Abstract
Bacillus spp. are widely marketed and used in agricultural systems as antagonists to various phytopathogens, but it can also benefit the plant as plant growth promoters. Therefore, the longer presence of the bacterium in the rhizosphere would result in a prolonged growth-promoting benefit, but little is yet known about its persistence in the rhizosphere after seed coating. The objectives of this study were to evaluate the tomato growth promotion mediated by Bacillus licheniformis FMCH001 and Bacillus subtilis FMCH002 and the survival rate of these bacteria both in shoots and in the rhizosphere. The Bacillus strains used throughout this study were obtained from Quartzo® produced by Chr. Hansen. The application of a mixture of B. subtilis and B. licheniformis (Quartzo®) at concentrations 1 × 108, 1 × 109, and 1 × 1010 CFU mL−1, as well as the application of B. subtilis and B. licheniformis individually at concentration 1 × 108 CFU mL−1, increased fresh and dry masses of shoot and root system, volume of root system, and length of roots of tomato plants when compared to control. Both Bacillus strains produced IAA after 48 h of in vitro. Bacillus colonies obtained from plant sap were morphologically similar to colonies of B. subtilis and B. licheniformis strains and were detected in inoculated on plants and not detected in control ones. A similar pattern was obtained through DNA-based detection (qPCR). Therefore, B. subtilis and B. licheniformis were able to produce auxin, promote tomato growth, and colonize and persist in the rhizosphere.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant growth-promoting rhizobacteria (PGPR) are beneficial bacteria that promote plant growth, protect crops against plant pathogens, and improve soil health [1,2,3,4,5]. The use of PGPR is a sustainable tool to mitigate the dependence on chemical fungicides and fertilizers and/or increase agricultural production [6].
Among the PGPR, Bacilli are recognized as the most important species [7,8,9,10], being widely marketed and used in agricultural systems as biofertilizers and/or antagonists to various phytopathogens [11, 12]. Bacillus spp. produce spores which are resistant to several environmental conditions [13, 14]. Besides, effectively colonizing roots, they are also efficient biocontrol agents being recognized by the ability to promote plant growth [15,16,17,18,19,20,21,22]. Bacillus-mediated plant growth promotion can occur through direct and indirect mechanisms [23,24,25]. Indirectly, Bacillus spp. minimize the problems that phytopathogens cause to plant growth, consequently increasing plant health [23, 26]. Directly, Bacillus spp. produce siderophore and phytohormones (indole-3-acetic acid, cytokinin, gibberellic acid), solubilize phosphorus and other nutrients that stimulate plant growth, and increase root volume [20, 27,28,29,30,31,32,33,34]. The change in the architecture of the root system also influences the plant’s ability to exploit the soil by improving water and nutrient uptake [35].
Several species of Bacillus have been reported in the promotion of plant growth, such as B. velezensis AP-3 and Bacillus spp. in tomato [19]; B. subtilis in tomato [36] and additionally in okra and spinach [37]; B. velezensis AP-3, S2547, and S2545 in cotton [20]; B. simplex in pea [38]; B. amyloliquefaciens in Lemna minor [27]; B. licheniformis in chrysanthemum [39], tomato [40], and maize [11]; and B. cenocepacia in tomato [40]. In many of these publications, it was observed that the Bacillus isolates produced phytohormones and siderophores and/or solubilized phosphorus.
Several Bacillus strains are applied in biological control and in the promotion of plant growth [41]. However, the success of these microorganisms in an agricultural application depends on the colonization efficiency and persistence in the rhizosphere of plants, which are considered one of the great challenges for biological control and one of the main reasons for instability in the activity of bacterial inoculants in the soil [42]. Rhizosphere colonization is crucial for PGPR and plant interactions [43].
The objectives of this study were to evaluate the tomato growth promotion mediated by Bacillus licheniformis FMCH001 and Bacillus subtilis FMCH002 and the survival rate of these bacteria both in shoots and in the rhizosphere.
Materials and methods
The assays were conducted at Embrapa Meio Ambiente, Laboratório de Microbiologia Ambiental “Raquel Ghini,” located in Jaguariúna, SP, Brazil.
Microorganisms
Bacillus licheniformis FMCH001 and Bacillus subtilis FMCH002 strains used throughout this study were obtained from Quartzo® produced by Chr. Hansen (Valinhos, SP, Brazil) and commercialized by FMC Química do Brasil Ltda. (Campinas, SP, Brazil). The isolates and the product formulated with the mixtures of the two Bacillus isolates were provided by Chr. Hansen. The isolates were applied separately to demonstrate the potential of each isolate to produce indole-acetic acid and promote tomato growth.
Indole-acetic acid production
To evaluate the production of indole-acetic acid (IAA), the isolates of B. subtilis and B. licheniformis were multiplied in 250-mL flasks containing 100 mL of Czapek medium (30 g of sucrose, 2 g of NaNO3, 1 g of K2HPO4, 0.5 g of KCl, 0.5 g of MgSO4, 0.02 g of FeSO4, and 1000 mL of distilled water) supplemented with 0.5 g of L-tryptophan L−1 medium. The flasks were incubated on a shaker table (TE-1401, Technal®, Piracicaba, SP, Brazil) at 150 rpm and 25 ± 2 °C for 2 days. Subsequently, the cultures were centrifuged at 7000 rpm for 10 min, the supernatants were collected, and the IAA concentration was determined using the Salkowski reagent (1 mL of 0.5 mol L−1 FeCl3.6H2O in 50 mL of 35% HClO4), according to Gordon and Weber (1951). The reaction was established by adding 100 μL of culture supernatant or Czapek medium (control), and 100 μL of the Salkowski reagent. Subsequently, the mixture was kept at room temperature for 30 min, and the absorbance was determined in a spectrophotometer at 530 nm. The concentration of IAA in the supernatant was calculated based on a standard curve prepared with IAA (Sigma-Aldrich®, St. Louis, Missouri, USA) (98% purity) at concentrations of 5, 10, 20, 50, and 100 μg mL−1. The mean of the control readings was discounted from the mean of the isolate’s readings. The trial was conducted in a completely randomized design with three replications and repeated to confirm the data.
