Abstract
The current study aimed to evaluate the plant growth-promoting (PGP) potential of endophytic strain Bacillus subtilis KU21 isolated from the roots of Rosmarinus officinalis. The strain exhibited multiple traits of plant growth promotion viz., phosphate (P) solubilization, nitrogen fixation, indole-3-acetic acid (IAA), siderophore, hydrogen cyanide (HCN), lytic enzymes production, and 1-aminocyclopropane-1-carboxylate (ACC) deaminase activity. The isolate also exhibited antagonistic activity against phytopathogenic fungi, i.e., Fusarium oxysporum, Fusarium graminiarum, and Rhizoctonia solani. The P-solubilization activity of B. subtilis KU21 was further elucidated via detection of glucose dehydrogenase (gdh) gene involved in the production of gluconic acid which is responsible for P-solubilization. Further, B. subtilis KU21 was evaluated for in vivo growth promotion studies of tomato (test crop) under net house conditions. A remarkable increase in seed germination, plant growth parameters, nutrient acquisition, and soil quality parameters (NPK) was observed in B. subtilis KU21-treated plants over untreated control. Hence, the proposed module could be recommended for sustainable tomato production in the Northwest Himalayan region without compromising soil health and fertility.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The productivity of crops must be increased in developing countries to feed the growing population, and this is frequently achieved using chemical fertilizers [1, 2]. However, long-term use of these fertilizers has been proven to reduce bacterial diversity in soil and can also have negative environmental consequences, such as phosphorus and nitrogen leaching into groundwater leading to increased soil and groundwater contamination [3]. This compelled the scientists all over the world to search for environment friendly and cost-effective solutions to mitigate this in a sustainable manner. The use of efficient, nutrient-mobilizing microorganisms also known as plant growth-promoting bacteria (PGPB) is one way to reduce the need of chemical fertilizers and simultaneously restoring the soil health and fertility in a sustainable manner [4, 5].
PGPB which are found around plant roots (rhizospheric) and/or inside plant tissues (endophytic) are well known to improve plant/soil health and plant productivity [4, 6,7,8]. Without causing any visible symptoms, bacterial endophytes colonize plant tissues and form beneficial relationships with their host plant via improving nutrient uptake through fixation of nitrogen, solubilization of minerals such as phosphorus and potassium, production, and regulation of phytohormones, i.e., IAA and ethylene, respectively. Also, a multitude of plant endophytes prevents host plants against phytopathogens through the production of hydrolytic enzymes, HCN, siderophores, and activation of plant defense system [9].
It is believed that all plants on the earth are associated with one or more endophytic bacteria [10]. Of these, medicinal plants choose endophytes in a specific manner based on factors such as the root exudate composition and the secondary metabolites produced by the plant [11]. As a result, the endophytic bacterial communities linked to medicinal plants become more diverse according to their needs for nutrition, the type of soil, and the environment they inhabit. Therefore, medicinal plants are the possible source for discovering new endophytic bacterial species, which may subsequently be utilized as bioinoculants for environmentally friendly farming methods.
In the last decade, a vast number of PGP bacterial genera have been isolated from several medicinal plants such as Pseudomonas, Pantoea, Bacillus and Inquilinus, Burkholderia, Citrobacter, Beijerinckia, Cedecea, Kosakonia, Lysobacter, Oxynema, and Pesudoxanthomonas [12,13,14] among which Bacillus sp. subtilis is predominant. Furthermore, numerous studies have also shown the potential of B. subtilis isolated form medicinal plants such as Duranta plumeri, Lonicera japonica, Clerodendrum colebrookianum Walp, and Pulicaria incisa to enhance plant growth and macronutrients uptake in crops such as cucumber [15], wheat [16], tomato [17], and Zea mays [18], respectively.
In the District Solan of Himachal Pradesh (India), several plants of medicinal importance are cultivated, including R. officinalis (rosemary), which is high in polyphenols and flavonoids [19]. Rosemary harbors diverse genera of endophytic bacteria with multifarious PGP traits [9]. Furthermore, application of these bacteria (Bacillus subtilis) led to improved growth metrics and soil nutrient acquisition in R. officinalis [20]. In addition to rosemary, numerous cash crops are also produced commercially in Solan region. In terms of area and production, tomatoes are the most extensively produced vegetable in this region. To boost output and productivity, farmers employ an excessive amount of chemical fertilizers. As described earlier, chemical fertilizers not only lower plant nutritive value and harm human health, but also endanger sustainable crop production. Keeping this in view, the objective of the current study was to determine the impact of B. subtilis KU21 on the growth performance of tomato as an important economic cash crop. As a result, the hypothesis behind the current investigation is that B. subtilis KU21 is not host specific and can demonstrate growth-promoting activities when linked with tomato. The study's framework focuses on the development of a B. subtilis KU21-based biofertilizer whose application can be expanded to non-specific host.
