Abstract
The present study aimed to isolate and identify root endophytic bacteria with multifunctional plant growth promoting (PGP) traits from medicinal plant Rosmarinus officinalis grown in the North-Western Himalayas. A total of 42 strains were isolated, exhibiting variable degrees of PGP traits, including phosphate solubilization (10–375 µg/mL), indole-3-acetic acid (6–66 µg/mL), siderophore (32.37%–301.48% SU) production and antifungal activity in terms of percent growth inhibition (% GI) against Fusarium oxysporum (44.44%–77.77% GI), Fusarium graminearum (48.88%–71.42% GI) and Rhizoctonia solani (44.44%–77.7% GI). The 16S rDNA sequencing results showed lineage of these strains to 15 genera viz., Aneurinibacillus, Bacillus, Beijerinckia, Cedecea, Ensifer, Enterobacter, Kosakonia, Lactobacillus, Lysobacter, Oxynema, Pseudomonas, Pantoea, Paenibacillus, Pseudoxanthomonas and Serratia. Out of 42 strains, 11 potential strains were selected for in vivo growth studies of R. officinalis. The results showed that the inoculation of Bacillus subtilis KU21, Pseudomonas aeruginosa SI12, and Cedecea lapagei KU14 significantly increased the physical growth parameters of plant over uninoculated control viz., number of lateral of branches (43.95%–46.39%), stem height (29.04%–38.57%), root length (32.31%–37.14%), shoot (34.76%–40.91%) and root biomass (62.89%–70.70%). Physiological characteristics such as total chlorophyll (30.41%–30.96%), phenol (14.43%–24.55%) and carotenoids (34.26%–39.87%) content, also showed a relative increase as compared to uninoculated control; furthermore, the macronutrients (NPK) contents of the plant as well as soil also showed an increase. The developed module may be recommended for sustainable production of R. officinalis in the North-Western Himalayan region without hampering the soil health and fertility.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants develop an intricate association with a variety of microorganisms including rhizospheric, and endophytic, which have attracted the attention of scientific community due to their verified benefits (Cordero et al. 2017; Liotti et al. 2018). These microorganisms are natural inhabitants of host plant and can also develop in an endogenous fashion. This is true for endophytic microorganisms, which colonize plant tissues for at least a part of their life-cycle without any visible disease symptoms (Silva et al. 2019). Bacteria of endophyte community are diverse and able to disperse throughout plant tissues systemically (Bacon and White 2016). Such colonization provides many advantages as this community, independent of its environmental circumstances, can interact effectively with the plant (Santoyo et al. 2016). This improves plant growth and a defense against plant pathogens, which further enhances stress tolerance, and can promote the synthesis or development of bioactive compounds of interest (Khamwan et al. 2018; McMullin et al. 2018).
Various genera of endophytic bacteria have been isolated from diverse type of plants, facilitating the growth of host plants under different agro-climatic conditions (Afzal et al. 2019; Liu et al. 2017). These include Bacillus and Brevibacillus (ALKahtani et al. 2020), Burkholderia and Enterobacter (Adhikari and Pandey, 2020), Enterobacter (Panigrahi et al. 2019) Acinetobacter and Pantoea (Faria et al. 2021).
Several studies have shown that roots associated bacterial strains can promote plant growth by mechanisms such as phytostimulation, biofertilization, and biocontrol (Gaiero et al. 2013; Abbamondi et al. 2016). Such association has direct physiological effects on plant growth and production, such as: nitrogen fixation, phosphate solubilization (P-solubilization), production of ammonia, siderophores, phytohormones and hydrolytic enzymes (Silva et al. 2019; Glick 2012). These effects provide for the need of plant nutrition in a sustainable manner, thereby reducing the use of chemical fertilizers and pesticides. This in turn preserves the soil biological diversity, thus providing an alternative to the conventional methods of cultivation and compelling the researchers to look for eco-friendly and sustainable agriculture production methods. To this end, the use of plant growth promoting rhizobacteria (PGPR) has emerged as an attractive approach in rosemary production (Sadegh Kasmaei et al. 2019). They are often used as plant growth enhancers under both normal and stressful conditions (Ahemad and Kibret, 2014).
Medicinal plants choose endophytes in a particular way because the selection may be dependent on the secondary metabolites provided by the plant and the composition of the root exudates; therefore, these microbial communities diversify based on their nutritional requirements, the form of soil, and the atmosphere in which they are located (Maggini et al. 2018).
Rosmarinus officinalis (rosemary) is a member of Labiatae family and is one of the most popular medicinal herbs rich in polyphenols (carnosic acid and rosamarinic acid) and flavanoids. It is native to Mediterranean region and cultivated worldwide in cool regions at elevations of 1000–3000 m above sea level (masl) i.e. semiarid and sub humid bioclimatic regions. In Himachal Pradesh, rosemary is grown at an elevation range of 1050–2100 masl falling in the mid-hills sub humid and high hills temperate wet zone. Owing to its numerous biological activities (antibacterial, antiproliferative, anti-inflammatory, and antioxidant), this plant has gained more interest from commercial point of view (Bourhia et al. 2019). However, to date only few studies have explored the endosphere of rosemary regarding plant health (Ahmed et al. 2019). Some studies have been carried out previously to evaluate the impact of commercially available PGPR (Pseudomonas fluorescens) on growth parameters and quality of bioactive compounds (flavonoids, phenolic acids, especially rosmarinic acid) of R. officnalis (Dehghani et al. 2019; Sadegh Kasmaei et al. 2019). So far, no published study has correlated the effect of native PGP bacterial endophytes to the growth of R. officnalis in North-Western Himalayas. The hypothesis underlying the present study is that the bacterial endophytes of this plant are promising bio-inoculants that can improve plant health. The outline of this study concentrates on assessment of functional diversity of culturable root bacterial endophytes of R. officinalis and in vivo studies of potential strains on the productivity of R.officinalis and soil functions.
Materials and methods
Collection of root samples
Root samples of R. officinalis seedling were collected during the summer (June 2017) from rosemary cultivating sites (Kangra, Kullu, Solan, and Sirmour) of Himachal Pradesh. Two year old plants (in vegetative state) were selected for sampling from all locations. Geographical coordinates and environmental conditions of sampling sites are presented in Table S1. A total of twenty-four (3 samples × 8 sites) composite root samples were obtained from all the locations and stored in plastic bags at 4 °C for further assaying of bacterial community structure.
