Abstract
One marine bacteria Bacillus pumilus was isolated using allura red as ε-poly-L-lysine (ε-PL) secretion indicator. But actually the product was identified as poly-γ-L-diaminobutanoic acid (γ-PAB) by ionization-time-of-flight mass spectrometry, not coproduced with ε-PL. The polymerization degree of γ-PAB was 4–22, namely short-chain γ-PAB, compared with that in S. celluloflavus, and it exhibited stronger inhibitory activities against yeasts than long-chain γ-PAB but weaker activities against bacteria. The fermentative behavior of B. pumilus was investigated, and the γ-PAB production was 38.6 mg/L in shake flask and was enhanced to 284.2 mg/L in 5-L bioreactor by a pH control strategy. Interestingly, the suitable pH for B. pumilus to produce γ-PAB was 4.8, different from 4.0 for current Streptomyces strains, which suggests a potential new metabolic mechanism in B. pumilus as a novel γ-PAB producer. No studies on short-chain γ-PAB production in bacteria have been reported previously and we considered that this is a new discovery in the field of homopolymer research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Homopolymer amino acid is composed of a single type of amino acid in the skeleton, which is different from regular protein or peptide because it is not synthesized through ribosome peptide synthetases (NRPSs), namely poly(amino acid)s [1, 2]. In recent years, the poly(amino acid)s attract more and more attention because of their specific biological and chemical functions [3]. However, at present only four poly(amino acid)s have been discovered in natural products, specified as poly-ε-L-lysine (ε-PL) [3, 4], poly-γ-glutamic acid (γ-PGA) [3], poly-L-diaminopropionic acid (PDAP) [5] and poly-γ-L-diaminobutanoic acid (γ-PAB) [6], which were all produced by microorganism. Currently known, γ-PAB and PDAP were secreted by microorganism into their culture broths accompanied by ε-PL in Streptomyces celluloflavus and Streptomyces albulus PD-1, respectively [5, 6]. Subsequent studies have shown that, compared with ε-PL, the new two poly(amino acid)s have similar biological properties and similar synthesis mechanisms [5, 7].
So far, the majority reported poly(amino acid)s producing strains are limited in Streptomyces and Kitasatospora, such as S. albulus [8], S. lydicus [9], Kitasatospora PL623 [10], S. griseofuscus [11] and S. graminearus [12]. As is known to all, actinomycetes can produce a variety of antibiotics, for example streptomycin, rifamycin, gentamicin, natamycin and so on. Therefore, it is not strange that some species of Streptomyces can secrete ε-PL or γ-PAB, the antimicrobial substances. However, very rare bacteria germs had been reported able to produce poly(amino acid)s as well. One is Bacillus sp. SDNS which was isolated from marine water samples in Alexandria, Egypt [13]. The other is Bacillus cereus isolated from the hill region in CBD-Belapur, India [14]. Compared to Streptomyces, bacteria have advantages on easy cultivation, rapid growth and high fermentation efficiency. More importantly, the synthesis mechanism of poly(amino acid)s in bacteria may be different from that in Streptomyces. So the acquisition of a ε-PL (or other polymers) producing bacteria will be of great significance.
Previously, we reported a method to screening ε-PL producing strains in Streptomyces by methylene blue [11], but it was not efficient for bacteria screening because this dye had high toxic. Under these circumstances, we performed a new detection method by employing allura red as the indicator, which was negatively charged and non-toxic toward bacteria. Based on our previous screening experience, one bacteria identified as Bacillus pumilus was isolated from marine water samples, whose secretions were considered containing ε-PL. However, further research found that this poly(amino acid) was actually γ-PAB, rather than ε-PL.
Here, we describe the characterization of the novel biopolymer secreted by a marine bacteria Bacillus pumilus, which was to some extent different from the γ-PAB produced in S. celluloflavus such as degree of polymerization (DP), bacteriostatic activity and fermentation property. To the best of our knowledge, this is the first report that γ-PAB can be produced by bacteriological microorganism and non-coproduced with ε-PL.
Materials and Methods
Microorganisms and Culture Mediums
A bacteria strain, identified as Bacillus pumilus and named B. pumilus LS-1, was isolated from a marine water sample collected in an abandoned sea cucumber farm at Weihai, China. The nucleotide sequence of 16S rDNA gene for B. pumilus LS-1 was assigned to the GenBank with the accession no. MT795914. Other microorganisms used for antibacterial tests were purchased from China General Microbiological Culture Collection Center (CGMCC) (Beijing). Standard substance of ε-PL and L-diaminobutanoic acid (L-DAB)• HCl for chromatographic analysis was purchased from Macklin Reagent (Beijing) Co., Ltd. All other conventional reagents used for medium or biochemical detection were purchased from commercial supplier.
