Abstract
Temperature-responsive polymers are smart materials that respond to changes in temperature and have a wide range of applications, ranging from sensing to biomedical fields. In this work, we investigated the synthesis and temperature-responsive behavior of responsive elastomer based on N-isopropylacrylamide-grafted natural rubber. The grafting reaction was carried out using deproteinized natural rubber (DPNR) latex and potassium persulfate as free radical initiator. The temperature responsiveness of the graft copolymers was investigated using water swelling and contact angle measurements, and compared with that of pure DPNR. The lower critical solution temperature of the graft copolymer was found to be in the range 30–34 °C, whereas the DPNR was not responsive to temperature. Furthermore, the graft copolymer exhibited temperature responsiveness in a solid state. As the temperature responsiveness of the graft copolymer is close to the human body temperature, it can be used in biomedical applications. Dye adsorption studies revealed the Langmuir isotherm, indicating monolayer coverage. The technique proposed in this study produces a temperature-responsive natural rubber, with potential applications as a responsive material for use in sensing and biomedical products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stimuli-responsive materials are smart materials because they can sense and respond to changes in environmental conditions, such as pH, temperature, light, ionic strength, electric field, and magnetic field [1,2,3,4]. Changes in surrounding conditions trigger a change in the physical and chemical properties of these materials, such as size, shape, hydrophobicity/hydrophilicity, and degradation rate [2]. These responsive materials can, therefore, be used to fabricate responsive, active devices that can regulate and introduce new functionalities in a range of applications. Temperature is one of the most widely used external stimuli because it can be simply modulated by temperature-programmed equipment or ambient conditions. Temperature-responsive materials are generally those that undergo a volume transition or soluble-to-insoluble change in solvents at around the lower critical solution temperature (LCST). One of the most widely studied temperature-responsive polymers is based on poly(N-isopropylacrylamide) (PNIPAM) because it has an LCST of around 32 °C in water, which is near the human body temperature [5,6,7,8,9,10,11,12,13,14]. The non-toxicity, high water solubility, and biocompatibility of PNIPAM make it applicable in a range of biomedical applications. However, its mechanical properties are not particularly useful and, therefore, significant efforts have been directed toward improving its mechanical strength. A range of temperature-responsive copolymers have also been prepared that increase the strength of and impart new properties to hybrid materials. NIPAM has been copolymerized and graft copolymerized with several polymers, including cellulose [12], poly(N-vinylpyrrolidone) [15], poly(acrylic acid) [7], poly(ethylene oxide) [16], polysiloxanes [17], chitosan [11], and styrene–butadiene rubber [14]. NIPAM-based copolymers thus have the potential to be used for a wider range of applications.
Natural rubber (NR) is a well-known renewable elastomeric material obtained from the Hevea brasiliensis tree in the form of a milky white fluid called latex [18]. NR is a versatile material widely used in many applications. However, NR is poorly resistant to oxidation, ozone, weathering, and many chemicals and solvents because of unsaturation in cis-polyisoprene, a major polymer of NR [19, 20]. Several undesirable reactions, such as crosslinking or chain scission, occur on the unsaturated backbone of cis-polyisoprene. Although the presence of double bonds makes cis-polyisoprene susceptible to degradation, they are points at which new functionalities can be added. Several chemical reactions have been used to modify NR, producing hybrid structures with high strength and stability. Various monomers have been grafted with NR, including dimethyl aminoethyl methacrylate [21], glycidyl methacrylate [22], methyl methacrylate [23,24,25], styrene [26,27,28], 2-hydroxyethyl methacrylate [29, 30], vinyl alcohol [31], and maleic anhydride [32, 33]. Recently, our group reported the synthesis of temperature-responsive crosslinked natural rubber with NIPAM and N-vinylcaprolactam. However, no temperature-responsive materials have to date been reported via grafting reaction with NR.