Isolation of bacterial endophytes
Surface sterilization of roots was performed for isolation of bacterial endophytes following the standard method (de Favaro et al. 2012) with slight modification as follows: root samples were washed under running water, sterilized with 70% ethanol for 45 s, and 2% sodium hypochlorite for 5 min followed by washing 4–5 times with sterile distilled water. The surface sterility of roots was cross-checked by plating 100 μL of the final wash and incubated overnight at 30 ºC. No growth on plate indicated absence of microorganisms on the root's surface. One gram of surface sterilized root sample was placed in 9 mL of sterilized distilled water and was ground to produce slurry using pestle and mortar under aseptic conditions. The root suspension was diluted in tenfold series and bacterial count was determined by standard spread plate technique. An aliquot of 100 µL of suspension (10–2-10–4 dilutions) was spread on nutrient and tryptic soy agar medium (Collins and Lyne 1984; Zhao et al. 2015) and incubated at 30 ºC till the appearance of bacterial colonies (up to 5 days). Isolated bacteria were enumerated as colony forming units per gram of roots (cfu/g root).
A total of 42 distinct morphotypes were selected and purified on nutrient and tryptic soy agar medium. The pure culture of these strains was preserved on petri plates at 4 °C for further analysis.
Morpho-biochemical characterization of endophytic bacteria.
Microscopic examination of endophytic bacteria was done together with biochemical characterization according to Bergey’s Manual of Determinative Bacteriology (Holt et al. 1994). Antibiotic resistance pattern of isolates was determined by testing their tolerance to intrinsic levels of various antibiotic kits (Hexa universal 1, 2 and Hexa Pseudo 1, 2) (Himedia, India) using disc diffusion method (Bakthavatchalu et al. 2012).
Identification of endophytic bacteria
16S rDNA sequence analysis was employed for molecular identification of isolated bacteria. Genomic DNA was extracted using the conventional method (Sambrook et al. 1989) followed by PCR-mediated amplification with a set of universal primers (16SF: 5'- AGAGTTTGATCCTGGCTCAG-3'and16SF: 5'-AAGGAGGTGATCCAGCCGCA-3´) (Mehta et al. 2013). PCR reaction mix of 25 µL was prepared with 50 ng of template DNA, 20 pmol of each primer, 0.2 mM dNTPs, and 1U Taq polymerase in 1X PCR buffer. The reaction was cycled 35 times as denaturation at 94 ºC for the 30 s, annealing at 55 ºC for 30 s and extension at 72 ºC for 1 min 30 s followed by a final extension at 72 ºC for 10 min. The PCR product was analyzed by gel electrophoresis on 1.2% (w/v) agarose gel. A band of ~ 1400 bp was excised from the gel and purified using a gel extraction kit (RBCs Real Genomics, New Taipei City, Taiwan) and was sequenced by GeNei™ Laboratories (Bengaluru, India). Based on 16S rDNA sequences, phylogenetically related bacteria were aligned using a BLASTn search (Altschul et al. 1997). Multiple alignments with sequences of related taxa were implemented using ClustalW (Higgins et al. 1994). A neighbor-joining phylogenetic tree was constructed with other 16S rDNA sequences of the related taxa retrieved from the GenBank database using MEGA X software.
In vitro screening for traits involved in plant growth promotion
Isolated bacteria were further authenticated by subsequent in vitro experiments to see whether they exhibited qualities which identified them as possible plant growth promoting bacterial endophytes (PGPBEs). Each in vitro screening test was conducted in triplicates. P-solubilization activity was determined by the method of Pikovskaya (1948). Quantitative production of indole-3-acetic acid (IAA) was estimated using the colorimetric method described by Gorden and Palleg (1957). To test the efficacy of endophytic bacteria as nitrogen fixers, loop full of 24 h old culture of each isolate were streaked on nitrogen free agar medium (Table S2) and incubated for 72 h at 30 ºC. Colonies showing growth on inoculated medium after being transferred ten times in the same medium were potent nitrogen fixers (Jensen 1987). The ability of isolates to produce siderophore and hydrocyanic acid (HCN) was also assessed by Schwyn and Neilands (1987) and Bakker and Schipper (1987) methods, respectively. For lytic enzyme activity, spot inoculation was done on minimal agar medium (Table S2) amended with 0.3% colloidal chitin for chitinase (Robert and Selitrennikoff 1988), starch agar medium for amylase (Shaw et al. 1995) and skim milk agar plates for protease activity (Fleming et al. 1975). Ammonia production was observed according to the method of Cappuccino and Sherman (1992).
The antagonistic activity of the bacterial isolates against test fungal pathogen viz., F. oxysporum (ITCC 7337), F. graminearum (ITCC 5334) and R. solani (ITCC 5308), was done by agar dual plate method (Vincent 1947). A loopfull of 48 h old culture of each isolate was streaked a little below the centre of the prepoured petriplates of malt extract agar (MEA) and incubated at 37 ºC. After 24 h, a 5 mm diameter actively growing test fungal pathogen was placed simultaneously on one side of the streak. The petri plate inoculated with the test pathogen only was kept as control for comparison. The plates were incubated at 24 ± 1 ºC and % GI was calculated using following formula:
where,
C = Growth of fungus in control.
T = Growth of fungus in treatment.
Stimulation of plant growth
To test the efficacy of endophytic strains for stimulating plant growth, a pot experiment was conducted for a period of four months (March–June, 2018) at Department of Basic Sciences, College of Horticulture, Dr Yashwant Singh Parmar University of Horticulture and Forestry, Nauni-Solan, Himachal Pradesh (India).
Eleven isolates with best ex situ PGP traits were selected. The potting mixture was prepared by mixing sand, soil and farm yard manure (FYM) in a ratio of 1:2:1. The following mixture was then subjected to intermittent sterilization i.e. three successive autoclave cycles of 1 h each at 100 ºC with 24 h of incubation between each cycle (tyndallization). The pH of potting mixture was determined in 1:2.5 (soil:water) suspension and the electrical conductivity (E.C.) of the supernatant liquid was recorded and expressed in dSm−1 (Jackson 1973). Furthermore, organic carbon (O.C.) was determined by chromic acid titration method of Walkley and Black (1934). Available N, P, and K contents of soil were determined following standard procedures (Tandon 2009).
