, Kozeretska IA, Trokhymets V, Dykyy I, Plewka M, Devetter M, Duris Z, Janko K (2015) Antarctic bdelloid rotifers: diversity, endemism and evolution. Hydrobiologia 761:5–43. https://doi.org/10.1007/s10750-015-2463-2
Johnson Jr. TW, Seymour RL, Padgett DE (2002) Biology and systematics of the Saprolegniaceae http://dl.uncw.edu/digilib/biology/fungi/Bary.pdf
Joshi NA, Fass JN (2011) Sickle: A sliding-window, adaptive, quality-based trimming tool for FastQ files (Version 1.33) [Software]. https://github.com/najoshi/sickle.
Kagoshima H, Maslen R, Kito K, Imura S, Niki H, Convey P (2019) Integrated taxonomy combining morphological and molecular biological analyses of soil nematodes from maritime Antarctica. Polar Biol 42:877–887. https://doi.org/10.1007/s00300-019-02482-8
Kallio P, Saarnio E (1986) The effect on mosses of transplantation to different latitudes. J Bryol 14(1):159–178. https://doi.org/10.1179/jbr.1986.14.1.159
Kihm JH, Kim S, McInnes SJ et al (2020) Integrative description of a new Dactylobiotus (Eutardigrada: Parachela) from Antarctica that reveals an intraspecific variation in tardigrade egg morphology. Sci Rep 10:9122. https://doi.org/10.1038/s41598-020-65573-1
Kirk PM et al (2011) Dictionary of the Fungi, 10th edn. CAB International, Wallingford, UK, p 784
Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glöckner FO (2013) Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 41(1):e1–e1. https://doi.org/10.1093/nar/gks808
Knox JS, Paterson RA (1973) The Occurrence and Distribution of Some Aquatic Phycomycetes on Ross Island and the Dry Valleys of Victoria Land. Antarctica Mycologia 65(2):373–387
Kooijman AM, Beltman B, Westhoff V (1994) Extinction and reintroduction of the bryophyte Scorpidium scorpioides in a richfen spring site in the Netherlands. Biol Conserv 69:87–96
Leliaert F, Smith DR, Moreau H, Herron MD, Verbruggen H, Delwiche CF, De Clerck O (2012) Phylogeny and molecular evolution of the green algae. Crit Rev Plant Sci 31:1–46. https://doi.org/10.1080/07352689.2011.615705
Leray M, Ho S-L, Lin I-J, Machida RJ (2018) MIDORI server: a webserver for taxonomic assignment of unknown metazoan mitochondrial-encoded sequences using a curated database. Hancock J (Eds). Bioinformatics. 2018; pmid:29878054
Lewis K, Smith AJE (1977) Studies on some bulbiferous species of Pohlia section Pohliella. I Experimental Investigations J Bryol 9:539–556
Linhart J, Vlčková S, Uvíra V (2002) Bryophytes as a special mesohabitat for meiofauna in a rip-rapped channel. River Res Appl 18:321–330. https://doi.org/10.1002/rra.671
Longton RE (1981) Inter-population variation in morphology and physiology in the cosmopolitan moss Bryum argenteum Hedw. J Bryol 11:501–520
Maslen NR, Convey P (2006) Nematode diversity and distribution in the southern maritime Antarctic—clues to history? Soil Biology and Biochemistry 38:3141–3151
Medinger R, Nolte V, Pandey RV, Jost S, Ottenwalder B, Schlotterer C, Boenigk J (2010) Diversity in a hidden world: potential and limitation of next-generation sequencing for surveys of molecular diversity of eukaryotic microorganisms. Mol Ecol 19:32–40. https://doi.org/10.1111/j.1365-294X.2009.04478.x
Melton JT, Singla M, Wood FC, Collins SJ, Tekle YI (2019) Three New Freshwater Cochliopodium Species (Himatismenida. J Eukaryotic Microbiol, Amoebozoa) from the Southeastern United States. https://doi.org/10.1111/jeu.12764
Mendoza L, Vilela R (2013) The mammalian pathogenic oomycetes. Curr Fungal Infect Rep 7:198–208
Merinero S, Dahlberg CJ, Ehrlén J, Hylander K (2020) Intraspecific variation influences performance of moss transplants along microclimate gradients. Ecology 101(5):e02999. https://doi.org/10.1002/ecy.2999
Ochyra R, Lewis-Smith RI, Bednarek-Ochyra H (2008) The illustrated moss flora of Antarctica. Cambridge University Press, Cambridge
Ogaki MB, Pinto OHB, Vieira R et al (2021) (2021) Fungi Present in Antarctic Deep-Sea Sediments Assessed Using DNA Metabarcoding. Microb Ecol. https://doi.org/10.1007/s00248-020-01658-8