Materials and Methods
Bacterial Culture and Maintenance
Root endophytic PGP bacterium B. subtilis KU21 previously isolated from R. officinalis [9] was used in the present study. This strain was maintained on nutrient agar (NA) plates at 4 °C for further experimentation.
In Vitro PGP Traits
The in vitro PGP activities of the strain B. subtilis KU21 were evaluated. For qualitative P-solubilization assay, isolate was streaked on Pikovskaya’s agar and incubated for 72 h at 30 °C. A clear zone formed around the colony demonstrated the P-solubilization. Further quantitative assessment of P-solubilization was carried out in PVK broth supplemented with 5.0 g/L tricalcium phosphate (TCP) using vanadomolybdate method [21]. For IAA production, the isolate was grown in Luria–Bertani broth (LB broth) amended with 5 mM l-tryptophan, 0.065% sodium dodecyl sulfate, and 1% glycerol for 72 h at 30 °C under shaken conditions and was estimated colorimetrically using Salkowski reagent [22]. The ability of isolate to grow on DF (Dworkin and Foster) salt minimum medium with ACC (1-aminocyclopropane-1-carboxylate) as the only nitrogen source indicated ACC deaminase activity [23]. The nitrogen-fixing potential of isolate was first evaluated by its capability to grow on nitrogen-free medium (Jensen’s agar medium) [9] and then by acetylene reduction assay to check nitrogenase activity [24]. For nitrogenase activity, isolate was grown in duplicate vials for 5 days at 30 °C containing 15 mL of gas phase and were sealed with silicone rubber caps. The cultures were exposed to atmosphere of air containing 10% acetylene. After 2 and 5 days of exposure to acetylene, 1 mL samples were withdrawn from the culture vials and analyzed by gas chromatography with a hydrogen flame ionization detector. Reduction of acetylene to ethylene was determined by the peak height relative to a standard of 50 nmol of ethylene.
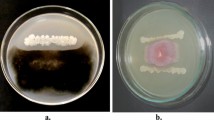
For qualitative siderophore production, isolate was spot inoculated on Chrome-Azurol S (CAS) agar plates and incubated for 72 h at 30 °C. Formation of yellow (hydroxamate), pink (catecholate), or white (carboxylate) zone around colony showed siderophore activity [25]. Percent siderophore unit (%SU) production was also determined in cell-free supernatant using spectrophotometric method as described by Schwyn and Neilands [25]. Lytic enzymes production, i.e., protease, amylase, and chitinase, was determined using spot inoculation on starch agar, skim milk agar, and minimal agar medium amended with 0.3% colloidal chitin, respectively, and incubation for 72 h at 30 °C. Formation of clear zone around colony indicated respective enzymes activity [9]. For HCN production, the isolate was streaked on NA plates amended with 4.4 g/L glycine. Whatman No.1 filter paper strips were soaked in 0.5% picric acid in 0.2% sodium carbonate and was placed inside the lid of the petriplates and incubated at 30 °C for 72 h. Change in color of filter paper from yellow to brown inferred HCN production [26]. Furthermore, antagonistic potential of isolate was also evaluated against F. oxysporum, F. graminiarum and R. solani using dual plate method [27]. Percent growth inhibition (%GI) was calculated according to Vincent [27].
Thin-Layer Chromatography (TLC) Based Analysis of IAA
TLC was used to assess in vitro IAA synthesis by B. subtilis KU21 using the method of Chaiharn and Lumyong [28] with some modifications. To extract crude IAA, strain B. subtilis KU21 was inoculated into nutrient broth enriched with filter sterilized l-tryptophan (0.2% v/v) and incubated for 72 h at 30 °C under shaken conditions. The culture suspension was then centrifuged for 30 min at 10,000 rpm to produce cell-free supernatant. 1 M NaOH was used to adjust the pH of the supernatant to 9.0. The IAA was extracted twice with ethyl acetate (100% v/v) at double the supernatant volume. The extracted ethyl acetate fraction's pH was reduced to 2.5 using acetic acid before being evaporated in a rotating evaporator at 40 °C. The dried extract was dissolved in 1 mL of methanol (analytical grade) and used for TLC analysis. Simultaneously, 50 ppm of IAA standard (Sigma-Aldrich) was prepared.