The properties of the potting mixture were: pH 7.01; E.C. 0.69 dSm−1; O.C. 1.12%; available N, P and K contents 290.32, 23.40 and 315.45 kg/ha, respectively. The soil used for pot (20.00 cm diameter and 16.00 cm deep) experiment belongs to Entisols order as per United States department of Agriculture Soil Taxonomy. 4 kg of potting mixture was filled in the pots before commencement of experiment. Two months old seedlings of R.officinalis were procured from the Department of Forest Products, College of Forestry, Dr Yashwant Singh Parmar University of Horticulture and Forestry, Nauni-Solan, Himachal Pradesh (India) and were washed under running tap water to remove the soil adhering to the roots. Pure cultures of bacterial isolates were inoculated into 100 mL flask containing nutrient broth and grown aerobically overnight on a rotary shaker (Thermo Scientific™ Sorvall™ ST 8) at 30 ºC (120 rpm). Bacteria were subsequently pelleted by centrifugation at 6000 rpm for 10 min. The pellets were washed with sterile distilled water three times, and the concentration of cells adjusted to 1 × 108 cfu/mL by dilution. This liquid formulation was used as inoculum (Mortensen 1997). Roots of seedlings were immersed in prepared inoculum for about 1 h before plantation. Two seedlings per pot were planted and allowed to grow for four months. Booster doses of liquid bacterial cultures of the same cell density were applied at the rate of 20 mL/plant near the root zone with 15 days interval after planting (3 times). Seedlings were watered daily during the first two weeks of planting followed by irrigation once every two days. The following 12 treatments were arranged in a completely randomized block design (CRD) with three replications for each treatment: T1, control (uninoculated); T2, Pseudomonas mediterranea KA7; T3, Pseudomonas oryzihabitans KU5; T4, Bacillus flexux KA10; T5, Pseudomonas aeruginosa SI12; T6, Pseudoxanthomonas japonensis KU13; T7, Pseudomonas putida KU2; T8, Cedecea lapagei KU14; T9, Pantoea agglomerans KA14; T10, Pseudomonas koreensis KA11; T11, Bacillus simplex KA2; T12, Bacillus subtilis KU21.
Observations on plant growth parameters such as shoot and root length (cm), biomass (g), and the number of lateral branches per plant were recorded by following standard methods. Oven-dried plant samples were ground and sieved for the estimation of macronutrients (NPK). The total concentration of N in plant samples was determined using micro-Kjeldhal's method (Helrich 1990). Plant samples were digested in a diacid mixture of HNO3:HCLO4 (4:1) for P and K analysis (Jackson 1973). P concentration was tested in the digested sample (Jackson 1973). K in the digest was analyzed using the flame photometer (Biogen, Microcontroller Flame Photometer) (Jackson 1967). Total chlorophyll, carotenoids, and total phenol content (TPC) of leaf samples were determined using methods of Withem et al. (1971), Yang et al. (1998), and Faust and Mikulewics (1967), respectively.
Isolation of endophytic bacteria from experimentally inoculated plants
To test whether the bacterial strains were capable of colonizing plant tissues, isolation of the total endophytic bacterial population was done after termination of the experiment. For this, root samples were collected from each treatment including control and standard serial dilution spread plate technique was employed as described in the previous section (isolation of bacterial endophytes). All bacterial endophytes recovered were compared with control data to distinguish growth of introduced bacteria from the presence of indigenous microorganisms. Colonies which showed morphological characteristics similar to treated strain were selected and identified biochemically as per Bergey’s Manual of Determinative Bacteriology (Holt et al. 1994). The dominance of inoculated strain was calculated according to Simpson’s index of dominance (D) as:
where, Pi is the relative abundance of isolates calculated according to the following equation Pi = ni/N.
ni, is the number of inoculated strain colonies and N, is the total number of endophyte colonies.
Statistical analysis
All the experiments were conducted under the statistical framework with three replications per treatment along with appropriate controls replicated three times. The data obtained from the laboratory experiments and the net house was subjected to one-way analysis of variance (ANOVA) using SPSS version 16 (SPSS Inc., Chicago, IL, USA) and Microsoft Excel 7.0 (Microsoft, Redmond, WA, USA). The means and standard deviation of data were also calculated. Comparisons of treatment means were performed by the Fisher's Projected LSD (least significant differences) test at P ≤ 0.05 level of significance. PCA (principal component analysis) was performed on pot experiment, to evaluate the relationship between effects of endophyte inoculation on several plant growth parameters. PCA was performed using PAST 3.0 software.
Results
Isolation and identification of endophytic bacteria
A total of 42 strains were isolated based on unique colony morphologies on medium used for isolation. Amongst these, 17 were Gram’s positive while 25 were Gram’s negative, varied from rods, cocci to coccobacilli. Endospore formation was observed in 16 isolates. Biochemically, low number of isolates were positive for indole (28.57%) and hydrogen sulfide (H2S) (9.52%) production. Greater numbers of bacterial isolates were positive nitrate reduction (80.95%) and oxidase production (61.90%). All the isolates were positive for catalase except KU20 and KU25. 59.52% were positive for methyl red, 40.47% showed positive test for voges proskauer (VP); only 21.43% bacterial isolates were able to hydrolyse gelatin. Almost all the isolates were able to ferment dextrose and sucrose, whereas a few isolates showed positive lactose fermentation test (Table S3).
Phylogenetic analysis based on 16S rDNA sequencing and alignment showed that these endophytes were affiliated to 2 phyla, i.e., Proteobacteria and Firmicutes, 15 genera viz., Aneurinibacillus, Bacillus, Beijerinckia, Cedecea, Ensifer, Enterobacter, Kosakonia, Lactobacillus, Lysobacter, Oxynema, Pseudomonas, Pantoea, Paenibacillus, Pseudoxanthomonas and Serratia and 32 species (Fig. 1a, b). The most dominant endophytic bacteria were reported in genera Bacillus and Pseudomonas representing 33.33% and 25.64% of the total isolates. A detailed account of the identities of these isolates has been presented in Table S4.
Phylogenetic tree of gram positive endophytic isolates (highlighted) and their closely associated taxa obtained from GenBank based on 16S rDNA sequence (a); Phylogenetic tree of gram negative endophytic isolates (highlighted) and their closely associated taxa obtained from GenBank based on 16S rDNA sequence (b)
Beneficial Plant traits of bacterial strains
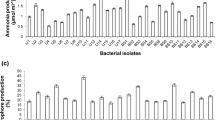
In vitro screening revealed that most of the strains exhibited multiple PGP activities (Fig. 2). All the strains substantially solubilized mineral P in PVK broth (70–375 µg/mL). All the strains of genera Cedecea, Ensifer, Enterobacter, Lactobacillus, Oxynema, Paenibacillus and Serratia showed IAA and siderophore production and ability to fix nitrogen. Majority of strains belonging to genera Bacillus, Pseudomonas, Pantoea and Pseudoxanthomonas were IAA (10, 10, 2 and 2 no. of strains) and siderophore (12, 11, 2 and 2 no. of strains) producers and fixed nitrogen (8, 10, 1 and 1 no. of strains), respectively. The strain of genus Beijerinckia produced siderophore and fixed nitrogen but was deficient in IAA production. Isolates of genera Aneurinibacillus, Lysobacter and Kosakonia produced IAA (1, 1and 3) and siderophore (1, 1 and 2 no.), respectively but did not showed nitrogen fixing ability. In total, 85.71% isolates produced IAA (10–66 µg/mL) and 92.85% were siderophore producers (35.71%-301.48% SU). 66.66% strains had ability to fix nitrogen on Jensen's nitrogen-free medium (Fig. 2).