Paiva TS, Borges B, Silva-Neto ID, Harada ML (2012) Morphology and 18S rDNA phylogeny of Hemicycliostyla sphagni (Ciliophora, Hypotricha) from Brazil with redefinition of the genus Hemicycliostyla. Int J Syst Evol Microbiol 62:229–241
Park JS, Simpson AGB (2011) Characterization of Pharyngomonas kirbyi (= “Macropharyngomonas halophila” nomen nudum), a very deep-branching, obligately halophilic heterolobosean flagellate. Protist 162:691–709
Park M, Lee H, Hong SG, Kim OS (2013) Endophytic bacterial diversity of an Antarctic moss. Sanionia Uncinata Antarctic Science 25(1):51
Parker WC, Watson SR, Cairns DW (1997) The role of hair-cap mosses (Polytrichum spp.) in natural regeneration of white spruce (Picea glauca (Moench) Voss). For Ecol Manage 92:19–28
Paterson RA, Knox JS (1971) Aquatic fungi: their occurrence on Ross Island and in the dry valleys. Antarctic Journal of the US 6:107
Priddle J, Dartnall HJG (1978) The biology of an Antarctic aquatic moss community. Freshw Biol 8(5):469–480. https://doi.org/10.1111/j.1365-2427.1978.tb01469.x
Putzke K, Vieira FCB, Pereira AB (2019) Vegetation recovery after the removal of a facility in Elephant Island, Maritime Antarctic. Land Degrad Dev 31:96–104
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucl Acids Res 41(D1):D590–D596
RAS (Eds.) (2020) Register of Antarctic Species. Accessed at http://ras.biodiversity.aq on 2020–09–28
Reinprayoon U, Permpalung N, Kasetsuwan N, Plongla R, Mendoza L, Chindamporn A (2013) Lagenidium sp. ocular infection mimicking ocular pythiosis. J Clin Microbiol 51:2778–2780. https://doi.org/10.1128/JCM.00783
Richard KJ, Convey P, Block W (1994) The terrestrial arthropod fauna of the Byers Peninsula. South Shetland Islands Polar Biol 14:371–379
Rippin M, Borchhardt N, Williams L, Colesie C, Jung P, Büdel B, Karsten U, Becker B (2018) Genus richness of microalgae and Cyanobacteria in biological soil crusts from Svalbard and Livingston Island: morphological versus molecular approaches. Polar Biol 41:909–923. https://doi.org/10.1007/s00300-018-2252-2
Rosa LH et al. (2019) Fungi in Antarctica: diversity, ecology, effects of climate change, and bioprospection for bioactive compounds. In: Rosa LH (ed) Fungi of Antarctica: diversity, ecology and biotechnological applications. Springer, pp 1–18
Rosa LH, da Silva TH, Ogaki MB et al (2020a) DNA metabarcoding uncovers fungal diversity in soils of protected and non-protected areas on Deception Island. Antarctica Sci Rep 10:21986. https://doi.org/10.1038/s41598-020-78934-7
Rosa LH, Pinto OHB, Šantl-Temkiv T et al (2020b) DNA metabarcoding of fungal diversity in air and snow of Livingston Island, South Shetland Islands. Antarctica Sci Rep 10:21793. https://doi.org/10.1038/s41598-020-78630-6
Ruppert K, Kline RJ, Rahman MS (2019) Past, present, and future perspectives of environmental DNA (eDNA) metabarcoding: A systematic review in methods, monitoring, and applications of global eDNA. Global Ecology and Conservation 17:1–29. https://doi.org/10.1016/j.gecco.2019.e00547
Schloss PD et al (2009) Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75(23):7537–7541
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Shaw JA, Cox CJ, Goffinet B, Buck WR, Boles S (2003) Phylogenetic evidence of a rapid radiation of pleurocarpous mosses (Bryophyta). Evolution 57:2226–2241
Smirnov AV, Nassonova E, Holzmann M, Pawlowski JAN (2002) Morphological, Ecological and Molecular Studies of Vannella simplex Wohlfarth-Bottermann 1960 (Lobosea, Gymnamoebia), with a new Diagnosis of this Species. Protist 153(4):367–377
Smith RIL (2003) The enigma of colobanthus quitensis and Deschampsia antarctica in Antarctica. In Huiskes, a.h.l., Gieskes, W.W.C., Rozema, J., Schorno, R.M.L., Van der Vies, S.M., Wolff, w.j., eds. Antarctic biology in a global context. Leiden: backhuys publishers, 234–239.
