Abstract
High conversion and high grafting efficiency attained by graft copolymerization of styrene onto deproteinized natural rubber (DPNR) was investigated with respect to the molecular weight of grafted polystyrene. The graft copolymerization was performed with tert-butyl hydroperoxide/tetraethylenepentamine as an initiator after deproteinization of natural rubber with urea. Grafted polystyrene was isolated from the resulting graft copolymer by ozonolysis reaction. After the ozonolysis of the graft copolymer of DPNR and polystyrene (DPNR-g-PS), the molecular weight of grafted polystyrene was determined by size exclusion chromatography. Effects of initiator and monomer concentrations were investigated with respect to the molecular weight of the grafted polystyrene, which was found to depend on not only the number of active site generated on the rubber particle but also the feed of styrene. Deactivation and chain transfer of the active sites were attributed to effective amount of styrene used for the graft copolymerization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Graft copolymerization of vinyl monomer onto natural rubber in latex stage is widely recognized to be important to prepare functional organic materials. For instance, nano-matrix structure [1] may be formed by the graft copolymerization followed by coagulation of the resulting latex in which the nano-matrix structure consists of a dispersoid of major natural rubber and a matrix of minor functional polymer. In the previous work [1, 2], we determined a suitable condition for the formation of nano-matrix structure, that is, initiator concentration of 3.3 × 10−5 mol/g rubber, monomer concentration of 1.5 mol/kg rubber, and reaction temperature of 303 K. Under the condition, high conversion and high grafting efficiency of the monomer, i.e., more than 90%, were accomplished for the graft copolymerization. To assure the condition, it is necessary to investigate the mechanism of the graft copolymerization of vinyl monomer onto deproteinized natural rubber.
The mechanism of the graft copolymerization may be investigated by characterizing the grafted polymer linking to the dispersoid of natural rubber. In this regard, size exclusion chromatography and nuclear magnetic resonance (NMR) spectroscopy after ozonolysis of graft copolymer are considered to be useful for the characterization of the grafted polymer.
In the previous work, Cameron and Qureshi [3] reported that the graft copolymerization of styrene to polyisoprene and polybutadiene was governed by polymerization time, initiator concentration, and monomer concentration based on the value of grafting efficiency and graft density. Kinetics and mechanism of graft copolymerization of methyl methacrylate onto rubber using various type of initiator system was studied by Nayak [4–7]. Prasassarakich et al. [8] enhanced a yield of graft copolymerization of styrene and acrylonitrile onto natural rubber by increasing initiator concentration, reaction temperature, and pressure. However, less information of the mechanism was proposed in the previous works.
To investigate the mechanism, we take a notice of the molecular weight of the grafted polymer in the graft copolymer, as the molecular weight of grafted polystyrene may be dependent upon the number of active site and feed of monomer. In the previous work, Barnard [9] characterized the graft copolymer by ozonolysis followed by molecular weight measurement of the ozonolysis product. The double bond of polyisoprene and polybutadiene as polymer backbone was reacted with ozone to form ozonide, which was reduced to aldehyde and ketone [10]. Therefore, in this study, we apply ozonolysis to DPNR-g-PS to investigate the mechanism of the graft copolymerization. After ozonolysis of DPNR-g-PS, molecular weight of the resulting product is determined by size exclusion chromatography. Effects of initiator and monomer concentrations on the molecular weight of the grafted polystyrene are investigated in which the initiator used was tert-butyl hydroperoxide/tetraethylenepentamine (TBHPO-TEPA), as it was confirmed to suppress side reactions during the graft copolymerization [11].
Experimental
Materials
Natural rubber latex used in the present study was a commercial high-ammonia (Golden Hope, Malaysia) natural rubber latex. The urea-deproteinized natural rubber (U-DPNR) [12, 13] was prepared by incubation of the latex with 0.1% w/w urea in the presence of 1% w/w sodium dodecyl sulfate (SDS; Chameleon, Osaka, Japan, 98%) at 303 K followed by centrifugation at 10,000 rpm. The cream fraction was re-dispersed in 1% w/w SDS to make 30% w/w dry rubber content (DRC) latex and was washed twice by centrifugation. The U-DPNR latex was diluted with distilled water to make 30% w/w DRC, and SDS was added up to 0.1% w/w.