All the bacterial strains were able to produce one or more cell wall degrading enzymes. The isolates belonging to several genera Paenibacillus, Aneurinibacillus, Bacillus, Beijerinckia, Cedecea, Kosakonia, Lysobacter, Oxynema, Pseudomonas, Pantoea, Pesudoxanthomonas and Serratia were positive for chitinase (1, 1, 10, 1, 1, 1, 1, 1, 7, 2, 2 and 1 no. of strains), protease (1, 1, 12, 1, 1, 1, 1, 1, 10, 2, 2 and 1 no. of strains) and amylase production (1, 1, 12, 1, 1, 1, 2, 1, 1, 8, 1 and 1 no. of strains), respectively. Genus Lactobacillus was unable to produce chitinase and protease but was positive for amylase (1 strain) production. Similarly, strains of genera Ensifer and Enterobacter produced chitinase (1, 1 no. of strains) and amylase (1, 1 no. of strains), respectively but lacked protease activity. In total, 73.81% strains exhibited chitinase activity, and 80.95% were protease and amylase producers. Majority of bacterial strains (88.09%) displayed ammonia production [Aneurinibacillus (1 strain), Bacillus (11 strains), Beijerinckia (1 strain), Cedecea (1strain), Ensifer (1strain), Enterobacter (1strain), Kosakonia (3 strain), Lactobacillus (1strain), Lysobacter (1strain), Oxynema (1strain), Pseudomonas (10 strain), Pantoea (1strain), Paenibacillus (1strain), Pseudoxanthomonas (2 strains) and Serratia (1strain)], whereas; only 40.47% were HCN producers [Aneurinibacillus (1 strain), Bacillus (7 strains), Cedecea (1 strain), Pseudomonas (6 strain) and Paenibacillus (1 strain) and Serratia (1 strain)] (Fig. 2).
The antagonistic activity was observed for endophytic strains against phytopathogenic fungi (F. oxysporum, F. graminearum and R. solani). 40.47% strains inhibited the growth of F. oxysporum (44.44%–77.77% GI), 33.33% and 26.19% strains were antagonist against F. graminearum (48.88%–71.42% GI) and R. solani (44.44%–77.77% GI), respectively. Only six strains (KA2, KA14, KU14, KU21, SI12 and SI13) showed antagonism against all fungal pathogens, out of which SI12 strain was most effective against all the three tested fungal phytopathogens (Table 1).
Effect of endophytic bacteria on plant growth promotion
A pot experiment was conducted to validate the in vitro PGP activities of selected strains (Pseudomonas mediterranea KA7, Pseudomonas oryzihabitans KU5, Bacillus flexux KA10, Pseudomonas aeruginosa SI12, Pseudoxanthomonas japonensis KU13, Pseudomonas putida KU2, Cedecea lapagei KU14, Pantoea agglomerans KA14, Pseudomonas koreensis KA11, Bacillus simplex KA2, Bacillus subtilis KU21) on the growth of R. officinalis. The in vitro PGP traits of selected strains have been depicted in Table 1.
Effect on physical characteristics
Most of the isolates significantly (P ≤ 0.05) increased growth parameters of R. officinalis over uninoculated control. Treatments receiving P. aeruginosa SI12 (T5) inoculation showed maximum stimulatory effects for overall plant growth parameters over uninoculated control, which was statistically at par with the treatments receiving C. lapagei KU14 (T8) and B. subtilis KU21 (T12) inoculation (Table 2). These incremental effects were 43.95%, 45.61%, and 46.39% for number of lateral branches, 38.30%, 29.04% and 38.57% on stem height, 34.76%, 37.21% and 40.91% for shoot biomass, 62.89%, 70.70% and 63.29% for root biomass, respectively over untreated control.
Effect on physiological characteristics
Photosynthetic pigments of R. officinalis leaves were determined to evaluate the impact of endophytic strains on photosynthetic efficiency of host plant. Data corresponding to biochemical parameters revealed that treatment T5 (P. aeruginosa SI12) had maximum significant (P ≤ 0.05) increase in total chlorophyll (33.09%), carotenoids (39.87%) and total phenol content (24.55%) of R. officinalis leaves over uninoculated control, which was at par with T8, T11 and T12 for chlorophyll and treatments T3, T7, T8, T11 and T12 in case of carotenoids (Fig. 3).
Effect of inoculation with endophytic bacteria on physiological characteristics of R. officinalis. Bars marked by different letters indicate significant differences based on LSD at P ≤ 0.05; error bars indicate means ± SE by LSD test (n = 3). T1 (Control); T2 (P. mediterranea KA7); T3 (P. oryzihabitans KU5); T4 (B. flexux KA10); T5 (P. aeruginosa SI12); T6 (P. japonensis KU13); T7 (P. putida KU2); T8 (C. lapagei KU14); T9 (P. agglomerans KA14); T10 (P. koreensis KA11); T11 (B. simplex KA2); T12 (B. subtilis KU21)
Principal component analysis of growth characteristics of R. officinalis in response to inoculation with endophytic bacteria
Principal component analysis of growth parameters of R. officinalis revealed that PC1 and PC2 accounted for 94.67% and 3.93% of the total data variation, respectively (Fig. 4). PC1 comprised treatments with T3 (P. oryzihabitans KU5), T6 (P. japonensis KU13), T7 (P. putida KU2), and T9 (P. agglomerans KA14) showed a strong relationship with several lateral branches and stem height. While in PC2, treatments with T4 (Bacillus flexus KA10), T7 (P. putida KU2), and T9 (P. agglomerans KA14) reported more influence on root biomass, total chlorophyll, and phenol content. This analysis showed that inoculation with potential plant growth-promoting bacterial endophytes had a significant effect on growth and productivity of R. officinalis.