Smith RIL, Convey P (2002) Enhanced sexual reproduction in bryophytes at high latitudes in the maritime Antarctic. J Bryol 24:107–117
Sollman P (2015) The genus Bryoerythrophyllum (Musci, Pottiaceae) in Antarctica. Polish Bot J 50(1):19–25
Tedersoo L et al (2018) High-level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Div 90:135–159
Terauds A, Chown SL, Morgan FJ, Peat H, Watts DJ, Keys H, Convey P, Bergstrom DM (2012) Conservation biogeography of the Antarctic. Divers Distrib 18:726–741. https://doi.org/10.1111/j.1472-4642.2012.00925.x
Tekle YI, Roger AO, LeckyAF, Kelly SD (2013) A New Freshwater Amoeba: Cochliopodium pentatrifurcatumn. sp. (Amoebozoa, Amorphea). Journal of Eukaryotic Microbiology, 60(4): 342–349.doi:https://doi.org/10.1111/jeu.12038
Thompson AR, Powell GS, Adams BJ (2019) Provisional checklist of terrestrial heterotrophic protists from Antarctica. Antarct Sci 31(6):287–303
Tin T, Fleming Z, Hughes K, Ainley D, Convey P, Moreno C, Pfeiffer S, Scott J, Snape I (2009) Impacts of local human activities on the Antarctic environment. Antarct Sci 21(1):3–33. https://doi.org/10.1017/S0954102009001722
Tomasel CM, Adams BJ, Tomasel FG, Wall DH (2013) The Life Cycle of the Antarctic Nematode Plectus murrayi Under Laboratory Conditions. J Nematol 45(1):39–42
Tong S, Vørs N, Patterson DJ (1997) Heterotrophic fagellates, centrohelid heliozoa and filose amoebae from marine and freshwater sites in the Antarctic. Polar Biol 18:91–106
Tsujimoto M, McInnes SJ, Convey P, Imura S (2014) Preliminary description of tardigrade species diversity and distribution pattern around coastal Syowa Station and inland Sør Rondane Mountains, Dronning Maud Land. East Antarctica Polar Biol 37:1361–1367
Usher M, Booth R (1986) Arthropod Communities in a Maritime Antarctic Moss-Turf Habitat: Multiple Scales of Pattern in the Mites and Collembola. J Anim Ecol 55(1):155–170. https://doi.org/10.2307/4699
Verleyen E, Van de Vijver B, Tytgat B, Pinseel E, Hodgson DA, Kopalová K, Chown SL, Van Ranst E, Imura S, Kudoh S, Van Nieuwenhuyze W, Sabbe K, Vyverman W (2021) Diatoms define a novel freshwater biogeography of the Antarctic. Ecography 44:548–560. https://doi.org/10.1111/ecog.05374
Vlčková S, Linhart J, Uvíra V (2002) Permanent and temporary meiofauna of an aquatic moss Fontinalis antipyretica. Hedw Acta u Palack Olom 39–40(1):131–140
Vyverman W, Verleyen E, Wilmotte A, Hodgson DA, Willems A, Peeters K, de Vijver BV, De Wever A, Leliaert F, Sabbe K (2010) Evidence for widespread endemism among Antarctic micro-organisms. Polar Sci 4(2):103–113. https://doi.org/10.1016/j.polar.2010.03.006
Waggoner BM, Poinar GO Jr (1993) Fossil habrotrochid rotifers in Dominican amber. Experientia 49:354–357. https://doi.org/10.1007/BF01923421
Wang S, Tang JY, Ma J, Li XD, Li YH (2018) Moss habitats distinctly affect their associated bacterial community structures as revealed by the high-throughput sequencing method. World J Microbiol Biotechnol 34(4):58
Weber AA, Pawlowski J (2013) Can abundance of protists be inferred from sequence data: a case study of Foraminifera. PLoS ONE 8:e56739. https://doi.org/10.1371/journal.pone.0056739
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols: a guide to methods and applications. Academic Press, New York, pp 315–322
Willoughby LG (1969) Pure culture studies on the aquatic phycomycete. Lagenidium Giganteum Trans Brit Mycol Soc 52(3):393–410. https://doi.org/10.1016/S0007-1536(69)80123-3
Yeates GW (1979) Terrestrial nematodes from the Bunger Hills and Gaussberg. Antarctica, New Zealand Journal of Zoology 6(4):641–643
Yilmaz P, Parfrey LW, Yarza P, Gerken J, Pruesse E, Quast C, Schweer T, Peplies J, Ludwig W, Glöckner FO (2014) The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucl Acids Res 42:D643–D648
Acknowledgements
We thank the Brazilian Navy, Brazilian Air Force and the staff at Ferraz station (GB Ferraz). This study received financial support from CNPq, PROANTAR, INCT Criosfera 2. P. Convey is supported by NERC core funding to the BAS ‘Biodiversity, Evolution and Adaptation’ Team. We also thank congresswoman Jô Moraes and the Biological Sciences Institute of the University of Brasilia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. Cann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Câmara, P.E.A.S., Convey, P., Rangel, S.B. et al. The largest moss carpet transplant in Antarctica and its bryosphere cryptic biodiversity. Extremophiles 25, 369–384 (2021). https://doi.org/10.1007/s00792-021-01235-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-021-01235-y