Mixture of B. subtilis and B. licheniformis on seed germination and emergence of tomato seedlings
Tomato seeds cultivar Santa Clara® were superficially disinfected in alcohol (70%) and in sodium hypochlorite (2.5%) for 2 min each. Then, they were washed three times in sterile distilled water and dried on sterile filter paper in an aseptic chamber. After drying, the seeds were placed in a 250-mL Erlenmeyer flask containing 100 mL of the suspension of Quartzo® (B. subtilis and B. licheniformis) at concentrations of 0, 1 × 108, 1 × 109, and 1 × 1010 CFU mL−1. After agitation at 100 rpm for 1 h, seeds were dried on filter paper for 1 h. Sowing was carried out in plastic boxes (Gerbox®), on a three-sheet germitest paper (Germiagro, Ribeirão Preto, SP), moistened with distilled water in an amount equivalent to 2.5 times the weight of the dry paper. The boxes were kept in a growth chamber at 25 ± 2 °C for 8 days. Seed germination was evaluated 5 and 8 days after the test was set up, and the results were expressed as percentage of normal seedlings [44]. The radicle protrusion was evaluated 3 days after sowing. The experiment was carried out in a completely randomized design with four replications, each replication represented by a plastic box with 50 seeds. Data were analyzed by regression at 5% probability. The test was repeated twice.
Bacillus subtilis and B. licheniformis in promoting the growth of tomato plants grown on rhizotrons
Tomato seedlings (cultivar Santa Clara®, Bragança Paulista, SP) were produced in multicell (200 cells) growing trays containing a commercial substrate (Tropstrato HT Hortaliças®). The seeds were sown without surface disinfection. Twenty-day-old seedlings were transferred to rhizotrons (built in 17.5 diameter PVC tubes, 100 cm long, cut longitudinally in half), containing a mixture of soil and the commercial substrate Terra Nostra® (Tatui, SP) in the proportion of 3:1 (v/v). The soil was collected in the experimental area at Embrapa Meio Ambiente, presenting the following chemical and physical attributes analyzed at 0–20 cm depth: pH in H2O = 4.3; OM = 32.3 g Kg−1; P = 9.36 mg dm−3; Ca = 3.09 cmolc dm−3; Mg = 1.48 cmolc dm−3; K = 128.55 mg dm−3; SB = 4.95 cmolc dm−3; H + Al = 6.10 cmolc dm−3; t = 4.99 cmolc dm−3; V% = 44.54.
The commercial product Quartzo® (B. subtilis and B. licheniformis) was applied at concentrations of 1 × 108, 1 × 109, and 1 × 1010 CFU mL−1. B. subtilis and B. licheniformis isolates were applied separately, each at a concentration of 1 × 108 CFU mL−1. Besides the application of 2.5 mL of Bacillus suspension on the seedling substrate, 10 mL of Bacillus were applied on container media immediately after transplanting and after 10 and 20 days. The individual isolates of B. subtilis and B. licheniformis were multiplied in a 125-mL Erlenmeyer flask containing 50 mL of GPL medium (10 g glucose; 10 g peptone; 5 g yeast extract; 3 g NaCl; 1 g of KH2PO4; 0.5 g of Mg SO4.7H2O; 1000 ml of distilled water, pH 6.0), under constant stirring at 150 rpm at 28 ± 2 °C. After 48 h, the content of the Erlenmeyer flask was transferred to a 50-mL Falcon tube and centrifuged for 10 min at 8000 rpm. Subsequently, the supernatant was discarded, and the pellet was resuspended in 0.85% (m/v) NaCl solution. Afterwards, the concentration was adjusted in a spectrophotometer (Shimadzu®; UV-1601 PC) OD540 = 2.250 nm, which corresponds to 1 × 108 CFU mL−1. This concentration was then confirmed by the serial dilution method.
The assays were conducted in a completely randomized design with five replicates and six treatments. The assay was performed twice, the first between November and December 2019 and the second between May and June 2021. The first and second tests were evaluated 32 and 36 days after transplanting the seedlings, respectively. Plant height, stem base diameter (mm), shoot and root system fresh and dry masses (g), volume of root system (mL) and root length (cm), and chlorophyll contents (using a Chlorophyll Meter SPAD-502 Plus) were evaluated.
Colonization of tomato plants by B. subtilis and B. licheniformis
A 2.5 mL suspension of the commercial product Quartzo®, at concentration of 1 × 1010 CFU mL−1, is applied according to Table 2 to assess the colonization of B. subtilis FMCH002 and B. licheniformis FMCH001 strains in the shoots and root system of tomato plants. Fourteen-day-old tomato seedlings Santa Clara® cultivar, previously grown on multicell growing trays (200 cells) containing substrate (Tropstrato HT Hortaliças®) autoclaved three times for 1 h for 3 consecutive days, were transferred to pots containing 500 mL of the same substrate.