PCA of growth characteristics of R. officinalis in response to endophytic bacterial isolates inoculation.1-T1 (Control), 2-T2 (P. mediterranea KA7), 3-T3(P. oryzihabitans KU5), 4-T4 (B. flexux KA10), 5-T5 (P. aeruginosa SI12), 6-T6 (P. japonensis KU13), 7-T7 (P. putida KU2), 8-T8 (C. lapagei KU14), 9-T9 (P. agglomerans KA14), 10-T10 (P. koreensis KA11), 11-T11 (B.simplex KA2), 12-T12 (B.subtilis KU21)
Effect on plant nutrient concentration
The data appended in Fig. 5 illustrate that treatment (T12) receiving B. subtilis KU21 inoculation had maximum significant increase in N, P and K content by 33.33%, 61.54% and 54.54%, respectively over untreated control and was at par with T5 (P. aeruginosa SI12), T8 (C. lapagei KU14) and T11 (B. simplex KA2) for N content and with T5 (P. aeruginosa SI12), T7 (P. putida KU2), T8 (C. lapagei KU14), T9 (P. agglomerans KA14) and T11 (B. simplex KA2) for P content.
Effect of liquid bacterial inoculum on nutrient concentration of R. officinalis. Bars marked by different letters indicate significant differences based on LSD at P ≤ 0.05; error bars indicate means ± SE by LSD test (n = 3). T1 (Control); T2 (P. mediterranea KA7); T3 (P. oryzihabitans KU5); T4 (B. flexux KA10); T5 (P. aeruginosa SI12); T6 (P. japonensis KU13); T7 (P. putida KU2); T8 (C. lapagei KU14); T9 (P. agglomerans KA14); T10 (P. koreensis KA11); T11 (B.simplex KA2); T12 (B. subtilis KU21)
Effect on soil properties and endophytic population
None of the treatments influenced the soil pH, E.C. and O.C. significantly in comparison with the initial soil test values (data not shown) recorded before the trial whereas the contents of available nutrients (NPK) increased significantly by the sole application of endophytic strains (Fig. 6). Maximum significant increase in available NPK content (16.79%, 36.26% and 7.56%) were recorded in treatment T12 (B. subtilis KU21) which was statistically at par with T8 (C. lapagei KU14) for N, T5 (P. aeruginosa SI12) and T8 (C. lapagei KU14) for P and T11 (B. simplex KA2) for K content. None of the treatments influenced the soil pH, E.C., O.C. significantly over soil initial test value (data not shown).
Effect of liquid bacterial inoculum on soil available nutrients (NPK). Bars marked by different letters indicate significant differences based on LSD at P ≤ 0.05; error bars indicate means ± SE by LSD test (n = 3). T1 (Control); T2 (P. mediterranea KA7); T3 (P. oryzihabitans KU5); T4 (B. flexux KA10); T5 (P. aeruginosa SI12); T6 (P. japonensis KU13); T7 (P. putida KU2); T8 (C. lapagei KU14); T9 (P. agglomerans KA14); T10 (P. koreensis KA11); T11 (B.simplex KA2); T12 (B. subtilis KU21)
Total endophytic bacterial count varied from 31.00 to 54 × 102 cfu/g root with the maximum count (54 × 102 cfu/g) in B. subtilis KU21 (T12) inoculated plants (Table 3).
Colonization studies with endophytic bacteria
In order to determine the ability to colonize and survive inoculated strains within plant roots, isolation of root endophytic bacteria from each treatment was done at termination of experiment. Control (uninoculated) was also used in colonization studies to ensure that the inoculated bacteria were recovered. In general, the representative control plant roots yielded no indigenous strain. Furthermore, the colony morphologies of the endophytes retrieved from inoculated plants were indistinguishable from the morphologies of the inoculated strains. The isolated strains with similar colony morphology were further identified biochemically and showed similar trends as that of inoculated ones (Table S3). The root endosphere of B. subtilis KU21 (T12) inoculated plants accounted for a maximum number (48 × 102 cfu/g) of bacterial colonies matching B. subtilis KU21 with maximum Simpson's index of dominance (0.79).
Intrinsic antibiotic resistance (IAR) of potential endophytic bacteria
Assessment of the IAR pattern of B. subtilis KU21, C. lapagei KU14 and P. aeruginosa SI12 showed considerable variation in terms of zone of clearance around the antibiotic discs (Table 4). It was observed that the tested strains were resistant to most of the antibiotics. But polymyxin B and colistin was lethal to P. aeruginosa SI12. Similarly, C. lapagei KU21 was susceptible to neomycin and Co-trimoxazole. Amikacin inhibited the growth of all three tested strains.
Discussion
Despite the great interest in plants used for the purpose of traditional medicine, little is known about the symbiotic associations of these plants with endophytic microorganisms (Silva et al. 2019). The present study evaluated the multifunctional potential of bacterial strains isolated from the roots of R. officinalis native to the North-Western Himalayan region of Himachal Pradesh, India. In this study, a collection of 42 root endophytic bacteria of R. officinalis were obtained from 4 different rosemary cultivating locations of Himachal Pradesh. These strains were identified using 16S rDNA sequencing and phylogenetic analysis. The isolates belonged to 15 genera and 32 species, mainly belonging to Proteobacteria and Firmicutes. These results confirmed rich endophytic pool in medicinal plants, in agreement with the previous studies (Elmagzob et al. 2019; Silva et al. 2019; Abdelshafy Mohamad et al. 2020). The predominant reported genera were Pseudomonas and Bacillus. Our findings are in line with the earlier studies which confirmed that Bacillus and Pseudomonas as PGPBEs have been widely found in medicinal plants such as Pinellia ternate, Lycium Chinese, Digitalis pupurae, (Miller et al. 2012); Ginkgo biloba (Yuan et al. 2012); Lonicera japonica (Zhao et al. 2015); Clerodendrum colebrookianum Walp. (Passari et al. 2016); Thyme vulgaris (Abdelshafy Mohamad et al. 2020); Fagonia mollis (ALKahtani et al. 2020); Pulicaria incisa (Fouda et al. 2021).