Solid agar plates named BTN medium, whose composition is (per liter): glucose 10 g, beef extract 2 g, tryptone 4 g and agar 20 g [15]. For screening of target strains, allura red solution and nystatin solution were added into BTN medium before pour Petri dish, and their final concentrations were 20 mg/L and 40 mg/L, respectively. The fermentation medium named M3G was composed of (per liter): glucose 80 g, (NH4)2SO4 10 g, yeast extract 5 g, MgSO4·7H2O 0.5 g, K2HPO4 0.8 g, KH2PO4 1.4 g, ZnSO4·7H2O 0.04 g and FeSO4·7H2O 0.03 g [15]. The pH of above three mediums was adjusted to 7.2 with NaOH solution before sterilization. What calls for special attention is that, replace the deionized water with sea water because B. pumilus is a marine strain.
Screening Method
Samples collected from seawater environments were spread on BTN agar plates containing allura red and nystatin after gradient dilution. The plates were placed in a biochemical incubator at 30℃ for cultivation. Four days later, observe the plates toward light and colonies which were surrounded by deep red haloes were picked up by sterile toothpicks and then inoculated on new BTN plates for streak cultivation.
Three loops of each strain were then inoculated into 40 ml of M3G medium on a rotary shaker at 200 rpm for 72 h. The fermentation broth was centrifuged and supernatant was used for preliminary verification to detect cationic polymers, by the reactions with dragendorff and methyl orange (MeO), respectively. Only those supernatants who could react with both of above reagents to generate red precipitate were adopted for the subsequent purification. The extracted products were then hydrolyzed to amino acid monomers by hydrochloric acid (6 mol/L) and subjected to amino acids detection employing thin layer chromatography (TLC) analysis. Ninhydrin solution was used as the chromogenic agent for spraying. Mobile phase was composed of water, glacial acetic acid, pyridine and butanol, respectively, with a volume ratio of 4:1:2:1.
Extraction and Purification of Products
The procedure for γ-PAB extraction was consistent with that of ε-PL, as described by Hirohara et al. [9]. After centrifugation, the supernatant was concentrated by a rotary evaporator and decolorized with activated carbon. pH of the filtrate was adjusted to 2.5 with HCl (1 M) and then mixed with methanol solution a by a volume ratio of 1:1. After stationary culture for 2 h, the turbid liquid was centrifuged and the resulting precipitate was dissolved in deionized water. Subsequent purification for γ-PAB was performed by ion-exchange chromatography on a TSK-gel protein column (C4-300, Tosoh, Japan), and a sodium phosphate buffer (50 mM, pH 7.2) was employed as equilibrium liquid. A gradient elution was carried out with supplement of NaCl solution. Finally, the purified γ-PAB was acquired by acetone precipitation and freeze drying, as a light yellow powder [6].
Identification of γ-PAB
The ionization-time-of-flight mass spectrometry (Axima-TOF, Shimadzu Biotech, Japan) based on matrix-assisted laser desorption (MALDI-TOF/MS) was employed to analyze the monomer information of polymer, in which the detailed steps were referred to Xia et al. [5]. High-performance liquid chromatography (HPLC) was used to determine hydrolysate of the oligomer to identify the amino acid composition, as described previously for identification of ε-PL [6]. Analytical reagent of L-diaminobutanoic acid (L-DAB) was adopted for reference material. A gel permeation chromatography (GPC) was employed to investigate the distribution of molecular weight on a TSK-gel CM-5PW column (Tosoh, Japan) [5].
Antibacterial Activity Tests
The antimicrobial activity of γ-PAB was determined by inhibiting other microorganisms including bacteria, mold and yeast evaluated, in which the semi-inhibitory concentration (IC50) was adopted as the evaluation criterion, as described previously for ε-PL or γ-PAB [5, 6]. Each of the tested microorganisms listed in Table 2 was inoculated into a liquid medium supplemented with γ-PAB in gradient concentrations at the beginning of cultivation. 24 h later, the OD of each cell suspension was measured by a visible spectrophotometer at 600 nm for biomass assessment. Tests analysis was performed in triplicate.