The experiment was carried out in a completely randomized design with four replicates. Forty four-day-old plants were collected from two replicates to perform the isolation of endophytic bacteria from the stem and rhizosphere in order to determine colony-forming units of Bacillus. From the other two replicates, 45-day-old plants were collected to determine the presence of B. subtilis (FMCH002) and B. licheniformis (FMCH001) strains in plant roots/rhizosphere via quantitative real-time PCR (qPCR).
The isolation of bacteria was carried out directly from the exuded sap of the plant by cutting the tomato plants transversally and collecting the exuded sap with micropipette. The collected sap was transferred to Petri dishes containing nutrient agar medium (Himidia, 26 Mumbai-India). The presence or absence of Bacillus colonies was evaluated after 48 h.
The determination of total bacteria in the rhizosphere was carried out by adding 5 g of roots and rhizosphere substrate in a 125-mL Erlenmeyer flask containing 45 mL of MgSO47H2O suspension (0.01 M). After stirring for 5 min on a shaker at 100 rpm and for 5 min in ultrasound, serial dilutions from 10−1 to 10−5 were performed. Then, 0.1 mL of each dilution was transferred to Petri dishes containing nutrient agar culture medium. The plates were kept in a growth chamber at 28 ± 2 °C, and colony counts were performed after 24 and 48 h. Only counts ranging from 30 to 300 colonies/plate were considered [45, 46]. The assays were carried out in a completely randomized design with three replicates, with each replicate represented by a Petri dish. For the quantification of the total bacteria, a dilution of 10−4 was used.
For the quantification of B. licheniformis (FMCH001) and B. subtilis (FMCH002) by qPCR, the extraction of total DNA from the samples was performed using 180 mg of root. Extraction was performed using the MP Biomedicals™ FastDNA™ SPIN soil kit according to the manufacturer’s instructions. The qPCR reaction was performed in a final volume of 12 μL, composed of 5.0 μL of DNA, 6.25 μL of SsoAdvanced™ Universal SYBR® Green Supermix (Bio-Rad), and 0.25 μL of each primer. Specific primer pairs were used for each strain. The sequence of the primers and the validation protocols are of FMC proprietary, and the specific quantification of the tested Bacillus spp. can be performed in collaboration with the company, if desired. For amplification, an initial denaturation cycle of 98 °C for 3 min was used. Afterwards, 45 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 25 s were used. All treatments were evaluated in biological and technical triplicate. The values obtained were estimated from standard curves for each strain, obtained from values from 1 × 103 to 1 × 108 CFU g−1 of root.
Statistical analyses
The data of the assay where the effect of the application of the mixture of B. subtilis with B. licheniformis on germination and emergence of tomato seedlings were evaluated by regression. Assays of IAA production, of plants grown in rhizotrons, and of bacteria colonization on tomato plants were performed in a completely randomized design and analyzed by comparing means using the Scott-Knott test (p < 0.05). Statistical analyses were performed using the statistical software RStudio® version 4.0.0.
Results
Indole-acetic acid production
The average production of IAA by B. subtilis (FMCH002) and B. licheniformis (FMCH001), in the first assay, was 9.60 and 16.88 µg of IAA mL−1 of supernatant, respectively, and in the second assay, the average production was 9.04 and 16.84 µg IAA mL−1 of supernatant, respectively. Both Bacillus strains produced IAA after 48 h of in vitro growth.
Mixture of B. subtilis and B. licheniformis on seed germination and emergence of tomato seedlings
Germination and root protrusion of tomato seeds treated with a mixture of B. subtilis and B. licheniformis increased linearly according to the doses applied (1 × 108 to 1 × 1010 CFU mL−1). The maximum germination of 91.5% was reached for concentrations of 1 × 109 and 1 × 1010 CFU mL−1. Regarding radicle protrusion, these same concentrations reached its plateau at 72.5% protrusion.
Bacillus subtilis and B. licheniformis in promoting the growth of tomato plants grown on rhizotrons
The application of a mixture of B. subtilis and B. licheniformis (Quartzo®) at concentrations 1 × 108, 1 x × 109, and 1 × 1010 CFU mL1, as well as the application of B. subtilis and B. licheniformis individually at concentration 1 × 108 CFU mL−1, increased (p < 0.05) fresh and dry masses of shoot and root system, volume of root system, and length of roots of tomato plants when compared to control (Table 1, Fig. 1).
The effect of Bacillus subtilis FMCH002 (BS) and Bacillus licheniformis FMCH001 (BL) on the growth of tomato plants 36 days after planting on rhizotrons. BS = B. subtilis 1 × 108 CFU mL−1. BL = B. licheniformis 1 × 108 CFU mL−1. BL + BS 1010, 109, and 108 = B. licheniformis + B. subtilis at concentrations of 1 × 1010, 1 × 109, 1 × 108 UFC mL−1, respectively
In the first assay, B. licheniformis, at a concentration of 1 × 108 CFU mL−1, increased the fresh and dry masses of shoots by 55% and 75%, respectively, when compared to control. The mixture of B. subtilis and B. licheniformis, at a concentration of 1 × 1010 CFU mL−1, increased the fresh and dry masses of the root system by 155% and 280%, respectively, when compared to control. Root system volume and root length were increased by 141% and 44%, respectively, when plants were amended with mixture of B. subtilis and B. licheniformis (Quartzo®) at 1 × 1010 CFU mL−1 concentration (Table 1).