The main purpose of the current study was to understand the interaction of endophytes with the host plant which involves mobilization of nutrients, production of phytohormones, siderophores, and antagonistic compounds. That is why bacterial strains were screened for in vitro traits of plant growth promotion with an aim to obtain potential candidates. These strains exhibited multifaceted PGP traits. Similar investigations reported that endophytic bacteria exhibited multiple traits of PGP (Ahmed et al. 2019; Fouda et al. 2021). Among all, the strains of Bacillus, Pseudomonas and Cedecea exhibited the highest amounts of P-solubilization, siderophore, IAA, HCN, ammonia and lytic enzymes (chitinase, protease, and amylase). Earlier studies have also indicated that several Bacillus species isolated from medicinal plants produced phytohormones, solubilized P and improved growth of tomato (Abdelshafy Mohamad et al. 2020) and Pseudowintera colorata (Purushotham et al. 2020). Similarly, Pseudomonas species produced IAA and increased plant biomass of medicinal plant Astragalus mongholicus (Sun et al. 2019). In contrast, few studies had reported PGP potential of Cedecea. For example Beniassa et al. (2019) reported the potential of Cedecea as P-solubilizer, nitrogen fixer and HCN producers under in vitro conditions.
In vitro screening for antifungal activity showed that six strains inhibited the growth of all tested fungal pathogens (F. oxysporum, F. graminearum, and R. solani). These antagonistic strains exhibited various antifungal properties viz., siderophore, chitinase, protease, amylase, HCN, and ammonia production, etc. Thus, these endophytes can protect the plant from phytopathogenic fungi either by degrading the cell wall or by stimulating systemic resistance in plants (Mohamad 2018). Similar work has been carried out by Egamberdieva et al. (2017), and Fouda et al. (2021) who have reported that endophytic strains associated with medicinal plants Ziziphora capital and Pulicaria incisa respectively, exhibited antifungal activity by producing several antimicrobial compounds. As per phytopathogenic data, the biocontrol ability of these strains can be further assessed on wide range of host plant to protect against fungal pathogens.
To authenticate the results of in vitro studies, we selected endophytic isolates possessing multifarious PGPTs for in vivo experiments. In pot experiment, the application of endophytic strains significantly increased the physical growth parameters of R. officinalis seedlings over untreated control, especially P. aeruginosa SI12, C. lapagei KU14 and B. subtilis KU21. The increased shoot/ root parameters in the inoculated plants is attributed to the release of a variety of plant growth regulators in the rhizosphere, resulting in an altered root architecture that may have prompted an expansion in the total root surface area and consequently, improved the water and nutrient uptake, especially N and P, with positive effects on plant growth as a whole (Montano et al. 2014; ALKahtani et al. 2020). Similar results were documented by Fouda et al. (2021) with the isolates B. cereus BI-8 and B. subtilis BI-10 isolated from medicinal plant Pulicaria incisa which showed an increase in various physical growth parameters of maize seedlings.
The inoculation of R. officinalis seedlings with indigenous endophytes was also reported to have affected several physiological properties of plants. The significantly increased contents of total chlorophyll were observed in plants inoculated with B. subtilis KU21, followed by P. aeruginosa SI12, C. lapagei KU14. The results of the present study corresponded with those of Zhang et al. (2008) and Jang et al. (2018), who reported that PGPR B. subtilis GB03 and B. subtilis JS improved photosynthetic capability by augmenting photosynthetic efficiency and chlorophyll levels in Arabidopsis and Poplar, respectively. Plants possess a variety of antioxidant molecules predominantly phenols that alleviate the reactive oxygen species and defend host cells against adverse conditions. The results of the present study suggested that there were certain elicitors in the microbial cultures which played a vital role in enhancing the phenolic content of R. officinalis leaves. Several studies have likewise reported the promising effect of endophytic bacteria in boosting phenolics and flavanoid contents in Withania somnifera (L.), Dunal, and sweet basil (Gupta and Pandey 2015; Singh et al. 2016).
In case of nutrient acquisition, an improved NPK concentration in plants inoculated with B. subtilis KU21 followed by P. aeruginosa SI12, C. lapagei KU14 was observed. This enhanced capacity of the plant to attain and utilize more nutrients could be attributed to the bioinoculation effect on the stimulated root system (Egamberdieva et al. 2017). Moreover, these microbes are also capable of solubilizing mineral nutrients, resulting in increased levels of available NPK in the soil, thereby facilitating their availability to plants (Setiawati and Mutmainnah 2016; Adhikari and Pandey 2020). For example, Bacillus possessing mineral solubilizing and nitrogen-fixing ability, significantly increased NP content in maize (ALKahtani et al. 2020). The endophytic strains of the current investigation were found to be capable of solubilizing P and fixing N under in vitro conditions, thus providing more NP to R. officinalis seedlings.
In a nutshell, among the eleven selected bacterial endophytes for plant growth promotion experiment, B. subtilis KU21, P. aeruginosa SI12, and C. lapagei KU14 improved the growth parameters of R. officinalis significantly. The PGP bacteria resistant to antibiotics may have survival and competitive qualities required for a good bioinoculant to be used as biofertilizer (Kloepper et al. 1980). Further field trials for exploring the future application of these strains in enhancing R. officinalis productivity are well under way. The current study has also reported the first time occurrence of C. lapagei as an endophytic bacterium from R. officinalis. Although, some studies have reported C. lapagei as an inhabitant of rhizo and endorhizosphere possessing multiple plant growth-promoting traits (Zhang et al. 2008; Benaissa et al. 2019). The in vivo growth promotion studies for this strain have not been evaluated so far. The current findings point out the first ever evidence of the growth promoting potential of C. lapagei on R. officinalis under in vivo conditions.
Data availability
Not applicable.
Code availability
Not applicable.