Shake-Flask Fermentation and Batch Fermentation
Each colony was inoculated on BTN plates for streak cultivation for 3 days at 30℃. Three loops of each strain were then inoculated into 40 ml of M3G medium on a rotary shaker at 200 rpm for 72 h. The fermentation broth was centrifuged and supernatant was used for measurement of γ-PAB production. Quantitative analysis of γ-PAB was performed according to the procedure described by Takehara et al., the same as ε-PL [6]. Tests analysis was performed in triplicate.
3 L of M3G medium was poured into a 5-L automatic fermentation tank (Dongfang Bio-engineering Equipment, Zhenjiang, China) and was suffered from steam sterilization. 240 mL of seed medium which has cultivated for 24 h was inoculated into the tank. The initial parameters were as follows: pH was 6.8, temperature was 30℃, stirring speed was 200 rpm, and ventilation rate was 3 L/min. 10 mL of culture broth was sampled every 4 h to determine concentration of γ-PAB, biomass (OD600) and residual glucose. Changes of the pH during fermentation were detected by a pH electrode. To maintain the pH value at 4.6, 4.8, 5.0 and 5.2, respectively, NH4OH solution was supplemented into the culture broth by an automatic pump. The level of dissolved oxygen (DO) was detected using a DO electrode and was maintained at 10% by adjusting the stirrer speed automatically.
Results
Screening of Cationic Polymer Producers by Allura Red
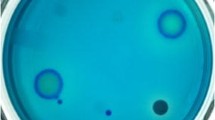
Cationic polymers including ε-PL are rich in positive charges; thus, they can reject cationic dyes to generate visible transparent haloes through electrostatic repulsion. By this intuitive phenomenon, methylene blue as an indicator was widely used for screening of ε-PL-producing strains [11, 16]. But actually, owing to the high toxicity of methylene blue, microorganisms could hardly grow on BTN agar plates even at a low concentration of 2 mg/L in our previous work. In this research, a new dye called allura red was adopted as the indicator, which provided a higher screening efficiency compared to methylene blue. Allura red is a food additive and widely used as edible pigment, so it is non-toxic toward humans and microbial. Colonies exhibited a normal growth on BTN agar even containing allura red as high as 20 mg/L. More importantly, in contrast to methylene blue, allura red is negatively charged so that it can attract cationic polymers such as ε-PL to generate more obvious red haloes (Fig. 1a). By this modified method, 32 strains from seawater environments were found able to secrete cationic substances.
a Red haloes in a BTN agar formed by the electrostatic attraction between allura red and cationic polymers, which were pointed by arrows. b The sediments reacted by alkaline substance and methyl orange or dragendorff reagent. c TLC profile of the amino acids. 1: non hydrolyzed extract; 2: hydrolyzed ε-PL standard; 3: L-lysine standard; 4: hydrolyzed extract (Color figure online)
Identification of the Cationic Substance
Our purpose was to screening ε-PL (or other polymers) producing bacteria, in which the red haloes were only a preliminary identification for positive-charged substances. ε-PL as a typical alkaloid can react with dragendorff reagent to form red precipitate, which is a specific reaction for this natural product. Besides, the mixture of cationic polymers and methyl orange can generate complex compound and result in precipitate as well, which is the basic principle for ε-PL measurement [1]. Therefore, the above two specific reactions were then employed to further confirm whether the positive-charged substances were cationic polymers or alkaloids. As a result, only one strain, designated as LS-1, whose culture broth showed positive phenomenon with the two reagents (Fig. 1b), was selected for further identification. The subsequent 16S rDNA sequence analysis (GenBank accession number: MT795914) indicated that the marine bacteria LS-1 was Bacillus pumilus, namely B. pumilus LS-1.
The product in culture broth of B. pumilus LS-1 was subjected to extraction and purification by an ion-exchange chromatography, which was consistent with the extraction methods for ε-PL. The purified product was a light yellow powder and was then hydrolyzed to amino acid monomers by hydrochloric acid (6 mol/L). The hydrolysate was spotted on silica gel plates for thin layer chromatography (TLC) analysis, with L-lysine as a reference material. However, the result of TLC was not what we expected because the hydrolyte was not L-lysine (Fig. 1c). We assumed that the unknown product showed potential as an oligomer owing to only one spot and the molecular weight of its monomer should be less than L-lysine because of the bigger Tf value (Fig. 1c). Through literature search, two new poly(amino acid)s named γ-PAB and PDAP attracted our attention [5, 6].