In the second assay, the results were similar to the first assay. However, the application of the mixture of B. subtilis and B. licheniformis at concentration of 1 × 109 CFU mL−1 differed from the other treatments for shoot fresh mass, with an increase of 67% when compared to control. For shoot dry mass, the mixture of B. subtilis and B. licheniformis, at a concentration of 1 × 109 CFU mL−1, showed an increase of 67% when compared to control. For root system dry mass, B. licheniformis at 1 × 108 CFU mL−1 differed from the other treatments, showing an increase of 230% when compared to control. Bacillus strains also increased root system fresh mass, root volume, and length, differing statistically from control.
Despite the height being higher in treatments with Bacillus, no significant differences were observed. When, in the first assay, a mixture of B. subtilis and B. licheniformis was applied at concentrations of 1 × 109 and 1 × 1010 CFU mL−1, an increment of approximately 22.6%, for both concentrations was observed when compared to control (Table 1). In the second assay, B. licheniformis, at a concentration of 1 × 108 CFU mL−1, increased height by 20% in relation to control. B. subtilis and B. licheniformis did not increase the stem diameter of the plants nor the chlorophyll content of the leaves.
Colonization of tomato plants by B. subtilis and B. licheniformis
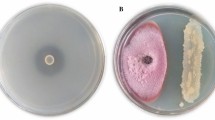
Bacillus colonies obtained from plant sap, using the technique of isolating bacteria directly, were morphologically similar to colonies of B. subtilis and B. licheniformis strains inoculated on plants as described by Bettiol et al. [47]. There was no detected bacterial growth from control plants (Fig. 2).
Colonies of bacteria isolated from the sap exuded after the cross section of the tomato stem obtained from different forms and application times of the mixture containing Bacillus subtilis FMCH002 and Bacillus licheniformis FMCH001 (Quartzo®) at a concentration of 1 × 1010 CFU mL-1. a Control; b application on the seeding substrate; c seeds treatment; d application on the seeding substrate and seeds treatment; e application 7 days after transferred to container media (7DAT); f application on the seeding substrate and 7 DAT; g seeds treatments and 7 DAT; h application on the container media; i application on the seeding substrate and container media; j seeds treatments and container media; k application on the seeding substrate, seeds treatment, container media, and 7 DAT
By counting colonies in Petri dishes, we observed that the highest concentration of total bacteria in the rhizosphere was 6 × 107 CFU g−1. In general, the same concentration was observed for all treatments, except the ones with seed treatment and in-furrow application, with concentrations of 2.8 × 106 and 4 × 105 CFU g−1 of rhizosphere, respectively. In this case, total bacterial concentration was similar to control (2.0 × 105 CFU g−1 of rhizosphere) (Table 2).
Using the qPCR technique, it was possible to quantify the number of bacterial cells g−1 of root. Although the Bacilli were detected from the biocontrol treated plants, it was not possible to correlate the different forms and times of application with the number of cells of bacterial strains in tomato roots. When the application of the Bacillus mixture was performed 7 days after transplanting, bacterial concentration of 7.46 × 109 g−1 in root was observed. With two applications of the Bacillus mixture, one on the seeds and the other on the container media substrate, 7 days after transplanting, the observed number of bacterial cells was 8.35 × 109 g−1 of root. The presence of Bacillus was not observed in the water-treated control. For the other treatments, the concentration ranged from 9.2 × 103 to 5.6 × 106 cells g−1 of root (Table 3).
Discussion
We describe the potential of B. subtilis (FMCH002) and B. licheniformis (FMCH001) to promote tomato growth when applied either in a formulated mixture (Quartzo®) and separately. The application of Bacillus strains not only promoted root growth and the increase in fresh and dry masses of the tomato root system and shoot, but also stimulated seed germination (Figs. 1 and 2, Table 2). The ability of Bacillus spp. to promote plant growth is reported by Franco-Sierra et al. [48], Liu et al. [49], Raji and Thangavelu [40], and Shahid et al. [50] among others.
The success of PGPR depends on an efficient colonization of the root system [51]. B. subtilis FMCH002 and B. licheniformis FMCH001 isolates efficiently colonized the root system of tomato plants (Tables 1, 2, and 3). The presence of Bacillus, both in the stem and in the rhizosphere of such plants, indicates the survival of the isolates (Tables 2 and 3). Kalam et al. [52] demonstrated that active colonization of tomato roots by B. subtilis is important for promoting plant growth and nutrition. However, the level of colonization of strains FMCH001 and FMCH002 was influenced by the number and form of application (Tables 2 and 3). The populations of the introduced bacteria in the rhizosphere was similar to that described by Abdallah et al. [41] who reported that a closely related bacterial species (Bacillus amyloliquefaciens) colonizing the rhizoplane of tomato plants and was detected at a rate of 1 × 105 to 1 × 107 CFU g−1 of root. Actually, it seems that the concentration of the bacterium in the rhizosphere is directly related to its efficacy, and tracking the population of the introduced bacterium can be proposed as a strategy to assure more consistent results in the field. Chen et al. [53] reported that root colonization by B. subtilis B579 at less than 104 CFU g−1 of rhizosphere did not affect cucumber production, while concentrations higher than that have promoted the plant performance.