In grafting monomers and polymers with NR, the main goal has been to improve the thermal, chemical, and mechanical properties of the NR, and the addition of responsive functions to NR via grafting reaction has never been reported. In this work, we propose a method for grafting NIPAM and NR as a means for preparing rubber-based, temperature-responsive materials. Grafting of hydrophilic monomers remains a challenge, because of the presence of surface proteins as rubber particle shells. Monomers are not well incorporated into the hydrophobic NR cores in latex and, therefore, often homopolymerize [34,35,36,37]. Therefore, in this work grafting was carried out using deproteinized NR latex and water-soluble potassium persulfate as an initiator. The LCST of the graft copolymers was measured and compared with that of pristine NR. Dye adsorption was investigated and the adsorption isotherms were derived. Based on these results, we show that temperature responsiveness can be introduced to NR, which will expand its range of applications.
Materials and methods
Materials
High-ammonia NR latex [60% dry rubber content (DRC)] was purchased from the Department of Agriculture, Thailand. All other chemicals were purchased from Sigma-Aldrich and used as received unless otherwise noted. NIPAM was purified by recrystallization in methanol. Organic solvents (Labscan, AR) were used as received. Deionized (DI) water was used throughout the experiments. PNIPAM was synthesized from NIPAM via free radical polymerization using benzoyl peroxide and used as a reference.
Deproteinization of natural rubber
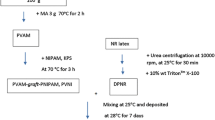
NR latex was added to a mixture of 0.1 wt% urea and 1 wt% sodium dodecyl sulfate (SDS) solutions. The mixture was incubated for 60 min at 25 °C and then diluted with DI water to obtain 30% DRC latex. After centrifugation at 10,000 rpm for 45 min at 25 °C, the cream fraction was re-dispersed in 1 wt% SDS and was centrifuged twice to obtain deproteinized natural rubber (DPNR) latex. Finally, the DPNR was kept in 0.1 wt% SDS with a final DRC of 32%.
Grafting of NIPAM onto DPNR
The DPNR (10 g) was diluted with DI water to approximately 16 wt% DRC and placed in a 250-mL round-bottom flask. The mixture was then degassed under a nitrogen atmosphere for at least 45 min. SDS (1 phr) as an emulsifier was then added to the mixture and stirred for 15 min. Afterwards, potassium persulfate (KPS, 1 phr) as an initiator was added continuously to the solution while stirring for 30 min at 75 °C.
The N-isopropylacrylamide (NIPAM, 100 phr) was diluted with DI water in a 250-mL round-bottom flask. SDS (1 phr) was then added to the solution and stirred for 15 min. KPS (1 phr) was slowly added and stirred at 95 °C for 30 min.
The NIPAM-initiator solution and the NR-initiator solution were mixed. The reaction mixture was heated at 75 °C for 12 h. Next, the reaction was cooled to 30 °C and precipitated in acetone. The solid polymer product was purified by stirring in acetone twice to remove unreacted monomer and contaminants. The grafted rubber was dried under vacuum at 60 °C for 24 h.
Grafting efficiency is the fraction of grafted NIPAM on the backbone NR chains. The grafting efficiency was calculated using the following equation [38]:
where I 4.0 is the integrated peak area of –CH–NH– from the NIPAM units and I 5.2 is the integrated peak area of –CH– in the isoprene units in the 1H-NMR spectrum.
Determination of LCST
The rubber samples were cut into small pieces (0.75 × 0.75 cm2) and compressed to displace air bubbles and reduce porosity by sandwiching them between two glass slides and heating at 60 °C overnight. The samples were then immersed in DI water for 2 h at temperatures of 28, 30, 32, 34, 36, and 38 °C to reach the saturation of water swelling. After gently removing the surface liquid with tissue paper, the weight of the materials was measured and the swelling percentage was calculated to determine the LCST, using the following equation [39]:
where W 1 and W 2 are the weight of the material before and after immersion.