Mass spectrometry is a powerful tool for confirming the structure of compounds and the results of subsequent MALDI-TOF/MS spectrometry confirmed the above assumption (Fig. 2). The signal peaks exhibited a permutation with equal charge-to-mass ratio of 101.15, indicating that the molecular weight of monomer residue was 101.15. Besides, the repetitive unit structure suggested that the product was an oligomer. This result further confirmed that the oligomer was not ε-PL because the units of lysine residues was 128.17 [6, 17]. From the above, the molecular weight of the monomer of the polymer was supposed to be 119.17 (101.15 + 18.02). According to literature reports, γ-PAB was a homopolymer of diaminobutanoic acid (DAB) and its residual molecular weight was 100.12 [6], which was highly consistent with our results. HPLC result further confirmed that the hydrolyte of this product was indeed L-DAB compared with the standard as a reference material (Fig. 3a). Therefore, to sum up, our original intention was to screening bacteriological microorganism for ε-PL production, but actually a γ-PAB producer was obtained by accident.
At present, three kinds of reported poly(amino acid)s, namely ε-PL, γ-PAB and PDAP, were all produced by Streptomyces, and the latter two polymers were always associated with ε-PL secretion; thus, they were considered as by-products [5, 6]. But in this research, we found a marine bacteria B. pumilus LS-1 could produce γ-PAB as well, and there was no concomitant with ε-PL. Besides, from the GPC spectrum we could get information of DP for γ-PAB (Fig. 3b). The molecular weight of the polymer was distributed between 400 and2300 Da, suggesting that the DP of γ-PAB in our research was 4–22. Compared with the reported γ-PAB in S.celluloflavus [6], γ-PAB produced by B. pumilus LS-1 has shorter chains and more widely distribution of DP (Table 1). We considered that this was a new discovery in the field of homopolymer research.
Antimicrobial Activity of Short-Chain γ-PAB
Previous studies have reported the antimicrobial activity of γ-PAB and PDAP, which produced by S.celluloflavus and S.albulus, respectively, and these two homopolymers have very similar bacteriostatic properties [5, 6]. Compared with ε-PL (DP: 25–35 and molecular weight: 3000–4500 Da), γ-PAB (DP: 19–24 and molecular weight: 1950–2330 Da) and PDAP (DP: 6–17 and molecular weight: 500–1500 Da) showed weaker antimicrobial activity against all of the tested bacteria but exhibited stronger inhibitory activity against yeasts. However, in this research, the more interesting point was the antibacterial differences between short-chain γ-PAB (DP: 4–22) and long-chain γ-PAB (DP: 19–24) (Table 1). And then, we adopted the same species of microorganisms to investigate the IC50 and made comparison with literature data [6].
Table 2 shows the antimicrobial activity of short-chain γ-PAB toward bacteria and yeasts. No matter for gram-negative bacteria or gram-positive bacteria, short-chain γ-PAB exhibited almost equal antibiotic titer to long-chain γ-PAB, suggesting that they were at a disadvantage by comparison with ε-PL. However, for yeasts, short-chain γ-PAB showed stronger antibacterial activity than long-chain γ-PAB did, especially toward Saccharomyces cerevisiae and Yarrowia lipolytica (Table 2). This finding might bring us a new revelation that the antibacterial ability of cationic polymer was not only related to electric density, but also has a correlation with the chain length. Therefore, it is foreseeable that combining γ-PAB with ε-PL has a higher efficiency in antibacterial applications owing to the complementary advantages, otherwise the bacteriostatic spectrum will be incomplete.
Effects of Organic Acids on γ-PAB Synthesis
Effects of organic acids in the citric acid cycle on γ-PAB synthesis in B. pumilus LS-1 were then investigated in shake-flask fermentation (Fig. 4). Citric acid is the first synthetic product in TCA cycle and its exogenous addition significantly improved γ-PAB yield, which was 39.5% higher than the control, while succinate exhibited a strongest inhibition to γ-PAB production among these organic acids, with a decreasement over 70%. Effects of other organic acids employed in TCA cycle such as malate, aconitate and α-ketoglutarate were between the above two (Fig. 4). A few acids not belonging to TCA cycle such as acetate and lactate also strongly prohibited B. pumilus LS-1 from producing γ-PAB, similar to succinate. In addition, according to the hypothesis described by Takehara et al. [18], L-DAB is probably the precursor of γ-PAB, and in this research the addition of L-DAB significantly increased the yield of the polymer by 40% (Fig. 4), which was a support for this hypothesis. However, more conclusive evidence will be confirmed by isotope labeling experiments in the future.