For TLC, 100 µL of the methanol extract and IAA standard was spotted on pre-coated silica gel TLC plate (F254, Merck, India) using capillary tube. The solvent front, which consisted of isopropanol and water (30:20 v/v), was allowed to flow for approximately 80% of the plate. Plates were sprayed with Salkowsky's reagent and watched for the emergence of pink dots in the dark. Rf (retardation factor) for IAA standard and crude extract was computed using the following formula:
Cloning of Glucose Dehydrogenase (gdh) Gene Responsible for Phosphate Solubilization from Genomic DNA of B. subtilis KU21
The complete gdh gene sequence of B. subtilis KU21 was amplified as described by Chauhan et al. [24] with some modifications. Briefly, the genomic DNA was isolated using conventional method [29] followed by PCR-mediated amplification using gene-specific primer set (gdhF: 5′-ATG TAT CCG GAT TTA AAA GGA-3′ and gdhR: 5′-TTA ACC GCG GCC TGC CTG GAA-3′). PCR mix of 25 µL was prepared with 50 ng of template DNA, 20 pmol of each primer, 0.2 mM dNTPs, and 1U Taq polymerase in 1× PCR buffer. The reaction was cycled 35 times as initial denaturation at 94 °C for 2 min, further denaturation for 45 s, annealing at 55 °C for 45 s and extension at 72 °C for 1 min followed by a final extension at 72 °C for 10 min. The PCR product was analyzed by gel electrophoresis on 2.0% (w/v) agarose gel and purified using a gel extraction kit (RBCs Real Genomics, New Taipei City, Taiwan). The purified fragment was ligated into pGEM-T cloning vector before transformation into chemically competent cells of Escherichia coli strain DH5α. Transformants were grown at 37 °C on Luria–Bertani (LB) agar containing ampicillin (100 µg/mL), IPTG (50 mM), and X-gal (80 µg/mL) for blue/white screening of recombinant colonies. Transformed E. coli strains were confirmed for the presence of insert of gdh gene using respective designed primers followed by sequencing (GeNei™ Laboratories, Bengaluru, India). The obtained sequence was aligned with corresponding sequences of gdh gene of Bacillus sp. from the database using BLASTn program [30]. Multiple alignment with gdh gene sequences of genus Bacillus was implemented using CLUSTAL W. A neighbor-joining phylogenetic tree was constructed with other gdh gene sequences of related taxa retrieved from GenBank using MEGA X software. The sequence was submitted to NCBI GenBank database (accession number: MN166090).
In Vivo Plant Growth Stimulation Studies
To assess the potential of B. subtilis KU21 for enhancing plant growth, a pot experiment was conducted using tomato (Solanum lycopersicum cv. Solan Lalima) as test crop during mid-summer season for a period of two months (June–August 2020) at net house of Department of Basic Sciences, College of Forestry, Dr YS Parmar University of Horticulture and Forestry, Solan, (Himachal Pradesh), India.
According to the United States Department of Agriculture Soil Taxonomy, the soil utilized in the pot experiment belongs to the Entisols order. The potting mixture was made by combining sand, soil, and farmyard manure (FYM) in a 1:2:1 ratio. The above combination was then tyndallized [9]. The pH of the potting mixture was tested in a 1:2.5 (soil:water) suspension, and the electrical conductivity (E.C.) of the supernatant liquid was measured and expressed in dSm−1 [31]. Furthermore, organic carbon (O.C.) was evaluated using the chromic acid titration method developed by Walkley and Black [32]. The available N, P, and K levels of soil were assessed using established protocols [33].
The inoculum of B. subtilis KU21 was prepared by inoculating into nutrient broth and incubated at 30 °C for overnight under shaken conditions. Pellet of bacterial cells was then obtained by centrifugation at 3000 rpm for 10 min. The pellet was subsequently washed with autoclaved distilled water and the cell suspension was finally adjusted to 1 × 108 CFU/mL by dilution. The suspension was employed as a seed treatment inoculum [24]. Tomato seeds cv. Solan Lalima were obtained from the Department of Vegetable Science, Dr YS Parmar University of Horticulture and Forestry, Solan, (Himachal Pradesh), India, and subjected to surface sterilization by sodium hypochlorite (2%) for 2 min, followed by washing with autoclaved distilled water. The sterilized seeds were immersed in 20 mL (for 2 h) of prepared bacterial inoculum (108 CFU/mL) supplemented with carboxymethyl cellulose (CMC) (0.2%) to facilitate adherence of bacterial cells to seeds. Tomato seeds immersed in sterilized distilled water supplemented with CMC was kept as control. Twenty treated seeds per treatment (7 replications of each treatment) were sown in each pot (containing 4 kg potting mixture). After 3–4 days of seedling emergence, thinning was done and three plants per pot were maintained. Booster doses of liquid bacterial culture of the same cell density was applied @ 20 mL/plant near the root zone with 15 days interval following planting (2 times).
At the termination of experiment, observations on physical parameters such as stem height and root length were measured from plant’s tip to the end of the stem and from collar area to the root end, respectively, using a foot ruler. Samples were dried in a hot air oven (LFAS, digital model) at 40 °C until a consistent weight was reached, and the dry weight (biomass) of the root and stem was calculated using a weighing balance machine (ATOM series electronic balance). Percent seed germination was recorded 3 days after sowing and was calculated as [34]:
The vigor index was also determined using the following formula [35]:
Oven-dried plant samples were ground and sieved for the estimation of macronutrients (NPK). The total concentration of N in plant samples was determined using micro-Kjeldhal’s method [35]. Plant samples were digested in a diacid mixture of HNO3:HClO4 (4:1) for P and K analysis [31]. P concentration was tested in the digested sample [31]. K in the digest was analyzed using the flame photometer (Biogen, Microcontroller Flame Photometer) [36].