Adsorption studies
Five indigo carmine solutions (10, 20, 30, 40, and 50 ppm) were prepared using DI water. Their absorbance was measured using UV–visible spectrophotometry. The NIPAM-g-DPNR samples (0.2 g) were immersed in the indigo carmine solutions (10 mL) and were then placed on a shaker for 1 week [39]. The rubber samples were removed and the absorbance was measured. The concentration of indigo carmine remaining in the solution was then calculated from the absorbance, using the calibration curve.
The amount of indigo carmine adsorbed on the NIPAM-g-DPNR sample was calculated using the following equation:
where q e (mg g−1) is the adsorption capacity of the rubber, C o (mg L−1) is the initial concentration of indigo carmine in the solution, C e (mg L−1) is the concentration of indigo carmine remaining in the solution, V (L) is the volume of the solution, and W (g) is the dry weight of the rubber sample. The data were fit using the Langmuir and Freundlich isotherm equations (Table 1).
Characterization
CHN data were obtained using a CHN-2000 LECO analyzer. 1H (400 MHz) nuclear magnetic resonance (NMR) spectra were obtained using an AVANCE Bruker NMR Spectrometer with CDCl3 as a solvent. The Fourier transform infrared (FT-IR) spectra were obtained using a Perkin Elmer FT-IR (Spectrum GX model) spectrometer and NaCl salt windows. Thermogravimetric analysis (TGA) of the polymers was carried out using an SDTA851e Mettler Toledo analyzer. The glass transition temperatures of the polymers were obtained using a DSC822e Mettler Toledo differential scanning calorimeter (DSC). In both cases, the samples were heated at a rate of 10 °C min−1 under nitrogen atmosphere. UV–visible spectra were obtained using a UV-1700 PharmaSpecUV–visible spectrometer. The water contact angle was measured using an OCA35 Dataphysics contact angle meter. The size of the water droplet was 8 µL.
Results and discussion
Synthesis and characterization of graft copolymer
The graft copolymer of NIPAM and NR was prepared via free radical grafting in an aqueous system using KPS as the initiator. The NR latex was deproteinized prior to grafting, because previous studies have shown that deproteinization removes surface protein, improving grafting efficiency [40, 41]. Deproteinization was carried out using urea as the deproteinizing agent. The deproteinized NR (DPNR) was characterized using CHN analysis. It was found that the nitrogen content was 43% lower in the DPNR than the NR, whereas carbon and hydrogen content remained almost unchanged (Table 2). This indicates that deproteinization of the NR removed approximately half of protein from the NR latex, comparable with previous report [41].
Grafting of N-isopropylacrylamide (NIPAM) onto DPNR was carried out in an aqueous system using KPS as free radical initiator (Fig. 1). The formation of grafting copolymer is believed to proceed by two possible pathways: a reaction between the free radicals and double bonds of the isoprene units, and a hydrogen abstraction mechanism [39, 42,43,44,45]. The NIPAM-grafted DPNR (NIPAM-g-DPNR) was purified by stirring twice in acetone to remove impurities, unreacted NIPAM, and PNIPAM homopolymer. The NIPAM-g-DPNR was characterized using FT-IR and 1H-NMR and compared with the DPNR and PNIPAM. The FT-IR spectrum of the NIPAM-g-DPNR showed signature signals of both DPNR and PNIPAM (Fig. 2). Signals at 3000 and 1660 cm−1 corresponded to C–H and C=C stretching vibrations, respectively, while those at 3300, 2962, 1650, and 1540 cm−1 corresponded to N–H stretching, C–H stretching, C=O stretching (amide I), and C=O stretching (amide II) vibrations, respectively. The FT-IR results are consistent with those reported in previous studies [46]. Images were taken of the NIPAM-g-DPNR, DPNR, and NIPAM (Fig. 1b). DPNR appeared as a clear solid and NIPAM as a white powder. The NIPAM-g-DPNR was a yellowish solid and stiffer than the DPNR.