The tomato growth promotion can occur through a direct production of plant hormones or increasing nutrient uptakes. B. subtilis and B. licheniformis are reported to be hormone and siderophore producers, phosphate solubilizers, and nitrogen fixers [29, 41, 54,55,56,57]. The isolates of B. subtilis FMCH002 and B. licheniformis FMCH001 produced 9.60 and 16.88 µg of IAA mL−1, which within the range of that hormone exogenous production by bacteria to promote plant growth [53]. Although the quantification of the IAA production was performed in situ and not in planta, the commercial product is made up of the cells and supernatant as active ingredients; therefore, at least in the supernatant fraction of the product, the plant hormone is likely to be encountered [49] and has respond at least in part to the root growth promotion observed activity [58]. Once in intimate association with the root, the bacterium can produce the hormone to sustain the hormone supply to the plant [27].
Among the benefits of exogenous IAA amendment to the rhizosphere, root elongation and shoot growth are the direct benefits, especially when the bacterium also interferes with the hormone transport within the plant [59]. Although the hormone is more implicated in plant growth, it also may play a role in stress tolerance, since defense- and cell wall–strengthening genes [60].
Noteworthy, for a plant growth promoter product to be commercially available to growers at an acceptable cost, it has to exert growth promoting benefits to multiple hosts. At least, one of the strains parts of the evaluated product (B. licheniformis FMCH001) has confirmed its growth promotion benefit to maize, particularly observed on roots, and such benefit was sustained even under drought stress [11]. Actually, the evaluated product is registered in Brazil for gall nematode management [61], and according to Brazilian legislation, it can be recommended for whatever crop for which the nematode is a problem. From our results, one of the mechanisms by which the product may act in the soil-borne disease control for which it is recommended is the compensatory or tolerance induced effect inferred from the growth promotion potential of the crop [62].
B. subtilis and B. licheniformis are widely marketed and used in agricultural systems as antagonists to various phytopathogens, such as nematodes, but it is also benefiting the plant as plant growth promoters. We observed that applications of B. subtilis FMCH002 and B. licheniformis FMCH001 through seed treatment, as well as applications on the seedling substrate, and container media substrate, promoted root system growth, and both isolates survived in the roots of treated plants.
B. subtilis and B. licheniformis are widely marketed and used in agricultural systems as antagonists to various phytopathogens, such as nematodes, but it is also benefiting the plant as plant growth promoters, and this can be a benefit to the plant in the absence of the pathogen and support a preventive-basis application of the product, which results in higher protection against the plant parasitic nematode and, in its absence, promotes plant growth. We observed that applications of B. subtilis FMCH002 and B. licheniformis FMCH001 through seed treatment, as well as bacterial amendment to the seedling substrate, and container media substrate, promoted root system elongation and sustained its population high in the roots of treated plants.
References
Kloepper JW, Hume DJ, Scher FM, Singleton C, Tipping B, Laliberte M, Frauley K, Kutcchaw T, Simonson C, Zaleska I, Lee L (1988) Plant growth-promoting rhizobacteria on canola (rapeseed). Plant Dis 72(1):42–46
Kloepper JW (1992) Plant growth-promoting rhizobacteria as biological control agents. In: Metting FB (ed) Soil microbial ecology: applications in agricultural and environmental management. Marcel Dekker Inc, New York, pp 255–274
Hayat R, Ali S, Amara U, Khalid R, Ahmed I (2010) Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol 60(4):579–598. https://doi.org/10.1007/s13213-010-0117-1
Hayat R, Ahmed I, Sheirdil RA (2012) An overview of plant growth promoting rhizobacteria (PGPR) for sustainable agriculture. Crop production for agricultural improvement. In: Ashraf M, Öztürk M, Ahmad M, Aksoy A (eds) Crop production for agricultural improvement. Springer, Dordrecht, pp 557–579. https://doi.org/10.1007/978-94-007-4116-4_22
Reddy MS, Yellareddygari SKR, Kumar KVK, Sudini H, Kloepper JW, Sairam KVSS, Wang Q, Arwiyanto T, Liu S, Sarma YR, Surendranatha Reddy EC, Vinh NC, Archana G, Naik MK, Soesanto L, Zhou XG, Dilantha Fernado WG, Inan-ul-Haq M, Park KS, Egamberdieva D, Sayyed RZ, Zhang S, Du B, Zhi-lin Y, Zhi-ling Y (2011) Commercial potential of biofertilizers and biofungicides (PGPR) for sustainable agriculture in Asia and the scope of Asian PGPR Society. In: Reddy MS, Wang Q (eds) Plant growth-promoting rhizobacteria (PGPR) for sustainable agriculture. Proceeding of the 2nd Asian PGPR Conference, 2011, Beijing, China, pp 21–24
