Abstract
γ-Polyglutamic acid (γ-PGA) is a biosynthetic outcome of glutamic acid polymerization by microbes. In the current study, we have isolated Bacillus methylotrophicus on solid differential media containing methylene blue. This is the first report mentioning the use of methylene blue to distinguish the monomeric and polymeric form of glutamic acid in the liquid medium using UV-Vis spectrophotometer. Our method can simplify the analytical process of γ-PGA confirmation using the aforementioned studies. This screening protocol is sensitive to the detection of γ-PGA quantities as low as 3 μg/mL; thus, the potent producers can be effectively screened. Furthermore, we have carried out process optimization of the present strain for γ-PGA production wherein we could obtain 1.4-fold improvement in the yield with respect to utilization of carbon source and 2.6-fold increase with respect to nitrogen source under submerged fermentation at a shake flask level. We have shown an increase in γ-PGA titer from 1.5 to 36 g/L using mannitol, monosodium glutamate, peptone, and tween 20.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glutamic acid is a monomeric unit of protein which on polymerization results in γ-polyglutamic acid (γ-PGA) [1]. γ-PGA is a superabsorbent, edible, and biodegradable polymer that is exploited for its properties in various fields of agriculture, cosmetics, medical, food, and waste water purification industries. γ-PGA can exist in two isoforms; namely α and γ depending upon the position of the carboxyl group that binds with the amine group forming a peptide. α-PGA is chemically synthesized with > 10 kDa molecular weight while γ-PGA can only be produced by the microbial process and its molecular weight ranges from ~ 100 to > 1000 kDa [2,3,4,5]. Gamma glutamyl linkage between the α-amino and γ-carboxy chains makes it a polyamino acid that cannot be degraded easily by naturally occurring proteases. However, the bacteria that produce γ-PGA generally contain specific enzymes that digest the polymer to monomeric units making it a biodegradable biopolymer.
Several Bacillus sp. have been reported to produce γ-PGA, nevertheless, few gram-negative bacteria, some archaea and eukaryotes can also bio-synthesize this polymer but not to a significant extent due to various reasons [6,7,8,9,10,11].
Role of γ-PGA extends from providing nutrition to survival in adverse conditions. Many bacteria possessing γ-PGA degrading enzymes utilize digested monomeric form of glutamic acid as a nutrient during its starvation period. Some microbes like Fusobacterium nucleatum produce γ-PGA for biofilm formation or to survive in adverse conditions like high salt concentrations [7, 12].
Owing to its importance in diverse fields, researchers have made a lot of efforts in screening the potent γ-PGA producing microorganisms followed by bio-polymer fermentation at shake flask levels. Traditional methods of screening for γ-PGA producing microorganisms are carried out in two steps. Firstly, the source containing mixed flora is plated on enrichment media and incubated under desired conditions. Secondly, γ-PGA producing microorganisms are selected on the basis of colony morphology. Later, bioprocess modeling is undertaken wherein, the microbes producing the most viscous broth are selected and the yield is further quantified using the HPLC [13]. However, these protocols are time consuming and laborious, proving to be a limitation towards screening of high yield producers of γ-PGA. This limitation was first addressed by Zeng et al. [14] who devised a high throughput screening of γ-PGA producing microorganisms using neutral red as an indicator in the plate. The downstream steps were also modified using ethanol for precipitation and dissolving the pellet further in water for quantification using UV-Vis spectrophotometer and HPLC. It is worth mentioning here that both γ-PGA and glutamic acid absorb UV at nearby wavelengths, i.e., 215 and 216 nm wavelengths, respectively. It is difficult to distinguish γ-PGA from UV-Vis studies if glutamic acid is also present in the same medium. Hence, there is an urgent need for a rapid identification of the biopolymer production directly in the fermentation broth to eliminate the number of steps involved in the analysis of the final product.
Therefore, the focus of our study was to establish a rapid screening protocol for γ-PGA production on the solid agar plate using methylene blue. γ-PGA, by virtue of the carboxylic group, has the tendency to form non-covalent interaction with the basic dyes like methylene blue. This aspect has been largely exploited for the adsorption of methylene blue by γ-PGA [15]. We have extrapolated this aspect of the non-covalent interaction between γ-PGA and methylene blue to the liquid culture-based qualitative analysis using UV-Vis spectrophotometer.
The studies for γ-PGA production are limited to B. subtilis and B. licheniformis with very few reports on B. methylotrophicus [16,17,18]. In the present study, we have also made an attempt to isolate and study the γ-PGA production with B. methylotrophicus.