The 1H-NMR spectrum of DPNR showed signals at 5.2, 2.1, and 1.7 ppm, corresponding to C–H, CH2, and CH3, respectively (Fig. 3), consistent with previous reports [2, 46, 47]. The 1H-NMR spectrum of the PNIPAM showed signals at 4.00, 1.82, 1.65, and 1.14 ppm, corresponding to CH–NH, C–H, CH2, and CH3, respectively. The 1H-NMR spectrum of NIPAM-g-DPNR showed signals corresponding to both DPNR and PNIPAM. The signal at 4.0 ppm corresponded to CH–NH in the N-isopropylacrylamide, while the signal at 5.2 ppm corresponded to the C–H of the isoprene units. These signals were used to calculate the grafting efficiency, which was found to be 18%.
To further confirm the formation of graft copolymer, a blend sample between DPNR latex and PNIPAM was prepared in water solution and dried as a thin film on a glass plate. The film was peeled off and purified via stirring twice in acetone and dried in an oven at 60 °C overnight. It was found that no PNIPAM appeared in the 1H-NMR spectrum as it was soluble in acetone (Fig. 3b). Therefore, the presence of both NIPAM and DPNR in the NIPAM-g-DPNR sample was attributed to the formation of a grafted structure that was inseparable by stirring in acetone. This also confirmed that stirring twice in acetone effectively removed the unbound PNIPAM homopolymer.
Effect of reaction conditions
The effect of reaction conditions and reagent concentrations on grafting efficiency was investigated. First, the effect of reaction temperature was investigated using a NIPAM concentration of 100 phr, KPS concentration of 1 phr, and reaction time of 12 h (Fig. 4a). It was found that when the reaction temperature was increased from 75 to 95 °C, which is the optimum working range for KPS [4], grafting increased from 12 to 21%. This dropped to 15% when the temperature increased further. Second, the grafting percentage as a function of NIPAM concentration was measured using a KPS concentration of 1 phr and reaction time of 12 h at 95 °C. It was found that, as the NIPAM concentration increased from 50 to 200 phr with respect to NR, grafting increased from 10 to 39% (Fig. 4b). Third, the effect of KPS concentration on grafting was studied using a NIPAM concentration of 100 phr and reaction time of 12 h at 95 °C (Fig. 4c). When the KPS concentration was increased from 0.5 to 2 phr, grafting increased from 10 to 25%. Lastly, the effect of reaction time on grafting was investigated using a NIPAM concentration of 100 phr, KPS concentration of 1 phr, and reaction temperature of 95 °C (Fig. 4d). When the reaction time was increased from 3 to 12 h, grafting increased from 12 to 45%.
Thermal properties
The thermal properties of the DPNR and NIPAM-g-DPNR were characterized using DSC and TGA. The DSC thermograms showed the glass transition temperatures of DPNR and NIPAM-g-DPNR to be −62.1 and −61.3 °C, respectively (Fig. 5). Low glass transition temperatures are due to the amorphous nature of NR. PNIPAM showed a broad endothermic transition between 60 and 150 °C, corresponding to the glass transition temperature, which is consistent with values reported in the literature [48]. A weak, broad endothermic transition between 70 and 150 °C was also observed from the NIPAM-g-DPNR sample, confirming the presence of small amounts of PNIPAM in the graft copolymer.
From TGA thermograms, the DPNR started to lose weight at 320 °C, consistent with previous reports (Fig. 6) [38, 39, 49]. PNIPAM started to lose weight at 100 °C due to breakage of the labile amide functional groups. The thermogram of the NIPAM-g-DPNR sample was similar to that of DPNR, but slightly less stable due to the presence of PNIPAM. This confirmed the presence of both DPNR and PNIPAM in the NIPAM-g-DPNR sample.