To examine root colonizing potential of endophytic strain B. subtilis KU21, isolation of culturable endophytic bacteria was carried out after termination of the experiment. For this, root samples were collected from each inoculated and uninoculated treatment and standard serial dilution spread plate technique was done on NA plates [9]. Recovered bacteria from treated and control treatments were compared (for presence/absence of inoculated strain B. subtilis KU21) by morpho-biochemically characterizing them as per Bergey’s Manual of Determinative Bacteriology [9]. Further identification of isolates was done at molecular levels using 16S rDNA sequence analysis as described by Sharma et al. [9]. Briefly, genomic DNA was obtained using the conventional method [29] and then PCR-mediated amplification was carried out [9] using a pair of universal primers (16SF: 5′-AGAGTTTGATCCTGGCTCAG-3′ and 16SR: 5′-AAGGAGGTGATCCAGCCGCA-3′). Using 50 ng of template DNA, 20 pmol of each primer, 0.2 mM dNTPs, and 1 U of Taq polymerase in 1× PCR buffer, a 25 µL PCR mix was created. 35 cycles of the reaction were performed, each consisting of 30 s of denaturation at 94 °C, 30 s of annealing at 55 °C, and 1 min and 30 s of extension at 72 °C. The last extension was carried out for 10 min at 72 °C. Agarose (1.2%) gel electrophoresis was performed to analyze obtained PCR product. A gel extraction kit (RBCs Real Genomics, New Taipei City, Taiwan) was used to extract the amplified fragment of about 1400 bp, which was then purified and sequenced (GeNei™ Laboratories in Bengaluru, India). Using a BLASTn search, phylogenetically related bacteria were aligned based on 16S rDNA sequences [30]. Utilizing MEGA X software, a neighbor-joining phylogenetic tree was created using 16S rDNA sequences from related taxa that were obtained from the GenBank database.
Statistical Analysis
All experiments were carried out within a statistical framework with seven number of replications and appropriate controls for both in vitro and in vivo studies. For pot trial, t test was performed using SPSS version 16 (SPSS Inc., Chicago, IL, USA) and Microsoft Excel 7.0. (Microsoft, Redmond, WA, USA).
Results
In Vitro PGP Traits
The selected strain B. subtilis KU21 demonstrated multiple PGP traits. The strain tested positive for P-solubilization (375 µg/mL), synthesis of siderophore (301.48%SU), HCN, and cell wall degrading enzymes such as protease, amylase, and chitinase synthesis. Strain also synthesized higher amount of IAA (52 µg/mL). The TLC profile revealed that the extract of ethyl acetate from B. subtilis KU21 showed a clear pink spot at the Rf value 0.71 which corresponds to the standard reference of Rf value 0.73, hence confirming IAA production (Fig. S1). The nitrogen-fixing ability was initially examined using bacterial growth on nitrogen-free agar medium and was finally confirmed via nitrogenase activity of reducing acetylene to 405 nmol ethylene mL−1 h−1. ACC deaminase activity was confirmed by the ability of strain B. subtilis KU21 to thrive on DF salt media (Table 1). The strain also exhibited broad-spectrum antifungal activity against F. oxysporum (60.00%GI), F. graminiarum (66.66%GI), and R. solani (62.00%GI) (Table 1).
Cloning of gdh Gene
Phosphate-solubilizing gdh gene fragment from genomic DNA of B. subtilis KU21 was successfully amplified and cloned in E. coli DH5α cells. Sequencing of cloned gdh gene concluded that it was 786 bp long encoding 261 amino acid proteins. Following sequence was analyzed with top seven hits as obtained in BLASTn analysis. Phylogenetic analysis revealed that gdh gene of B. subtilis KU21 showed maximum homology with gdh gene of Aneurinibacillus aneurinilyticus CKMV1 followed by B. subtilis strain CGMCC (Fig. 1).
In Vivo PGP Studies
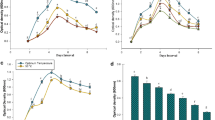
A pot experiment was carried out in net house to evaluate the growth response of tomato seeds bacterized with strain B. subtilis KU21. Overall growth metrics of tomato seedlings treated with strain B. subtilis KU21 were significantly (P ≤ 0.05) improved in comparison with uninoculated control (Table 2). Over the untreated control, the metrics increased by 4.67% for seed germination, 31.93% and 42.22% for stem height and shoot biomass, 38.00% and 48.05% for root length and biomass, and 44.56% for vigor index.