References
Abbamondi GR, Tommonaro G, Weyens N, Thijs S, Sillen W, Gkorezis P (2016) Plant growth-promoting effects of rhizospheric and endophytic bacteria associated with different tomato cultivars and new tomato hybrids. Chem Biol Technol Agric. https://doi.org/10.1186/s40538-015-0051-3
Abdelshafy Mohamad OA, Ma JB, Liu YH, Zhang D, Hua S, Bhute S (2020) Beneficialendophytic bacterial populations associated with medicinal plant Thymus vulgaris alleviate salt stress and confer resistance to Fusarium oxysporum. Front Plant Sci 11:1–17
Adhikari P, Pandey A (2020) Bioprospecting plant growth promoting endophytic bacteria isolated from Himalayan yew (Taxus wallichiana Zucc). Microbiol Res. https://doi.org/10.1016/j.micres.2020.126536
Afzal I, Shinwari ZK, Sikandar S, Shahzad S (2019) Plant beneficial endophytic bacteria: mechanisms, diversity, host range and genetic determinants. Microbiol Res. https://doi.org/10.1016/j.micres.2019.02.001
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J King Saud Univ Sci 26:1–20
Ahmed EAS, Hassan EAE-T, EI-Tobgy KMK, Ramadan EM (2019) Characterization of endophytic bacteria associated with some medicinal plants. Arab Univ J Agric Sci Ain Shams Univ Cairo Egypt 27(5):2513–2526
ALKahtani MDF, Fouda A, Attia KA et al (2020) Isolation and characterization of plant growth promoting endophytic bacteria from desert plants and their application as bioinoculants for sustainable agriculture. Agronomy 10(9):1325. https://doi.org/10.3390/agronomy10091325
Altschul SF, Thomas LM, Alejandro AS, Jinghui Z, Zheng Z, Webb M, David JL (1997) Gapped BLAST and PSIBLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Bacon CW, White JF (2016) Functions, mechanisms and regulation of endophytic and epiphytic microbial communities of plants. Symbiosis 68:87–98
Baker AW, Schippers S (1987) Microbial cyanide production in the rhizosphere about potato yield reduction and Pseudomonas sp. mediated plant growth stimulation. Soil Biol Biochem 19:451–457
Bakthavatchalu S, Shivakumar S, Sullia SB (2012) Identification of multi-trait PGPR isolates and evaluation of their potential as biocontrol agents. Acta Biologica Indica 1:61–67
Benaissa A, Djebbar R, Abderrahmani A (2019) Diversity of plant growth-promoting rhizobacteria of Rhus tripartitus in the arid soil of Algeria (Ahaggar) and their physiological properties under abiotic stresses. Adv Hortic Sci 32:525–534
Bourhia M, Laasri FE, Aourik H et al (2019) Antioxidant and antiprolife rative activities of bioactive compounds contained in Rosmarinus officinalis used in the Mediterranean Diet. Evid-Based Complementary Altern Med. https://doi.org/10.1155/2019/7623830
Cappuccino JC, Sherman N (1992) Microbiology. A Laboratory Manual, 3rd edn. Benjamin/Cummings, Pub, San Francisco, pp 125–179
Collins CH, Lyne PM (1984) Microbiological methods. Butterworths, London
Cordero I, Ruiz-Diez B, Balaguer L, Richter A, Pueyo JJ, Rincon A (2017) Rhizospheric microbial community of Caesalpinia spinosa (Mol.) Kuntze in conserved and deforested zones of the Atiquipa fog forest in Peru. Appl Soil Ecol 114:132–141
de Favaro LCL, de Sebastianes FLS, Araujo WL (2012) Epicoccum nigrum P16, a sugarcane endophyte, produces antifungal compounds and induces root growth. PLoS ONE. https://doi.org/10.1371/journal.pone.0036826
Dehghani Bidgoli R, Azarnezhad N, Akhbari M, Ghorbani M (2019) Salinity stress and PGPR effects on essential oil changes in Rosmarinus officinalis L. Agric Food Secur 8:1–7
Egamberdieva D, Wirth S, Behrendt U, Ahmad P, Berg G (2017) The antimicrobial activity of medicinal plants correlates with the proportion of antagonistic endophytes. Front Microbiol 8:199p
Elmagzob AAH, Ibrahim MM, Zhang GF (2019) Seasonal diversity of endophytic bacteria associated with Cinnamomum camphora (L.) Presl. Diversity. https://doi.org/10.3390/d11070112
Faria PSA, Marques V et al (2021) Multifunctional potential of endophytic bacteria from Anacardium othonianum Rizzini in promoting in vitro and ex vitro plant growth. Microbiol Res. https://doi.org/10.1016/j.micres.2020.126600
Faust SD, Mikulewicz EW (1967) Factors influencing the condensation of 4 aminoantipyrine with derivatives of hydroxybenzene – II. Influence of hydronium ion concentration on absorptivity. Water Res 1:509–522
Fleming HP, Etchells JL, Costilus RH (1975) Microbial inhibition by an isolate of Pediococcus from cucumber brines. J Appl Microbiol 30:1040–1042
Fouda A, Eid AM, Elsaied A et al (2021) Plant growth-promoting endophytic bacterial community inhabiting the leaves of Pulicaria incisa (lam.) dc inherent to arid regions. Plants. https://doi.org/10.3390/plants10010076
Gaiero JR, McCall CA, Thompson KA, Day NJ, Best AS, Dunfield KE (2013) Inside the root microbiome: Bacterial root endophytes and plant growth promotion. Am J Bot 100:1738–1750
Glick BR (2012) Plant growth-promoting rhizobacteria: Mechanisms and Applications. Scientificia. https://doi.org/10.6064/2012/963401
Gordon SA, Palleg LG (1957) Quantitative measurement of IAA .Plant Physiology 10:37–38.
Gupta R, Pandey R (2015) Microbial interference ameliorates essential oil yield and diminishes root-knot infestation in sweet basil under field conditions. Biocontrol Sci Technol 25:1165–1179
Helrich K (1990) Official and tentative methods of analysis. Association of Official Analytical Chemists. William Star Wetglad, Washington
Higgins DJ, Thompson T, Gibson JD, Thompson DGH, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties, and weight matrix choice. Nucleic Acids Res 22:4673–4680
Holt JG, Krieg NR, Sneath PHA, Staley JT (1994) Bergey’s manual of Determinative Bacteriology, 9th edn. Williams and Wilkins, Baltimore
Jackson ML (1967) Soil chemical analysis. Oxford and IBH Publishing House, Bombay
Jackson ML (1973) Soil chemical analysis. Prentice-Hall of India, New Delhi
Jang JH, Kim SH, Khaine I et al (2018) Physiological changes and growth promotion induced in poplar seedlings by the plant growth-promoting rhizobacteria Bacillus subtilis JS. Photosynthetica 56(3):1188–1203. https://doi.org/10.1007/s11099-018-0801-0
Jensen ES (1987) Inoculation of pea by Rhizobium in planting furrow. Plant Soil 97:63–70
Khamwan S, Boonlue S, Riddech N, Jogloy S, Mongkolthanaruk W (2018) Characterization of endophytic bacteria and their response to plant growth promotion in Helianthus tuberosus L. Biocatal Agric Biotechnol 13:153–159
Kloepper JW, Schrot MN, Miller TD (1980) Effect of rhizosphere colonization by plant growth promoting rhizobacteria on potato development and yield. Phytopathology 70:1080–1082