Graft copolymerization
Graft copolymerization of U-DPNR latex was carried out with styrene as a monomer using TBHPO-TEPA as an initiator. U-DPNR latex was charged with N2 gas for 1 h at 303 K. The initiator and monomer were added to the latex, respectively. The reaction was carried out by stirring the latex at about 400 rpm for 2 h at 303 K. The unreacted styrene was removed by using a rotary evaporator under reduced pressure. The as-prepared graft copolymer (gross polymer) was obtained by dipping a glass tube into the reacted latex and dried under reduced pressure at ambient temperature for more than a week. The gross polymer was extracted with acetone/2-butanone 3:1 mixture in a Soxhlet apparatus under nitrogen atmosphere in the dark and dried under reduced pressure for a week in which the removal of almost all free polystyrene, isolated from natural rubber, was completed by the extraction for 24 h.
Characterization
Ozonization was carried out by blowing an equimolar amount of ozone in ozonated oxygen through a 0.4% w/v methylene chloride solution of the extracted graft copolymer at 243 K. Reductive degradation of the resulting ozonide was performed by reaction with lithium aluminum hydride (LiAlH4) in diethyl ether followed by decomposition of residual LiAlH4 with water. After reductive degradation, grafted polystyrene, thus isolated from graft copolymer, was dissolved in a small amount of chloroform. The chloroform solution was centrifuged, and the polymer was precipitated with methanol.
Measurements of molecular weight and molecular weight distribution of the rubber were made with a TOSOH GPC consisting of a TOSOH CCPD pump; RI-8012 differential refractometer and UV-8011 UV detector. The measurement was made at 303 K, and the flow rate of the mobile phase, THF, was 0.5 ml/min.
Proton-nuclear magnetic resonance (1H-NMR) measurement was carried out at 323 K by a JEOL EX-400 NMR spectrometer at pulse repetition time of 7 s.
Results and discussion
Figure 1 shows a plot of styrene conversion and grafting efficiency vs initiator concentration. Conversion of styrene and grafting efficiency in graft copolymer were estimated as follows:
where weight of polystyrene in gross polymer and feed of monomer were determined by gravimetric method, as the values of styrene conversion and grafting efficiency in graft copolymers estimated from both gravimetric method, Fourier transform, and NMR spectroscopies were significantly similar.
The conversion and grafting efficiency depended on initiator concentration in which a maximum value was obtained at 3.3 × 10−5 mol/g rubber of initiator concentration. Therefore, at this suitable initiator concentration, an effect of monomer concentration on styrene conversion and grafting efficiency was investigated.
Figure 2 shows monomer-concentration dependency of styrene conversion and grafting efficiency. The conversion and the grafting efficiency were a function of styrene feed. The maximum value was achieved at monomer concentration of 1.5 mol/kg rubber. Therefore, the suitable condition for high conversion and grafting efficiency was confirmed to be 3.3 × 10−5 mol/g rubber in initiator concentration and 1.5 mol/kg rubber in monomer concentration, as reported in the previous paper [1, 2].
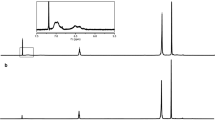
Figure 3 shows 1H-NMR spectra for U-DPNR-g-PS copolymer achieved from 1.5 mol/g rubber in monomer concentration and 3.3 × 10−5 mol/g rubber in initiator concentration before and after ozonolysis. Before ozonolysis, signals characteristic of cis-1,4-isoprene units appeared at 1.76, 2.10 and 5.13 ppm, which were assigned to methyl, methylene, and unsaturated methine protons of isoprene units, respectively. Broad signals around 6–7 ppm were assigned to phenyl proton of styrene units. In contrast, after ozonization, the peak at 5.13 ppm corresponding to unsaturated methine protons of isoprene units disappeared; however, the peak at 3.6 ppm corresponding to proton of hydroxyl group after reduction of aldehyde and ketone from ozonization appeared. This indicates that double bonds of cis-1,4-isoprene units are broken by ozonolysis, and grafted polystyrene is isolated from U-DPNR-g-PS. Thus, the ozonolysis-size exclusion chromatography was applied to investigate the mechanism of the graft copolymerization of styrene onto DPNR with respect to molecular weight of the grafted polystyrene.
Figure 4 shows the initiator-concentration dependency of number-average molecular weight (M n) of polystyrene grafted on natural rubber. The M n of grafted polystyrene decreased with increasing initiator concentration. This may be explained to be due to the deactivation and chain transfer; at low initiator concentration, the number of active site on the rubber particle was too small to react with all styrene monomer, whereas it was too large at high initiator concentration.
Figure 5 shows a plot of M n of grafted polystyrene vs monomer concentration. The M n of grafted polystyrene prepared at 0.5 mol/kg rubber of styrene was low, whereas that at 2.5 mol/kg rubber was high. To explain the monomer concentration dependency, we estimated the number of active site as follows:
where [M] is monomer concentration added initially in the system; conversion was determined by gravimetric method, and M n was estimated by ozonolysis-size exclusion chromatography.