The NPK content of tomato seedlings was similarly considerably impacted by strain B. subtilis KU21 inoculation over untreated seedlings (Fig. 2). The N, P, and K contents of strain B. subtilis KU21 bacterized seedlings increased by 14.51%, 25.80%, and 18.50%, respectively, as compared to the uninoculated control.
The initial characteristics of potting mixture used in the present study were as follows: pH 7.18, E.C. 0.70 dSm−1, O.C. 1.08%, and available N, P, and K contents of 242.18, 23.08, and 310.23 kg/ha, respectively. After strain B. subtilis KU21 bacterization, the pH, E.C., and O.C. of the soil did not alter (data not shown). Treatment B. subtilis strain KU21, on the other hand, was shown to considerably increase accessible N (13.14%), P (20.41%), and K (11.75%) content as compared to the untreated control (Fig. 3).
To determine the colonization potential of inoculated strain within plant roots, isolation and identification of root endophytic bacteria was done from strain inoculated and uninoculated seedlings at the end of pot trial. The control treatment yielded 3.8 × 103 cfu/g root endophytic bacteria; however, no identical B. subtilis KU21 inoculated strain was found (Table 2). Furthermore, the colony morphology of the root endophytes, i.e., 2.3 × 103 cfu/g of the total isolates (5.5 × 103 cfu/g) from inoculated seedlings was identical with the B. subtilis KU21. The isolated endophytes with similar colony morphology were initially identified biochemically, followed by 16S rDNA sequencing which also showed similar pattern as that of inoculated strain B. subtilis KU21 (Table S1, Fig. S2).
Discussion
In our earlier research, B. subtilis KU21 considerably improved R. officinalis growth and the availability of soil nutrients [4, 13]. Furthermore, the current work was carried out to investigate the non-specific host PGP capability of B. subtilis KU21 utilising tomato as a test crop. Our study demonstrates the enhancement in plant growth parameters and soil nutrient uptake by tomato being inoculated with endophytic strain B. subtilis KU21. The PGP potential of B. subtilis has been widely documented in several crops without endangering the health of the soil, plants, or people [37,38,39].
Since direct (P-solubilization, nitrogen fixation, IAA, and siderophore production) and indirect (antimicrobial activities against phytopathogens via hydrolytic enzymes and ammonia production) mechanisms of plant growth promotion are well documented for endophytic bacteria [9, 18, 40], the isolate B. subtilis KU21 was first assessed for above mentioned PGP traits in vitro. Strain B. subtilis KU21 solubilized P and formed considerably high amount of IAA, which was validated using the TLC technique. Nitrogen fixation in B. subtilis KU21 was also detected in an acetylene reduction test for nitrogenase activity. The importance of P-solubilization, IAA production, and nitrogen fixation by B. subtilis strains derived from various medicinal plants in exhibiting several PGP properties and boosting plant growth has been well established [9, 37, 41].
The findings in the present study indicate that B. subtilis KU21 solubilized P which might be mediated by metabolic production of organic acids. The cloning and sequencing of gdh gene in B. subtilis KU21 as reported in the present study, therefore, hint toward the acid production theory involving the gluconic acid biosynthesis by the glucose dehydrogenase enzyme. Mobilization of insoluble phosphates has been reported by researchers to be because of the production of gluconic acid, which results from the extracellular oxidation of glucose via the quinoprotein glucose dehydrogenase [42,43,44,45,46]. Polymerase chain reaction method confirmed the presence of gdh gene in strain B. subtilis KU21. Detection of gdh gene has also been reported earlier in the genus Bacillus by Mehta et al. [47].
Besides facilitating PGP properties, isolate B. subtilis KU21 was characterized as potential biocontrol agent against some agriculturally important phytopathogenic fungi, i.e., F. oxysporum, F. graminiarum, and R. solani. The biocontrol potential of B. subtilis KU21 might be associated with the production of secondary metabolites, i.e., antibiotics, siderophore, HCN, and lytic enzymes [9, 20]. Earlier studies of Sharma et al. [38] and Kumar et al. [39] had also reported antifungal properties of B. subtilis strains isolated from medicinal plants Podophyllum hexandrum and Hippophae rhamnoides L., respectively.
To assess in vitro PGP traits of strain B. subtilis KU21, in planta evaluation was carried out in tomato. The results indicated that the application of strain B. subtilis KU21 considerably enhanced the percent germination as well as growth of tomato seedlings in terms of plant height, root, and stem biomass and vigor index to varying degrees in comparison with untreated control. This enhancement could be ascribed to biopriming of seeds with liquid suspension of B. subtilis KU21, as biopriming creates ideal environment for bacterial inoculation and colonization [48, 49], which ultimately increase the amount of accessible P by producing organic acids and dissolving more insoluble P in the soil. Another possibility is the production of phytohormones such as IAA and ACC deaminase. ACC deaminase reduces ethylene levels in roots, resulting in increased root length, vigor, and surface area [50, 51]. Several studies have reported the promising effects of biopriming with B. subtilis in several crops such as barley [52], tomato [38, 39], finger millet [53], and oregano [54].