Glick BR (2014) Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res 169(1):30–39. https://doi.org/10.1016/j.micres.2013.09.009
Shi JW, Lu LX, Shi HM, Ye JR (2022) Effects of plant growth-promoting rhizobacteria on the growth and soil microbial community of carya illinoinensis. Curr Microbiol 79(11):1–12. https://doi.org/10.1007/S00284-022-03027-9
Liu J, Zhang J, Zhu M et al (2022) Effects of plant growth promoting rhizobacteria (PGPR) strain bacillus licheniformis with biochar amendment on potato growth and water use efficiency under reduced irrigation regime. Agron 12(5):1031. https://doi.org/10.3390/AGRONOMY12051031
de Lima BC, Moro AL, Santos ACP, Bonifacio A, Araujo ASF, de Araujo FF (2019) Bacillus subtilis ameliorates water stress tolerance in maize and common bean. J Plant Interact 14(1):432–439. https://doi.org/10.1080/17429145.2019.1645896
Rabbee MF, Sarafat Ali M, Choi J, Hwang BS, Jeong SC, Baek K, hyun. (2019) Bacillus velezensis: a valuable member of bioactive molecules within plant microbiomes. Mol 24(6):1046. https://doi.org/10.3390/MOLECULES24061046
Akhtar SS, Amby DB, Hegelund JN, Fimognari L, Großkinsky DK, Westergaard JC, Muller R, Moelbak L, Liu F, Roitsch T (2020) Bacillus licheniformis FMCH001 increases water use efficiency via growth stimulation in both normal and drought conditions. Front Plant Sci 11:297. https://doi.org/10.3389/fpls.2020.00297
Miljaković D, Marinković J, Balešević-Tubić S (2020) The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 8(7):1037. https://doi.org/10.3390/microorganisms8071037
Piggot PJ, Hilbert DW (2004) Sporulation of Bacillus subtilis. Curr Opin Microbiol 7(6):579–586. https://doi.org/10.1016/j.mib.2004.10.001
Choudhary DK, Johri BN (2009) Interactions of Bacillus spp and plants – with special reference to induced systemic resistance (ISR). Microbiol Res 164(5):493–513. https://doi.org/10.1016/j.micres.2008.08.007
Ongena M, Jacques P (2008) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16(3):115–125. https://doi.org/10.1016/j.tim.2007.12.009
Cawoy H, Bettiol W, Fickers P, Ongena M (2011) Bacillus based biological control of plant diseases. In: Stoytcheva M (ed) Pesticides in the modern world – pesticide use and management. InTech, Rijeka, pp 273–302
Radhakrishnan R, Hashem A, AbdˍAllah EF (2017) Bacillus: a biological tool for crop improvement through bio-molecular changes in adverse environments. Front Physiol 8:667. https://doi.org/10.3389/fphys.2017.00667
Hashem A, Tabassum B, FathiAbdˍAllah E (2019) Bacillussubtilis: a plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J Biol Sci 26(6):1291–1297. https://doi.org/10.1016/j.sjbs.2019.05.004
Medeiros CAA, Bettiol W (2021) Multifaceted intervention of Bacillus spp. against salinity stress and Fusarium wilt in tomato. J Appl Microbiol 31(5):2387–2401. https://doi.org/10.1111/jam.15095
GuimarãesPacifico M, Eckstein B, Bettiol W (2021) Screening of Bacillus for the development of bioprotectants for the control of Fusarium oxysporum f. sp. vasinfectum and Meloidogye incognita. Biol Control 164:104764. https://doi.org/10.1016/j.biocontrol.2021.104764
Aloo BN, Makumba BA, Mbega ER (2019) The potential of Bacilli rhizobacteria for sustainable crop production and environmental sustainability. Microbiol Res 219:26–39. https://doi.org/10.1016/j.micres.2018.10.011
Yasmin H, Naeem S, Bakhtawar M, Jabeen Z, Nosheen A, Naz R, Keyani R, Mumtaz S, Hassan MN (2020) Halotolerant rhizobacteria Pseudomonas pseudoalcaligenes and Bacillus subtilis mediate systemic tolerance in hydroponically grown soybean (Glycine max L.) against salinity stress. Penna S, ed. PLoS One 15(4):e0231348. https://doi.org/10.1371/journal.pone.0231348
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ - Sci 26(1):1–20. https://doi.org/10.1016/j.jksus.2013.05.001
Torres M, Llamas I, Torres B, Toral L, Sampedro I, Béjar V (2020) Growth promotion on horticultural crops and antifungal activity of Bacillus velezensis XT1. Appl Soil Ecol 150:103453. https://doi.org/10.1016/j.apsoil.2019.103453
Soni R, Keharia H (2021) Phytostimulation and biocontrol potential of Gram-positive endospore-forming Bacilli. Planta 54(3):49. https://doi.org/10.1007/s00425-021-03695-0
Kamilova F, Okon Y, de Weert S, Hora K (2015) Commercialization of microbes: manufacturing, inoculation, best practice for objective field testing, and registration. In: Lugtenberg B (ed) Principles of plant-microbe interactions. Springer, Cham, pp 319–327. https://doi.org/10.1007/978-3-319-08575-3_33
Idris EE, Iglesias DJ, Talon M, Borriss R (2007) Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol Plant-Microbe Interact 20(6):619–626. https://doi.org/10.1094/MPMI-20-6-0619
Olanrewaju OS, Glick BR, Babalola OO (2017) Mechanisms of action of plant growth promoting bacteria. World J Microbiol Biotechnol 33(11):197. https://doi.org/10.1007/s11274-017-2364-9