Liotti RG, da Silva Figueiredo MI, da Silva GF, de Mendonça EAF, Soares MA (2018) Diversity of cultivable bacterial endophytes in Paullinia cupana and their potential for plant growth promotion and phytopathogen control. Microbiol Res 207:8–18
Liu Y, Guo J, Li L, Asem MD, ZhangnY MOA (2017) Endophytic bacteria associated with endangered plant Ferula sinkiangensis in an arid land: diversity and plant growth-promoting traits. J Arid Land 9:432–445
Maggini V, Miceli E, Fagorzi C, Maida I, Fondi M, Perrin E (2018) Antagonism and antibiotic resistance drive a species-specific plant microbiota differentiation in Echinacea spp. FEMS Microbiol Ecol. https://doi.org/10.1093/femsec/fiy118
McMullin DR, Nguyen HDT, Daly GJ, Menard BS, Miller JD (2018) Detection of foliar endophytes and their metabolites in Picea and Pinus seedling needles. Fungal Ecol 31:1–8
Mehta P, Walia A, Chauhan A, Shirkot CK (2013) Plant growth promoting traits of phosphate-solubilizing rhizobacteria isolated from apple trees in trans Himalayan region of Himachal Pradesh. Arch Microbiol 5:357–369
Miller KI, Qing C, Sze DM, Neilan BA (2012) Investigation of the biosynthetic potential of endophytes in traditional Chinese anticancer herbs. PLoS ONE. https://doi.org/10.1371/journal.pone.0035953
Mohamad OAA, Li L, Ma JB, Hatab S et al (2018) Evaluation of the antimicrobial activity of endophytic bacterial populations from chinese traditional medicinal plant Licorice and characterization of the bioactive secondary metabolites produced by Bacillus atrophaeus against Verticillium dahlia. Front Microbiol. https://doi.org/10.3389/fmicb.2018.00924
Montano PF, Villeges AC, Bellogin RA et al (2014) Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol Res 169:325–326
Mortensen CN (1997) Seed bacteriology laboratory guide. Danish Government Institute of Seed Pathology for Developing Countries. Denmark, Copenhagen, p 68
Panigrahi S, Mohanty S, Rath CC (2019) Characterization of endophytic bacteria Enterobacter cloacae MG00145 isolated from Ocimum sanctum with Indole Acetic Acid (IAA) production and plant growth promoting capabilities against selected crops. S Afr J. https://doi.org/10.1016/j.sajb.2019.09.017
Passari AK, Mishra VK, Leo VV, Gupta VK, Singh BP (2016) Phytohormone production endowed with antagonistic potential and plant growth-promoting abilities of culturable endophytic bacteria isolated from Clerodendrum colebrookianum Walp. Microbiol Res 193:57–73
Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. J Microbio 7:362–370
Purushotham N, Jones E, Monk J, Ridgway H (2020) Community structure, diversity and potential of endophytic bacteria in the primitive New Zealand medicinal plant Pseudowintera colorata. Plants. https://doi.org/10.3390/plants9020156
Robert WK, Selitrennikoff CP (1988) Plant and bacterial chitinase differ in antifungal activity. J Gen Microbiol 134:169–176
Sadegh Kasmaei L, Yasrebi J, Zarei M et al (2019) Influence of plant growth promoting rhizobacteria, compost, and biochar of Azolla on Rosemary (Rosmarinus Officinalis L.) growth and some soil quality indicators in a calcareous soil. Commun Soil Sci Plant Anal 50:119–131
Sambrook J, Fritsch EF, Maniatis T (1989) Diversity, biocontrol, and plant growth-promoting abilities of xylem residing bacteria from solanaceous crops. Int J Microbiol Res 38:14p
Santoyo G, Moreno-Hagelsieb G, del Carmen O-M, Glick BR (2016) Plant growth-promoting bacterial endophytes. Microbiol Res 183:92–99
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Setiawati C, Mutmainnah L (2016) Solubilization of potassium-containing mineral by microorganisms from the sugarcane rhizosphere. Agric Sci Procedia 9:108–117
Shaw FJ, Lin PF, Chen CS, Chen CH (1995) Purification and characterization of an extracellular α- amylase from thermos species. Bot Bull Acad Sin 36:195–200
Silva CF, Vitorino LC, Mendonça MAC, Araujo WL, Dourado MN, Albuquerque LC (2019) Screening of plant growth-promoting endophytic bacteria from the roots of the medicinal plant Aloe vera. S Afr J Bot. https://doi.org/10.1016/j.sajb.2019.09.019
Singh A, Gupta R, Srivastava M, Gupta MM, Pandey R (2016) Microbial secondary 637 metabolites ameliorate growth, in planta contents and lignification in Withania somnifera (L.) 638 Dunal. Physiol Mol Biol Pla 22:253–260
Sun H, Kong L, Du H, Chai Z, Gao J, Cao Q (2019) Benefits of Pseudomonas poae s61 on Astragalus mongholicus growth and bioactive compound accumulation under drought stress. J Plant Interact 14:205–212
Tandon HLS (2009) Methods of analysis of soil, plant, water, fertilizers, and organic manures. New Delhi, Fertiliser Development and Consultation Organisation
Vincent JM (1947) Distortion of fungal hyphae in the presence of certain inhibitors. Nature 150:850p
Witham FH, Bladydes DF, Delvins RM (1971) Experiments in plant physiology. Van Nostrand Reinhold; p, New York, p 245
Yang CM, Chang KW, Yin MH, Huang HM (1998) Methods for the determination of the chlorophylls and their derivatives. Taiwania 43(2):116–122
Yuan B, Wang Z, Qin S, Gui-H Z, Feng YJ, Li-H W, Ji-H J (2012) Study of the anti-sapstain fungus activity of Bacillus amyloliquefaciens CGMCC 5569 associated with Ginkgo biloba and identification of its active components. Bioresour Technol 114:536–541
Zhang H, Xie X, Kim MS, Kornyeyev DA, Holaday S, Pare PW (2008) Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant 6:264–273
Zhao L, Xu Y, Lai XH, Shan C, Deng Z, Ji Y (2015) Screening and characterization of endophytic Bacillus and Paenibacillus strains from medicinal plant Lonicera japonica for use as potential plant growth promoters. Braz J Microbiol. https://doi.org/10.1590/S1517-838246420140024
Acknowledgements
The authors are thankful to the Indian Council of Agricultural Research, New Delhi, India, for providing financial assistance through the All India Network Project on Soil Biodiversity and Biofertilizer.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization: AC; Methodology: MS, GS, Formal analysis and investigation: AC; Writing—original draft preparation: MS; Writing—review and editing: GS; Supervision: AC.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, M., Sood, G. & Chauhan, A. Bioprospecting beneficial endophytic bacterial communities associated with Rosmarinus officinalis for sustaining plant health and productivity. World J Microbiol Biotechnol 37, 135 (2021). https://doi.org/10.1007/s11274-021-03101-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-021-03101-7