Effect of pH on Cell Growth and γ-PAB Production
During the fermentation process for ε-PL production in Streptomyces strains, the pH can naturally decrease from 6.8 to 3.0 if pH is not controlled. But actually, the pH level was the most critical factor for ε-PL synthesis regulation and the suitable pH for cell growth is over 5.0, while for ε-PL synthesis was 4.0 [19, 20]. Consistent with that of ε-PL, the most optimal pH for γ-PAB production in S.celluloflavus is 4.0 as well [19]. However, our previous investigation in shake-flask fermentation showed that the ultimate pH of culture broth was 4.4 for B. pumilus LS-1 producing γ-PAB, far away from the 4.0 that of S.celluloflavus. Thus, it was very necessary to determine the suitable pH to produce γ-PAB in B. pumilus LS-1 at a higher degree. In this research, the pH was maintained at 4.6, 4.8, 5.0 and 5.2, respectively, by NH4OH solution in a controllable fermentor and the results are shown in Fig. 5. It was indeed obvious that the pH levels significantly influenced the γ-PAB production in B. pumilus LS-1. Following with the rise of pH, fermentation periods got shorter and shorter, from 33 h of pH 4.6–21 h of pH 5.2 (Fig. 5a), which of course meant that the consumption of carbon source was also accelerated (Fig. 5b). Similar to Streptomyces strains, higher pH was more suitable for cell growth and the highest OD600 for B. pumilus LS-1 at pH 5.2 reached 86.2, which was 44.2% higher than that at pH 4.6 (Fig. 5c). When pH was maintained at 4.8, the highest yield of ε-PL was achieved as 284.2 mg/L, 3.7-fold over that at pH 4.4 (Fig. 5d). Therefore, the optimal pH for γ-PAB production in B. pumilus LS-1 was 4.8, which was significantly different from the 4.0 in S.celluloflavus [6], suggesting that they have different physiological metabolic mechanisms or enzymatic characteristics.
Discussions
Poly(amino acid)s attract people’s great interest because of their unique biochemical properties. The most widely studied oligomer is ε-PL, whose producer was first discovered in 1977, and until now most founded ε-PL-producing strains belong to actinomycetes. Only two bacteria strains are reported to produce ε-PL, namely Bacillus sp. SDNS [13] and Bacillus cereus [14]. Further research found that there were two newly poly(amino acid)s identified as γ-PAB and PDAP, which were coproduced with ε-PL in Streptomyces, respectively [5, 6]. In 2008, the synthesis mechanism of ε-PL in S. albulus NBRC14147 was illuminated by Yamanaka [2], in which the non-ribosomal peptide synthetases (NRPS) naming Pls on cell membrane catalyze L-lysine to produce ε-PL. This catalytic mechanism has aroused great interest in the academic community and the subsequent research on PDAP synthesis in S. albulus PD-1 clarified a similar synthetic property with NRPS [7]. Therefore, the synthesis mechanism of ε-PL in bacteria or γ-PAB in microorganism is expected to be studied. From the above, acquisition of a ε-PL producing bacteria will be of great significance, which was the reason why we carried out mass of work to screening poly(amino acid)s producing strains.
However, we were committed to isolate ε-PL producing strains aiming bacteria for a long time using methylene blue as indicator but failed to obtain the colonies with transparent holes [11, 16]. In most cases, Streptomyces strains were easy to be obtained because ε-PL production in wild Streptomyces strains was high, usually 200–700 ml/L [11], and the electrostatic repulsion between ε-PL and methylene blue was strong so that visible holes could be formed. But for bacteria, ε-PL production in wild strains was very low, only 20–30 mg/L [13, 14], as a result it was very difficult to form visible holes owing to the weak interaction. Therefore, select a more sensitive indicator was the key to obtain bacteria for ε-PL production and the dye named allura red was usable (Fig. 1). By using the newly developed method, we carried out an exhaustive screening work from soil and marine water samples. In order to obtain bacteria, 40 mg/L of nystatin was added into BTN medium with the purpose of inhibiting mold. Finally, 32 bacteria strains were isolated by visible haloes, which proved the high efficiency of this method. And surprisingly, the B. pumilus LS-1, which was acquired from marine, was supposed to be a ε-PL producer, but actually a γ-PAB producer, because these two poly(amino acid)s had very similar biological properties.