Figure 6 shows monomer-concentration dependency of number of active site and styrene conversion. At low monomer concentration, the number of active site was about 5 × 10−4 mol/mol rubber. At 1.5 mol/kg rubber of styrene, the number of active site increased dramatically. This implies that the low conversion and low grafting efficiency at low monomer feed may be attributed to the deactivation of number of active site due to less amount of styrene. In contrast, at high monomer feed, low conversion and low grafting efficiency may be explained to be due to a chain transfer.
To confirm the occurrence of chain transfer at high monomer feed, M n of homopolystyrene dissolving in acetone/2-butanone solution from Soxhlet extraction was determined by size exclusion chromatography. Figure 7 shows M n of the grafted polystyrene in which M n of a soluble fraction (homopolystyrene) after the Soxhlet extraction is also shown in the figure. The M n of the grafted polystyrene increased monotonically, as feed of monomer increased, whereas the M n of the homopolystyrene did not. At low feed of styrene, the M n of the grafted polystyrene was higher than that of the homopolystyrene. This suggests that at low monomer concentration, almost all styrene monomer can react completely with the created active site on the rubber particle. However, chain-transfer reactions from the growing polymer radical to initiator, monomer, and growing polymer may occur near the rubber particle, as shown in Fig. 8. In this way, polymer chains, which are formed with initiator fragments, have lower molecular weight compared to that of ideally formed polymers in the absence of the chain transfer. In fact, the molecular weight of the resulting polystyrene was lower than the ideal value (M n = 1 × 104). In contrast, at high monomer concentration, the molecular weight of the grafted polystyrene linking to rubber particle was high and that of homopolystyrene as a by-product was also high. This may be explained to be due to excess of styrene monomer, that is, not only transfer reactions, as shown in Fig. 8, but also homopolymerization of styrene monomer may occur in micelle as an ordinary emulsion polymerization, as summarized in Fig. 9.
Conclusion
Mechanism of the graft copolymerization of styrene onto U-DPNR in latex stage, using tert-butyl hydroperoxide/tetraethylenepentamine as an initiator, was investigated with respect to molecular weight of grafted polystyrene determined by ozonolysis-size exclusion chromatography. Based on the results, initiator and monomer concentrations were proven to play an important role in the conversion and grafting efficiency of styrene for the graft copolymerization of natural rubber. The deactivation of initiator and chain transfer were proven to occur at low and high monomer concentrations, respectively. The highest conversion and grafting efficiency of styrene for graft copolymer were achieved at styrene feed of 1.5 mol/kg rubber and initiator concentration of 3.3 × 10−5 mol/g rubber.
References
Kawahara S, Kawazura T, Sawada T, Isono Y (2003) Polymer 44:4527
Pukkate N, Kitai T, Yamamoto Y, Kawazura T, Sakdapipanich J, Kawahara S (2007) Eur Polym J 43:3208
Cameron GC, Qureshi MY (1980) J Polym Sci Polym Chem Ed 18:2143
Lenka S, Nayak PL, Basak A (1986) J Polym Sci Part A: Polym Chem 24:3139
Nayak PL, Basak A (1986) J Appl Polym Sci 32:4271
Lenka S, Nayak PL, Mohanty IB, Mishra SN (1985) J Appl Polym Sci 30:2711
Lenka S, Nayak PL, Das AP, Mishra SN (1985) J Appl Polym Sci 30:429
Prasassarakich P, Sintoorahat P, Wongwisetsirikul N (2001) J Chem Eng Jpn 34:249
Barnard D (1956) J Polym Sci 22:213
Pine SH (1987) Organic Chemistry, 5th edn. McGraw-Hill International Edition, New York
Fukushima Y, Kawahara S, Tanaka Y (1998) J Rubber Res 1:154
Kawahara S, Klinklai W, Kuroda H, Isono Y (2004) Polym Adv Technol 15:181
Klinklai W, Saito T, Kawahara S, Tashiro K, Suzuki Y, Sakdapipanich JT, Isono Y (2004) J Appl Polym Sci 93:555
Acknowledgments
This study work was supported in part by COE program and Nagaoka University of Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pukkate, N., Yamamoto, Y. & Kawahara, S. Mechanism of graft copolymerization of styrene onto deproteinized natural rubber. Colloid Polym Sci 286, 411–416 (2008). https://doi.org/10.1007/s00396-007-1787-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-007-1787-5