In terms of macronutrient content, seedlings treated with B. subtilis KU21 had significantly more NPK than untreated control plants. This is because of absorbing and utilizing more nutrients from soil because of biopriming which increased root surface area and root hair formation [4]. In addition, B. subtilis KU21 have the potential to solubilize mineral nutrients, increasing the amount of soil accessible NPK levels and permitting the availability of those nutrients to plants. The present results are corroborated with Sood et al. [55], who also reported the potential of B. subtilis of increasing soil available macronutrients (NPK) and enhancing their uptake in wheat.
Conclusion
In essence, our results unequivocally proved our hypothesis correct that strain B. subtilis KU21 build beneficial association with non-native host and stimulated plant growth. Thus, strain B. subtilis KU21 could be used to build an environmentally acceptable and cost-effective system for biofertilization of tomato. Field trials are also being conducted to confirm the efficacy of B. subtilis KU21 in increasing crop productivity.
References
Sun R, Zhang X, Guo X, Wang D, Chu H (2015) Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol Biochem 88:9–18
Zhou J, Jiang X, Zhou B, Zhao B, Ma M, Guan D, Li J, Chen S, Cao F, Shen D et al (2016) Thirty-four years of nitrogen fertilization decreases fungal diversity and alters fungal community composition in black soil in northeast China. Soil Biol Biochem 95:135–143
Rafi MM, Krishnaveni MS, Charyulu PBBN (2019) Phosphate-solubilizing microorganisms and their emerging role in sustainable agriculture. In: Buddolla V (ed) Recent developments in applied microbiology and biochemistry. Academic Press, Cambridge, MA, USA, pp 223–233
De La T, Neyser VR, Clara IR, Martha R, Carlos A, Federico AG, Hector P, Reiner R (2016) Effect of plant growth-promoting bacteria on the growth and fructan production of Agave americana L. Braz J Microbiol 47:587–596
Gamez R, Cardinale M, Montes M, Ramirez S, Schnell S, Rodriguez F (2019) Screening, plant growth promotion and root colonization pattern of two rhizobacteria (Pseudomonas fluorescens Ps006 and Bacillus amyloliquefaciens Bs006) on banana cv. Williams (Musa acuminata Colla). Microbiol Res 220:12–20
Abdelaal KAA, Tawfik SF (2015) Response of sugar beet plant (Beta vulgaris L.) to mineral nitrogen fertilization and bio-fertilizers. Int J Curr Microbiol Appl Sci 4:677–688
Abdelaal KAA (2015) Pivotal role of bio and mineral fertilizer combinations on morphological, anatomical and yield characters of sugar beet plant (Beta vulgaris L.). Middle East J Agric Res 4:717–734
Abdelaal KAA, Badawy SA, Abdel Aziz RM, Neana SMM (2015) Effect of mineral nitrogen levels and PGPR on morphophysiological characters of three sweet sorghum varieties (Sorghum bicolor L. Moench). J Plant Prod 6:189–203
Sharma M, Sood G, Chauhan A (2021) Bioprospecting beneficial endophytic bacterial communities associated with Rosmarinus officinalis for sustaining plant health and productivity. World J Microbiol Biotechnol 37:135. https://doi.org/10.1007/s11274-021-03101-7
Cun H, Munir S, He P et al (2022) Diversity of root endophytic bacteria from maize seedling involved in biocontrol and plant growth promotion. Egypt J Biol Pest Control 32:129. https://doi.org/10.1186/s41938-022-00622-7
Maggini V, Miceli E, Fagorzi C, Maida I, Fondi M, Perrin E (2018) Antagonism and antibiotic resistance drive a species-specifc plant microbiota differentiation in Echinacea spp. FEMS Microbiol Ecol. https://doi.org/10.1093/femsec/fy118
Angelique R, Henry DN, Nikos K, Katerina G, Eleni M, Varela AA, Carolin S, Vassilios PP et al (2021) Endophytic bacteria from the roots of the medicinal plant Alkanna tinctoria Tausch (Boraginaceae): exploration of plant growth promoting properties and potential role in the production of plant secondary metabolites. Front Microbiol. https://doi.org/10.3389/fmicb.2021.633488
Hari SB, Briste PS, Sumi AA et al (2023) Endophytic bacteria isolated from medicinal plants induce plant growth promotion and southern blight disease suppression in tomato. J Plant Pathol 105:197–210. https://doi.org/10.1007/s42161-022-01248-2
Abdel-Hamid SM, Fouda A, El-Ela AHK, El-Ghamry, et al (2021) Plant growth-promoting properties of bacterial endophytes isolated from roots of Thymus vulgaris L. and investigate their role as biofertilizers to enhance the essential oil contents. Biomol Concepts 12:175–196. https://doi.org/10.1515/bmc-2021-0019