To the best of our knowledge, this is the first report that γ-PAB can be produced by bacteriological microorganism, and non-coproduced with ε-PL. Besides, the DP of γ-PAB produced in B. pumilus LS-1 was 4–22, namely short-chain γ-PAB, compared with that in S. celluloflavus [6]. We considered that this was a new discovery in the field of homopolymer research. It is worthy to mention that the short-chain γ-PAB showed stronger antibacterial activity than long-chain γ-PAB did, especially toward the yeasts (Table 2). Therefore, it is foreseeable that combining γ-PAB with ε-PL has a higher efficiency in antibacterial applications owing to the complementary advantages.
According to Yamanaka et.al. [2, 21], the low pH environment is conducive to the accumulation of intracellular energy such as ATP, because the synthesis of ε-PL needs large amount of ATP consumption, which is a character of NRPS. Therefore the optimal pH for ε-PL production was around 4.0, and so did γ-PAB in S. celluloflavus [6]. But in our research, the fermentation performance of B. pumilus LS-1 differs from the reported Streptomyces strains owing to that the optimal pH for γ-PAB synthesis was 4.8 [6, 21], which suggests that it was a γ-PAB producer with new physiological metabolic mechanisms or enzymatic characteristics. These related investigations are undergo in our laboratory.
The γ-PAB production of B. pumilus LS-1 in shake flask was 38.6 mg/L and 284.2 mg/L in batch fermentation, which is still too low to meet the industrial needs. But as is known to all, compared to Streptomyces, bacteria have advantages on easy cultivation, rapid growth and high fermentation efficiency. So in the future meaningful work should concentrate on strain breeding and fermentation optimization to improve the γ-PAB production in B. pumilus LS-1. In conclusion, B. pumilus LS-1 is a potential bacteria worth studying the synthetic mechanism of short-chain γ-PAB and developing new industrial applications.
Data Availability
All data generated or analysed during this study are included in this published article.
References
Kunioka M (1997) Biosynthesis and chemical reactions of poly(amino acid)s from microorganisms. Appl Microbiol Biotechnol 47:469–475. https://doi.org/10.1007/s002530050958
Yamanaka K, Maruyama C, Takagi H (2008) epsilon-poly-l-lysine dispersity is controlled by a highly unusual nonribosomal peptide synthetase. Nat Chem Biol 4:766–772. https://doi.org/10.1038/nchembio.125
Obst M, Steinbüchel A (2004) Microbial degradation of poly(amino acid)s. Biomacromol 5:1166–1176. https://doi.org/10.1021/bm049949u
Hiraki J, Ichikawa T, Ninomiya S (2003) Use of ADME studies to confirm the safety of polylysine as a preservative in food. Regul Toxicol Pharm 37:328–340. https://doi.org/10.1016/S0273-2300(03)00029-1
Xia J, Xu H, Feng X, Xu Z, Chi B (2013) Poly(L-diaminopropionic acid), a novel non-proteinic amino acid oligomer co-produced with poly(ε-l-lysine) by Streptomyces albulus PD-1. Appl Microbiol Biot 97:7597–7605. https://doi.org/10.1007/s00253-013-4936-4
Takehara M, Saimura M, Inaba H, Hirohara H (2008) Poly(γ-L-diaminobutanoic acid), a novel poly(amino acid), coproduced with poly(ε-L-lysine) by two strains of Streptomyces celluloflavus. FEMS Microbiol Lett 286:110–117. https://doi.org/10.1111/j.1574-6968.2008.01261.x
Xu Z-X, Sun Z-Z, Li S, Xu Z, Cao C-G, Xu Z-Q, Feng X-H, Xu H (2015) Systematic unravelling of the biosynthesis of poly(L-diaminopropionic acid) in Streptomyces albulus PD-1. Sci Rep 5:17–28. https://doi.org/10.1038/srep17400
Chen X-S, Ren X-D, Zeng X, Mao Z-G (2013) Enhancement of ε-poly-L-lysine production coupled with precursor L-lysine feeding in glucose-glycerol co-fermentation by Streptomyces sp. M-Z18. Bioprocess Biosyst Eng 36:1843–1849. https://doi.org/10.1007/s00449-013-0958-7