Jha CK, Saraf M (2015) Plant growth promoting rhizobacteria (PGPR). J Agric Res Dev 5(2):108–119
Saleem M, Meckes N, Pervaiz ZH, Traw MB (2017) Microbial interactions in the phyllosphere increase plant performance under herbivore biotic stress. Front Microbiol 8:41. https://doi.org/10.3389/fmicb.2017.00041
Bavaresco LG, Osco LP, Araujo ASF, Mendes LW, Bonifacio A, Araújo FF (2020) Bacillus subtilis can modulate the growth and root architecture in soybean through volatile organic compounds. Theor Exp Plant Physiol 32(2):99–108. https://doi.org/10.1007/s40626-020-00173-y
Ambawade MS, Pathade GR (2015) Production of gibberellic acid by Bacillus siamensis BE 76 isolated from banana plant (Musa spp). Int J Sci Res 4(7):394–398
Kashyap BK, Solanki MK, Pandey AK, Prabha S, Kumar P, Kumari B (2019) Bacillus as plant growth promoting rhizobacteria (PGPR): a promising green agriculture technology. In: Ansari R, Mahmood I (eds) Plant health under biotic stress. Springer, Singapore, pp 219–236. https://doi.org/10.1007/978-981-13-6040-4_11
Zerrouk IZ, Rahmoune B, Auer S, Rößler S, Lin T, Baluska F, Dobrev PL, Motyka V, Ludwig-Müller J (2020) Growth and aluminum tolerance of maize roots mediated by auxin- and cytokinin-producing Bacillus toyonensis requires polar auxin transport. Environ Exp Bot 176:104064. https://doi.org/10.1016/j.envexpbot.2020.104064
Araujo FF, Bonifacio A, Bavaresco LG, Mendes LW, Araujo ASF (2021) Bacillus subtilis changes the root architecture of soybean grown on nutrient-poor substrate. Rhizosphere 18:100348. https://doi.org/10.1016/j.rhisph.2021.100348
Samaras A, Roumeliotis E, Ntasiou P, Karaoglanidis G (2021) Bacillus subtilis MBI600 Promotes growth of tomato plants and induces systemic resistance contributing to the control of soilborne pathogens. Plants 10(6):1113. https://doi.org/10.3390/plants10061113
Adesemoye AO, Obini M, Ugoji EO (2008) Comparison of plant growth-promotion with Pseudomonas aeruginosa and Bacillus subtilis in three vegetables. Brazilian J Microbiol 39(3):423–426. https://doi.org/10.1590/S1517-83822008000300003
Schwartz AR, Ortiz I, Maymon M, Herbold CW, Fujishige NA, Vijanderan JA, Villella W, Hanamoto K, Diener A, Sanders RE, Mason DA, Hirsch AM (2013) Bacillus simplex - a little known PGPB with anti-fungal activity-alters pea legume root architecture and nodule morphology when coinoculated with Rhizobium leguminosarum bv viciae. Agronomy 3(4):595–620. https://doi.org/10.3390/agronomia3040595
Zhou C, Zhu L, Xie Y, Li F, Xiao X, Ma Z, Wang J (2017) Bacillus licheniformis SA03 confers increased saline-alkaline tolerance in chrysanthemum plants by induction of abscisic acid accumulation. Front Plant Sci 8:1143. https://doi.org/10.3389/fpls.2017.01143
Raji M, Thangavelu M (2021) Isolation and screening of potassium solubilizing bacteria from saxicolous habitat and their impact on tomato growth in different soil types. Arch Microbiol 203(6):3147–3161. https://doi.org/10.1007/s00203-021-02284-9
Ben Abdallah D, Frikha-Gargouri O, Tounsi S (2018) Rizhospheric competence, plant growth promotion and biocontrol efficacy of Bacillus amyloliquefaciens subsp. plantarum strain 32a. Biol Control 124:61–67. https://doi.org/10.1016/j.biocontrol.2018.01.013
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moënne-Loccoz Y (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321(1–2):341–361. https://doi.org/10.1007/s11104-008-9568-6
Kamilova F, Validov S, Azarova T, Mulders I, Lugtenberg B (2005) Enrichment for enhanced competitive plant root tip colonizers selects for a new class of biocontrol bacteria. Environ Microbiol 7(11):1809–1817. https://doi.org/10.1111/j.1462-2920.2005.00889.x
Brasil (2009) Regras Para Análise de Sementes. Ministério da Agricultura, Abastecimento Pecuária. https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/arquivos-publicacoes-insumos/2946_regras_analise__sementes.pdf. Accessed 2 Dec 2020
Clark FE (2016) Agar-plate method for total microbial count. In: Norman AG (ed) Methods of soil analysis: part 2 chemical and microbiological properties, 9.2, pp 1460–1466. https://doi.org/10.2134/agronmonogr9.2.c48
Schortemeyer M, Hartwig UA, Hendrey GR, Sadowsky MJ (1996) Microbial community changes in the rhizospheres of white clover and perennial ryegrass exposed to free air carbon dioxide enrichment (FACE). Soil Biol Biochem 28(12):1717–1724. https://doi.org/10.1016/S0038-0717(96)00243-X
Bettiol W, Morandi MAB, Pinto ZV, Lucon CMM (2022) Controle de qualidade e conformidade de produtos e fermentados à base de Bacillus spp.: proposta metodológica. Embrapa (Comunicado Técnico 59), Jaguariúna, p 15. https://ainfo.cnptia.embrapa.br/digital/bitstream/item/239978/1/Bettiol-Controle-qualidade-2022-2.pdf. Accessed 2 Nov 2022
Franco-Sierra ND, Posada LF, Santa-María G, Romero-Tabarez M, Villegas-Escobar V, Álvarez JC (2020) Bacillus subtilis EA-CB0575 genome reveals clues for plant growth promotion and potential for sustainable agriculture. Funct Integr Genomics 20(4):575–589. https://doi.org/10.1007/s10142-020-00736-x