Ansary WR, Prince FRK, Haque E, Sultana F, West HM et al (2018) Endophytic Bacillus spp. from medicinal plants inhibit mycelial growth of Sclerotinia sclerotiorum and promote plant growth. Z Naturforsch C. https://doi.org/10.1515/znc-2018-0002
Zhao L, Xu Y, Lai XH, Shan C, Deng Z, Ji Y (2015) Screening and characterization of endophytic Bacillus and Paenibacillus strains from medicinal plant Lonicera japonica for use as potential plant growth promoters. Braz J Microbiol. https://doi.org/10.1590/S1517-838246420140024
Passari AK, Mishra VK, Leo VV, Gupta VK, Singh BP (2016) Phytohormone production endowed with antagonistic potential and plant growth-promoting abilities of culturable endophytic bacteria isolated from Clerodendrum colebrookianum Walp. Microbiol Res 193:57–73
Fouda A, Eid AM, Elsaied A et al (2021) Plant growth-promoting endophytic bacterial community inhabiting the leaves of Pulicaria incisa (lam.) dc inherent to arid regions. Plants 10(1):76. https://doi.org/10.3390/plants10010076
Bourhia M, Laasri FE, Aourik H et al (2019) Antioxidant and antiproliferative activities of bioactive compounds contained in Rosmarinus oficinalis used in the Mediterranean diet. Evid Based Complement Altern Med. https://doi.org/10.1155/2019/7623830
Sharma M, Sood G, Chauhan A (2023) Novel synergism of Cedecea lapagei KU14 and Bacillus subtilis KU21 for sustainable productivity of Rosmarinus officinalis in Northwest Himalayas. Rhizosphere. https://doi.org/10.1016/j.rhisph.2023.100683
Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. J Microbiol 7:362–370
Gordon SA, Palleg LG (1957) Quantitative measurement of IAA. Plant Physiol 10:37–38
Dworkin M, Foster JW (1958) Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol 75(5):592–603
Chauhan A, Guleria S, Balgir P et al (2017) Tricalcium phosphate solubilization and nitrogen fixation by newly isolated Aneurinibacillus aneurinilyticus CKMV1 from rhizosphere of Valeriana jatamansi and its growth promotional effect. Braz J Microbiol 48:294–304
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Baker AW, Schippers S (1987) Microbial cyanide production in the rhizosphere about potato yield reduction and Pseudomonas sp. mediated plant growth stimulation. Soil Biol Biochem 19:451–457
Vincent JM (1947) Distortion of fungal hyphae in the presence of certain inhibitors. Nature 150:850
Chaiharn M, Lumyong, (2011) Screening and optimization of indole-3-acetic acid production and phosphate solubilisation from rhizobacteria aimed at improving plant growth. Curr Microbiol 62:173–181
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, New York
Altschul SF, Thomas LM, Alejandro AS, Jinghui Z, Zheng Z, Webb M, David JL (1997) Gapped BLAST and PSIBLAST: a new generation of protein database search programmes. Nucleic Acids Res 25:3389–3402
Jackson ML (1973) Soil chemical analysis. Prentice Hall of India Pvt. Ltd., New Delhi, India, pp 49–95
Walkley A, Black TA (1934) An estimation of soil organic matter and purposed modification of chromic acid titration method. Soil Sci 37:29–38
Tandon HLS (2009) Methods of analysis of soil, plant, water, fertilizers, and organic manures. Fertiliser Development and Consultation Organisation, New Delhi
Babi AA, Anderson JD (1973) Vigour determination in soybean seed by multiple criteria. Crop Sci 13:630–633
Helrich K (1990) Official and tentative methods of analysis. Association of official analytical chemists. William Star Wetglad, Washington
Jackson ML (1967) Soil chemical analysis. Oxford and IBH Publishing House, Bombay
de O Nunes PS, de Medeiros FHV, de Oliveira TS, de Almeida Zago JR, Bettiol W (2023) Bacillus subtilis and Bacillus licheniformis promote tomato growth. Braz J Microbiol 54:397–406. https://doi.org/10.1007/s42770-022-00874-3
Sharma R, Sharma P, Chauhan A et al (2017) Plant growth promoting activities of rhizobacteria isolated from Podophyllum hexandrum growing in North-West regions of the Himalaya. Proc Natl Acad Sci India Sect B Biol Sci 87:1443–1457. https://doi.org/10.1007/s40011-016-0722-2
Kumar A, Guleria S, Mehta P et al (2015) Plant growth-promoting traits of phosphate solubilizing bacteria isolated from Hippophae rhamnoides L. (Sea-buckthorn) growing in cold desert Trans-Himalayan Lahul and Spiti regions of India. Acta Physiol Plant 37:48. https://doi.org/10.1007/s11738-015-1793-z
Ahmed EAS, Hassan EAE-T, EI-Tobgy KMK, Ramadan EM (2019) Characterization of endophytic bacteria associated with some medicinal plants. Arab Univ J Agric Sci Ain Shams Univ Cairo Egypt 27:2513–2526