Temperature responsiveness
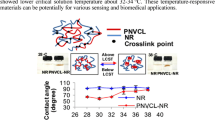
The temperature responsiveness of NR and NIPAM-g-DPNR in water were characterized at temperatures of 28, 30, 32, 34, 36, and 38 °C. At 28 °C, the NIPAM-g-DPNR swelled by 12% of its original weight, or about ten times more than the DPNR, due to the presence of hydrophilic NIPAM units. The NIPAM-g-DPNR can be considered a hydrogel-like material. The percentage swelling of the NR did not change with temperature, whereas that of the NIPAM-g-DPNR started to decrease at a water temperature of 30 °C and became constant at 34 °C (Fig. 7). The LCST of NIPAM-g-DPNR was, therefore, determined to fall in the range 30–34 °C, which is similar to the coil-to-globule transition temperature of PNIPAM. These results suggest that the presence of NR did not significantly affect the LCST of the PNIPAM. This is consistent with reports on other PNIPAM-based materials [9, 50,51,52,53].
The LCSTs of NR and NIPAM-g-DPNR were also determined from the temperature-controlled contact angle. At 28 °C, the contact angles of NR and NIPAM-g-DPNR were 98° and 63°, respectively, indicating a greater hydrophobicity of NR than NIPAM-g-DPNR (Fig. 8). The contact angles of the NR sample were almost constant across the measured temperature range. In contrast, the contact angles of the NIPAM-g-DPNR sample started to increase at the temperature of 32 °C reaching 97° at 38 °C, which is close to those of the NR sample. The increase in contact angle was due to an increase in hydrophobicity of the NIPAM units resulting from breakage of the hydrogen bonds between the water molecules and NIPAM. Based on this abrupt change in contact angles, the LCSTs were determined to be approximately 32–36 °C, similar to those determined by the water swelling method.
The temperature responsiveness of the DPNR and NIPAM-g-DPNR specimens was monitored on a hot plate, whose temperature was slowly increased from 25 to 40 °C over a period of 7 min. The NIPAM-g-DPNR sample changed its shape close to its LCST, whereas the DPNR sample retained its original shape throughout (Fig. 9). This indicates that NIPAM-g-DPNR remains temperature responsive in the solid phase.
Dye adsorption studies
Next, the absorption of indigo carmine dye by NIPAM-g-DPNR was investigated. Indigo carmine was chosen to represent a water-soluble anionic model drug. The rubber samples were incubated in indigo carmine solution for 1 week at room temperature. The Langmuir and Freundlich isotherms were used to investigate the interaction between the indigo carmine and the NIPAM-g-DPNR. The Langmuir adsorption isotherm is based on the assumption that a monolayer coverage is formed on the surface of the adsorbent whereas the Freundlich adsorption isotherm describes the characteristics of multilayer adsorption [54,55,56]. On the basis of the R-square (R 2) of these models (Fig. 10), the Langmuir model showed the highest value of R 2 as 0.754, being the best fit to the experimental data between the two models. Our analysis suggests that the adsorption of indigo carmine onto the surface of the NIPAM-g-DPNR was of the monolayer form. NIPAM-g-DPNR is thus a potentially useful temperature-responsive material for biomedical applications.
Conclusions
In this work, graft copolymers between NIPAM and DPNR were successfully prepared via reaction in emulsion using KPS as initiator. The graft materials were rubber-like with thermal properties close to pristine NR. The temperature responsiveness of the graft copolymers in water and solid state showed an LCST of 32–34 °C close to that of PNIPAM. The dye adsorption studies revealed the Langmuir isotherm, indicating monolayer coverage. Therefore, these temperature-responsive, hydrogel-like rubbers have many potential biomedical and sensing applications. This includes smart bandages and facial masks that release medicine or therapeutic ingredients upon contact with human skin. Other applications include smart tires for automobiles that change adhesion in response to temperature. The strategy presented in this work allows multi-stimuli-responsive rubbers to be prepared through reactions between multiple responsive materials. This greatly extends the properties of the rubbers.