Liu Z, Wang H, Xu W, Wang Z (2020) Isolation and evaluation of the plant growth promoting rhizobacterium Bacillus methylotrophicus (DD-1) for growth enhancement of rice seedling. Arch Microbiol 202(8):2169–2179. https://doi.org/10.1007/s00203-020-01934-8
Shahid I, Han J, Hanooq S, Malik KA, Borchers CH, Mehnaz S (2021) Profiling of metabolites of Bacillus spp. and their application in sustainable plant growth promotion and biocontrol. Front Sustain Food Syst 5:37. https://doi.org/10.3389/fsufs.2021.605195
Zhou D, Huang XF, Chaparro JM, Zhou D, Huang XF, Chaparro JM, Badri DV, Manter DK, Vivanco JM, Guo J (2016) Root and bacterial secretions regulate the interaction between plants and PGPR leading to distinct plant growth promotion effects. Plant Soil 401(1–2):259–272. https://doi.org/10.1007/s11104-015-2743-7
Kalam S, Basu A, Podile AR (2020) Functional and molecular characterization of plant growth promoting Bacillus isolates from tomato rhizosphere. Heliyon 6(8):e04734. https://doi.org/10.1016/j.heliyon.2020.e04734
Chen F, Wang M, Zheng Y, Luo J, Yang X, Wang X (2010) Quantitative changes of plant defense enzymes and phytohormone in biocontrol of cucumber Fusarium wilt by Bacillus subtilis B579. World J Microbiol Biotechnol 26(4):675–684. https://doi.org/10.1007/s11274-009-0222-0
Sukkasem P, Kurniawan A, Kao TC, Chuang H, wen, (2018) A multifaceted rhizobacterium Bacillus licheniformis functions as a fungal antagonist and a promoter of plant growth and abiotic stress tolerance. Environ Exp Bot 155:541–551. https://doi.org/10.1016/j.envexpbot.2018.08.005
Bhattacharya A, Giri VP, Singh SP, Pandey S, Chauhan P, Soni SK, Srivastava S, Singh PC, Mishra A (2019) Intervention of bio-protective endophyte Bacillus tequilensis enhance physiological strength of tomato during Fusarium wilt infection. Biol Control 139:104074. https://doi.org/10.1016/j.biocontrol.2019.104074
Cui W, He P, Munir S, He P, Li X, Li Y, Wu J, Wu Y, Yang L, He P, He Y (2019) Efficacy of plant growth promoting bacteria Bacillus amyloliquefaciens B9601–Y2 for biocontrol of southern corn leaf blight. Biol Control 139:104080. https://doi.org/10.1016/j.biocontrol.2019.104080
Pérez-Hernández Y, Díaz-Solares M, Rondón-Castillo AJ, Fuentes-Alfonso L, González-Sierra L, Guzmán-Cedeño ÁM (2020) Aislamiento de cepas de Bacillus spp. a partir del bioproducto IHPLUS® con potencialidades para el desarrollo agropecuario e industrial. Pastos y Forrajes 43(1):56–65
Idris EE, Bochow H, Ross H, Borriss R (2004) Use of Bacillus subtilis as biocontrol agent. VI. Phytohormonelike action of culture filtrates prepared from plant growth-promoting Bacillus amyloliquefaciens FZB24, FZB42, FZB45 and Bacillus subtilis FZB37. J Plant Dis Prot 111(6):583–597
Zhang H, Kim MS, Krishnamachari V, Payton P, Sun Y, Grimson M, Farag MA, Ryu CM, Rm A, Melo IS, Paré PW (2007) Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 226(4):839–851. https://doi.org/10.1007/s00425-007-0530-2
Backer R, Rokem JS, Ilangumaran G, Lamont J, Praslickova D, Ricci E, Subramanian S, Smith DL (2018) Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci 9:1473. https://doi.org/10.3389/fpls.2018.01473
AGROFIT - Sistema de agrotóxicos fitossanitário. Ministério da Agricultura, Pecuária e Abastecimento (2022). https://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons. Accessed 16 May 2022
López-Bucio J, Campos-Cuevas JC, Hernández-Calderón E, Velásquez-Becerra C, Farías-Rodriguez R, Macías-Rodríguez LI, Valencia-Cantero E (2007) Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin-and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol Plant-Microbe Interact 20(2):207–217. https://doi.org/10.1094/MPMI-20-2-0207
Acknowledgements
Peterson Sylvio de Oliveira Nunes acknowledges Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarship. Wagner Bettiol (CNPq 307855/2019-8) and Flavio H V Medeiros (CNPq 317266/2021-7) acknowledge Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq for the productivity fellowship. The authors acknowledge Chr. Hansen for donating the commercial product and Bacillus isolates, as well as for performing the qPCR analyses.
Author information
Authors and Affiliations
Contributions
WB and PSON conceived, designed, and performed the greenhouse and laboratory experiments. TSO and JRAZ performed the qPCR analysis. PSON and FHVM analyzed the data. WB, JRAZ, TSO, and FHVM contributed with reagents/materials/analysis tools. PSON, WB, and FHVM wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Luis Nero
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de O. Nunes, P.S., de Medeiros, F.H.V., de Oliveira, T.S. et al. Bacillus subtilis and Bacillus licheniformis promote tomato growth. Braz J Microbiol 54, 397–406 (2023). https://doi.org/10.1007/s42770-022-00874-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-022-00874-3