Samaras A, Roumeliotis E, Ntasiou P, Karaoglanidis G (2021) Bacillus subtilis MBI600 promotes growth of tomato plants and induces systemic resistance contributing to the control of soilborne pathogens. Plants 10:1113. https://doi.org/10.3390/plants10061113
Rodriguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Sashidhar B, Podile AR (2010) Mineral phosphate solubilization by rhizosphere bacteria and scope for manipulation of the direct oxidation pathway involving glucose dehydrogenase. J Appl Microbiol 109:1–12
Patel DK, Archana G, Kumar GN (2008) Variation in the nature of organic acid secretion and mineral phosphate solubilization by Citrobacter sp. DHRSS in the presence of different sugars. Curr Microbiol 56(2):168–174
Choi O, Kim J, Kim JG, Jeong Y, Moon JS, Park CS, Hwang I (2008) Pyrroloquinoline quinone is a plant growth promotion factor produced by Pseudomonas fluorescens B16. Plant Physiol 146:657–668
Park KH, Lee CY, Son HJ (2009) Mechanism of insoluble phosphate solubilization by Pseudomonas fluorescens RAF15 isolated from ginseng rhizosphere and its plant growth-promoting activities. Lett Appl Microbiol 49(2):222–228
Mehta P, Walia A, Chauhan A, Shirkot CK (2013) Plant growth promoting traits of phosphate-solubilizing rhizobacteria isolated from apple trees in trans Himalayan region of Himachal Pradesh. Arch Microbiol 195(5):357–369
Moeinzadeh A, Sharif-Zadeh F, Ahmadzadeh M et al (2010) Biopriming of sunflower (Helianthus annuus L.) seed with Pseudomonas fluorescens for improvement of seed invigoration and seedling growth. Aust J Crop Sci 4:564
Mahmood A, Turgay OC, Farooq M, Hayat R (2016) Seed biopriming with plant growth promoting rhizobacteria: a review. FEMS Microbiol Ecol 92:1–14
Li J, Ovakim DH, Charles TC et al (2000) An ACC-Deaminase minus mutant of Enterobacter cloacae UW4 no longer promotes root elongation. Curr Microbiol 41:101–105
Penrose DM, Glick BR (2001) Levels of 1-aminocyclopropane-1-carboxylic acid (ACC) in exudates and extracts of canola seeds treated with plant growth-promoting bacteria. Can J Microbiol 47:368–372
Mirshekari B, Hokmalipour S, Sharifi RS et al (2012) Effect of seed biopriming with plant growth promoting rhizobacteria (PGPR) on yield and dry matter accumulation of spring barley (Hordeum vulgare L.) at various levels of nitrogen and phosphorus fertilizers. J Food Agric Environ 10:314–320
Chaudhary R, Kumar V, Gupta S, Naik B, Prasad R, Mishra S, Saris PEJ, Kumar V (2023) Finger millet (Eleusine coracana) plant–endophyte dynamics: plant growth, nutrient uptake, and zinc biofortification. Microorganisms 11:973. https://doi.org/10.3390/microorganisms11040973
Çakmakçi R, Haliloglu K, Turkoglu A, Ozkan G, Kutlu M, Varmazyari A, Molnar Z, Jamshidi B, Pour-Aboughadareh A, Bocianowski J (2023) Effect of different plant growth-promoting rhizobacteria on biological soil properties, growth, yield, and quality of oregano (Origanum onites L.). Agronomy 13(10):2511. https://doi.org/10.3390/agronomy13102511
Sood G, Kaushal R, Panwar G, Dhiman M (2018) Effect of indigenous plant growth-promoting rhizobacteria on wheat (Triticum aestivum L.) productivity and soil nutrients. Commun Soil Sci Plant Anal. https://doi.org/10.1080/00103624.2018.1556282
Acknowledgements
The authors are thankful to the Indian Council of Agricultural Research, New Delhi, India, for providing financial assistance through the All-India Network Project on Soil Biodiversity and Biofertilizer.
Author information
Authors and Affiliations
Contributions
AC designed the study. MS conducted the experiments. MS and GS drafted the manuscript. AC contributed to the revision of the manuscript. All authors read and approved the final draft of the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors report there are no competing interests to declare.
Ethics Approval and Consent to Participate
Not applicable as the study not applied on human or animals study. The article does not include any studies on human participants or animals conducted by any of the authors.
Consent for Publication
All authors agree for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, M., Sood, G. & Chauhan, A. Assessment of Plant Growth Promotion Potential of Endophytic Bacterium B. subtilis KU21 Isolated from Rosmarinus officinalis. Curr Microbiol 81, 207 (2024). https://doi.org/10.1007/s00284-024-03734-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-024-03734-5