References
Almeida H, Amaral MH, Lobão P (2012) Temperature and pH stimuli-responsive polymers and their applications in controlled and self-regulated drug delivery. J Appl Pharm Sci 02:01–10
Chen J-K, Chang C-J (2014) Fabrications and applications of stimulus-responsive polymer films and patterns on surfaces: a review. Materials 7:805–875
Ward MA, Georgiou TK (2011) Thermoresponsive polymers for biomedical applications. Polymers 3:1215
Mark B, Overberger CG, Menges G (1986) Encyclopedia of polymer science and engineering. Wiley 6:492
Chee C-K, Rimmer S, Soutar I, Swanson L (2006) Synthesis and conformational behaviour of luminescently labelled poly[styrene-graft-(N-isopropyl acrylamide)] copolymers. Polym Int 55:740–748
Abdulkadir A, Hazer B (2008) Poly(N-isopropylacrylamide) thermoresponsive cross-linked conjugates containing polymeric soybean oil and/or polypropylene glycol. Eur Polym J 44:1701–1713
Zhang J, Chu LY, Cheng CJ, Mi DF, Zhou MY, Ju XJ (2008) Graft-type poly(N-isopropylacrylamide-co-acrylic acid) microgels exhibiting rapid thermo- and pH-responsive properties. Polymer 49:2595–2603
Liu R, Fraylich M, Saunders BR (2009) Thermoresponsive copolymers: from fundamental studies to applications. Colloid Polym Sci 287:627–643
Yi G, Huang Y, Xiong F, Liao B, Yang J, Chen X (2011) Preparation and swelling behaviors of rapid responsive semi-IPN NaCMC/PNIPAm hydrogels. J Wuhan Univ Technol Mater Sci Ed 26:1073–1078
Abdullah Al N, Lee KS, Mosaiab T, Park SY (2013) pH and thermo-responsive poly(N-isopropylacrylamide) copolymer grafted to poly(ethylene glycol). J Appl Polym Sci 130:168–174
Ifuku S, Miwa T, Morimoto M, Saimoto H (2013) Thermoresponsive chitosan/N-isopropylacrylamide copolymer through atom transfer radical polymerization. Int J Biol Macromol 52:14–19
Kang H, Liu R, Huang Y (2013) Synthesis of ethyl cellulose grafted poly (n-isopropylacrylamide) copolymer and its micellization. Acta Chim Sin 71:114–120
Wang Y, Qin J, Wei Y, Li C, Ma G (2013) Preparation strategies of thermo-sensitive P(NIPAM-co-AA) microspheres with narrow size distribution. Powder Technol 236:107–113
Hermann A, Mruk R, Roskamp RF, Scherer M, Ma L, Zentel R (2014) Poly(N -isopropylacrylamide)-modified styrene-butadiene rubber as thermoresponsive material. Macromol Chem Phys 215:32–43
Jin S, Liu M, Chen S, Gao C (2008) Synthesis, characterization and the rapid response property of the temperature responsive PVP-g-PNIPAM hydrogel. Eur Polym J 44:2162–2170
Qin S, Geng Y, Discher DE, Yang S (2006) Temperature-controlled assembly and release from polymer vesicles of poly(ethylene oxide)-block- poly(N-isopropylacrylamide). Adv Mater 18:2905–2909
Hodorog ADR, Ibanescu C, Danu M, Simionescu BC, Rocha L, Hurduc N (2012) Thermo-sensitive polymers based on graft polysiloxanes. Polym Bull 69:579–595
Graves DF (2007) Rubber. In: Kent JA (ed) Handbook of industrial chemistry and biotechnology. Springer, New York, pp 689–718
Gamlin C, Markovic MG, Dutta NK, Choudhury NR, Matisons JG (2000) Structural effects on the decomposition kinetics of EPDM elastomers by high-resolution TGA and modulated TGA. J Therm Anal Calorim 59:319–336
Kangwansupamonkon W, Gilbert RG, Kiatkamjornwong S (2005) Modification of natural rubber by grafting with hydrophilic vinyl monomers. Macromol Chem Phys 206:2450–2460