Hirohara H, Munenori T, Masayuki S (2006) Biosynthesis of poly(ε-L-lysine)s in two newly isolated strains of Streptomyces sp. Appl Microbiol Biotechnol 73:321–331. https://doi.org/10.1007/s00253-006-0479-2
Zhang Y, Feng X-H, Xu H, Yao Z (2010) ε-Poly-L-lysine production by immobilized cells of Kitasatospora sp. MY 5–36 in repeated fed-batch cultures. Bioresour Technol 14:5523–5527. https://doi.org/10.1016/j.biortech.2010.02.021
Li S, Tang L, Chen X-S, Liao L-J, Li F, Mao Z-G (2011) Isolation and characterization of a novel ε-poly-L-lysine producing strain: Streptomyces griseofuscus. J Ind Microbiol Biotechnol 38:557–563. https://doi.org/10.1007/s10295-010-0803-9
Li S, Li F, Chen X-S, Wang L, Tang L, Mao Z-G (2012) Genome shuffling enhanced ε-Poly-L-Lysine production by improving glucose tolerance of Streptomyces graminearus. Appl Biochem Biotechnol 166:414–423. https://doi.org/10.1007/s12010-011-9437-2
Nermeen A, Abeer E, Samia S, Dunja M, Soraya A (2012) Antibacterial and anticancer activity of ε-poly-L-lysine (ε-PL) produced by a marine Bacillus subtilis sp. J Basic Microb 52:1–10. https://doi.org/10.1002/jobm.201100290
Anuj HC, Madhavi R (2015) Enhancement of ε-poly-L-lysine (ε-PL) production by a novel producer Bacillus cereus using metabolic precursors and glucose feeding. Biotech 5:839–846. https://doi.org/10.1007/s13205-015-0291-8
Wang L, Chen X-S, Wu G-Y, Zeng X, Ren X-D, Li S, Mao Z-G (2016) Genome shuffling and gentamicin-resistance to improve ε-poly-L-lysine productivity of Streptomyces albulus W-156. Appl Biochem Biotechnol 180:1601–1617. https://doi.org/10.1007/s12010-016-2190-9
Nishikawa M, Ogawa K (2002) Distribution of microbes producing antimicrobial ε-Poly-L-Lysine polymers in soil microflora determined by a novel method. Appl Environ Microb 68:3575–3581. https://doi.org/10.1128/AEM.68.7.3575-3581
Shima S, Sakai H (1981) Poly-L-lysine produced by Streptomyces. part III. chemical studies. Agric Biol Chem 45:2497–2502. https://doi.org/10.1080/00021369.1981.10864930
Takehara M, Hibino A, Saimura M, Hirohara H (2010) High-yield production of short chain length poly(ε-L-lysine) consisting of 5–20 residues by Streptomyces aureofaciens, and its antimicrobial activity. Biotechnol Lett 32:1299–1303. https://doi.org/10.1007/s10529-010-0294-9
Kahar P, Lwata T, Hiraki J (2001) Enhancement of ε-poly-L-lysine production by Streptomyces albulus strain 410 using pH control. J Biosci Bioeng 91:190–194. https://doi.org/10.1016/S1389-1723(01)80064-5
Kito M, Takimoto R, Yoshida T, Nagasawa T (2002) Purification and characterization of ε-poly-L-lysine-degrading enzyme from the ε-poly-L-lysine tolerant Chryseobactrium sp QJ7. J Biosci Bioeng 1:92–104. https://doi.org/10.1016/S1389-1723(03)90105-8
Yamanaka K, Kito N, Imokawa Y (2010) Mechanism of ε-poly-L-lysine production and accumulation revealed by identification and analysis of an ε-poly-L-lysine degrading enzyme. Appl Environ Microbiol 68:5669–5675. https://doi.org/10.1128/AEM.00853-10
Acknowledgement
We appreciated Liang Wang for mass spectrometry analysis in the manuscript.
Funding
This work was financially supported by Shandong Natural Science Foundation (ZR2019BC044).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no competing interests.
Ethical Standards
All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, S., Yao, Y., Hu, S. et al. Short-Chain Poly(γ-diaminobutanoic acid), A Poly(amino acid) Produced by a Marine Bacteria Bacillus pumilus. Curr Microbiol 78, 1142–1149 (2021). https://doi.org/10.1007/s00284-021-02371-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-021-02371-6