Oliveira PC, Guimaraes A, Cavaille JY, Chazeau L, Gilbert RG (2005) Poly(dimethylaminoethyl methacrylate) grafted natural rubber from seeded emulsion polymerization. Polymer 46:1101–1105
Juntuek P, Ruksakulpiwat C, Chumsamrong P, Ruksakulpiwat Y (2011) Glycidyl methacrylate grafted natural rubber: synthesis, characterization, and mechanical property. J Appl Polym Sci 122:3152–3159
Satraphan P, Intasiri A, Tangpasuthadol V, Kiatkamjornwong S (2009) Effects of methyl methacrylate grafting and in situ silica particle formation on the morphology and mechanical properties of natural rubber composite films. Polym Adv Technol 20:473–486
Kochthongrasamee T, Prasassarakich P, Kiatkamjornwong S (2006) Effects of redox initiator on graft copolymerization of methyl methacrylate onto natural rubber. J Appl Polym Sci 101:2587–2601
Zhang S, Cao L, Shao F, Chen L, Jiao J, Gao W (2008) Grafting of methyl methacrylate onto natural rubber in supercritical carbon dioxide. Polym Adv Technol 19:54–59
Suksawad P, Yamamoto Y, Kawahara S (2011) Preparation of thermoplastic elastomer from natural rubber grafted with polystyrene. Eur Polym J 47:330–337
Arayapranee W, Rempel GL (2008) Morphology and mechanical properties of natural rubber and styrene-grafted natural rubber latex compounds. J Appl Polym Sci 109:1395–1402
Pukkate N, Kitai T, Yamamoto Y, Kawazura T, Sakdapipanich J, Kawahara S (2007) Nano-matrix structure formed by graft-copolymerization of styrene onto natural rubber. Eur Polym J 43:3208–3214
Promdum Y, Klinpituksa P, Ruamcharoen J (2009) Grafting copolymerization of natural rubber with 2-hydroxyethyl methacrylate for plywood adhesion improvement. Songklanakarin J Sci Technol 31:453–457
Amnuaypanich S, Ratpolsan P (2009) Pervaporation membranes from natural rubber latex grafted with poly(2-hydroxyethyl methacrylate) (NR-g-PHEMA) for the separation of water-acetone mixtures. J Appl Polym Sci 113:3313–3321
Wongthep W, Srituileong S, Martwiset S, Amnuaypanich S (2013) Grafting of poly(vinyl alcohol) on natural rubber latex particles. J Appl Polym Sci 127:104–110
Wongthong P, Nakason C, Pan Q, Rempel GL, Kiatkamjornwong S (2012) Grafting of maleic anhydride onto deproteinized natural rubber via differential microemulsion polymerization. Adv Trends Eng Mater Appl 183–190
Nakason C, Kaesaman A, Supasanthitikul P (2004) The grafting of maleic anhydride onto natural rubber. Polym Test 23:35–41
Nakason C, Kaesaman A, Yimwan N (2003) Preparation of graft copolymers from deproteinized and high ammonia concentrated natural rubber latices with methyl methacrylate. J Appl Polym Sci 87:68–75
Oshio A, Kitai T, Kawahara S, Kuroda H (2006) Investigation of high graft-copolymerization of styrene onto natural rubber. In: Polymer preprints, vol 55. Japan, p 3606
Pukkate N, Yamamoto Y, Kawahara S (2008) Mechanism of graft copolymerization of styrene onto deproteinized natural rubber. Colloid Polym Sci 286:411–416
Wongthong P, Nakason C, Pan Q, Rempel GL, Kiatkamjornwong S (2013) Modification of deproteinized natural rubber via grafting polymerization with maleic anhydride. Eur Polym J 49:4035–4046
Kookarinrat C, Paoprasert P (2015) Versatile one-pot synthesis of grafted-hydrogenated natural rubber. Iran Polym J 24:123–133
Nuntahirun P, Yamamoto O, Paoprasert P (2016) Preparation and temperature-responsive behavior of crosslinked polymers between poly(N-isopropylacrylamide) and natural rubber. Macrol Res 24:816–823
Fukuhara L, Miyano K, Yamamoto Y, Ishii H, Kawahara S (2015) Preparation of purified natural rubber by removal of proteins. Kobunshi Ronbunshu 72:1–6
Kawahara S, Klinklai W, Kuroda H, Isono Y (2004) Removal of proteins from natural rubber with urea. Polym Adv Technol 15:181–184
Halimatuddahliana IH, Akil HM (2005) The effect of dicumyl peroxide vulcanization on the properties and morphology of polypropylene/ethylene–propylene diene terpolymer/natural rubber blends. Int J Polym Mater 54:1169
Liu H, Chuai C, Iqbal M, Wang H, Kalsoom BB, Khattak M, Khattak MQ (2011) Improving foam ability of polypropylene by crosslinking. J Appl Polym Sci 122:973–980
Tamboli SM, Mhaske ST, Kale DD (2011) Improving foam ability of polypropylene by crosslinking. J Appl Polym Sci 122:973–980
Manaila E, Stelescu MD, Craciun G, Surdu L (2014) Effects of benzoyl peroxide on some properties of composites based on hemp and natural rubber. Polym Bull 71:2001–2022
Li D, Zhang X, Yao J, Simon GP, Wang H (2011) Stimuli-responsive polymer hydrogels as a new class of draw agent for forward osmosis desalination. Chem Commun 47:1710–1712
Park YI, Zhang B, Kuo C-Y, Martinez JS, Park J, Mallapragada S, Wang H-L (2013) Stimuli-responsive poly-N-isopropylacrylamide: phenylene vinylene oligomer conjugate. J Phys Chem C 117:7757–7763
Seddiki N, Aliouche D (2013) Synthesis, rheological behavior and swelling properties of copolymer hydrogels based on poly(N-isopropylacrylamide) with hydrophilic monomers. Bull Chem Soc Ethiop 27:447
Chanroj T, Paoprasert P (2016) Chlorohydrination of natural rubber latex using sodium hypochlorite for fuel-resistant properties. Rubber Chem Technol 89:251–261
Xia X, Hu Z (2004) Synthesis and light scattering study of microgels with interpenetrating polymer networks. Langmuir 20:2094–2098
Cai Y, Shen W, Loo SL, Krantz WB, Wang R, Fane AG, Hua X (2013) Towards temperature driven forward osmosis desalination using Semi-IPN hydrogels as reversible draw agents. Water Res 47:3773–3781
Hebeish A, Farag S, Sharaf S, Shaheen TI (2014) Thermal responsive hydrogels based on semi interpenetrating network of poly (NIPAm) and cellulose nanowhiskers. Carbohydr Polym 102:159–166
Zadrazil A, Stepánek F (2010) Investigation of thermo-responsive optical properties of a composite hydrogel. Colloid Surf A 372:115–119
Chen X (2015) Modeling of experimental adsorption isotherm data. Information 4:14–22
Dada AO, Olalekan AP, Olatunya AM, Dada O (2012) Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J Appl Chem 3:38–45
Itodo AU, Itodo HU (2010) Sorption energies estimation using Dubinin–Radushkevich and Temkin adsorption isotherms. Life Sci J 7:31–39
Acknowledgements
This work is financially supported by the Thailand Research Fund (TRF) and the Faculty of Science and Technology, Thammasat University (TRG5880199) and the Thailand Graduate Institute of Science and Technology (TGIST: SCA-CO-2558-996-TH). The authors acknowledge the Central Scientific Instrument Center (CSIC), Department of Chemistry, Faculty of Science and Technology, and Thammasat University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nuntahirun, P., Yamamoto, O. & Paoprasert, P. Temperature-responsive N-isopropylacrylamide-grafted natural rubber. Polym. Bull. 75, 1387–1401 (2018). https://doi.org/10.1007/s00289-017-2099-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-017-2099-7