Material and Methods
Microorganisms and Culture Condition
Japanese dish Natto, procured from Japan, and soil samples, collected from India, were used to isolate the γ-PGA producing microorganisms. Soil samples were collected from the rhizosphere of pepper, cabbage, sweet corn, fenugreek leaves, barley, tomato, and sugarcane plants from the agricultural fields in Pirangut, Pune, India. One gram of the soil sample was suspended in 20 mL of 0.85% (w/v) saline. This solution was made in a 50 ml falcon tube and the tube was later kept under shaking conditions (200 rpm) at room temperature for 4 h. 0.1 mL of suspended soil sample solution was inoculated in 10 mL of liquid basal media containing 10 g/L glucose, 5 g/L yeast extract, 5 g/L monosodium glutamate (MSG), 0.5 g/L K2HPO4, 0.5 g/L KH2PO4, and 0.1 g/L MgSO4.7H2O. The pH was maintained at 6.5. Swabs of Natto were also inoculated in the basal media as mentioned above, followed by spreading 0.1 mL of the culture on agar plates. The plates were incubated at 37 °C for 24 h. The microbes were later isolated and purified by streaking on basal agar media containing 0.04 g/L methylene blue. The liquid culture studies were performed in 250 mL shake flasks with 100 mL filling volume. The submerged fermentation was carried out for 96 h at 37 °C and 120 rpm shaking.
Identification of the γ-PGA Producing Microorganism
The isolated microbial strain producing γ-PGA was identified using 16S rRNA (ribosomal DNA)-based gene sequencing. Genomic DNA of the selected strain was subjected to polymerase chain reaction (PCR) to amplify the 16SrRNA gene fragment using 27F and 1492R primers (Fermentas). Amplicons obtained were cloned into pTZ57R/T vector using Thermo Scientific InsTAclone PCR cloning Kit. Restriction digestion of the positive clones was carried out using Bam H1 and EcoR1. Confirmed clones were sequenced using M13 forward and reverse primers followed by BLAST analysis. The γ-PGA producing culture was later deposited to National Collection of Industrial Microorganisms (NCIM), Pune, and is available with the accession number NCIM 5536.
Process Optimization at Shake Flask Level with B. methylotrophicus
The isolate of the bacterial strain, B. methylotrophicus, was subjected to process optimization at a shake flask level for γ-PGA production. To study the effect of different carbon sources; sucrose, galactose, fructose, glucose, xylose, lactose, maltose, mannitol, inositol, and arabitol were assessed for the biopolymer production at a concentration of 50 g/L each. All the other components of the media were kept constant. In addition to the carbon source, the other components present in the production medium were 60 g/L monosodium glutamate, 10 g/L sodium chloride, 60 g/L peptone, and 0.1% (v/v) tween 20. In the second subsequent experiment, the impact of the nitrogen sources was assessed. The inorganic nitrogenous sources namely, ammonium chloride, ammonium nitrate, potassium nitrate, sodium nitrate, ammonium sulfate, and organic nitrogenous sources like peptone and yeast extract were assessed for γ-PGA production. In this set, 50 g/L mannitol, 60 g/L monosodium glutamate, 10 g/L sodium chloride, and 0.1% (v/v) tween 20 were the other components of the production media. The initial pH was set at 6.5.The other aspects of the bioprocess which were also optimized were aeration and the agitation speed. The temperature for all the experiment setup was kept at at 37 °C.
Aeration and Size of Inoculum
Aeration studies were initially carried out using media containing 50 g/L mannitol and 60 g/L peptone, 60 g/L monosodium glutamate, 10 g/L sodium chloride, and 0.1% (v/v) tween 20. Fermentation media was taken in 500 mL flask with the volume of 50, 100, 150, and 200 mL. Two percent (v/v) of inoculum size which was grown overnight was used to start the fermentation process. Inoculum media comprised of 10 g/L glucose, 5 g/L yeast extract, 5 g/L monosodium glutamate, 0.5 g/L K2HPO4, 0.5 g/L KH2PO4, 0.1 g/L MgSO4.7H2O, and the media pH was adjusted to 6.5. Based on the maximum concentration of γ-PGA production obtained in the sets, 50 mL of production media was taken in 500 mL flask to provide maximum aeration to the culture in the subsequent experiments. The flasks containing media were incubated at 125 rpm at 37 °C, and the experiment was continued till 96 h.
γ-PGA Purification
After fermentation, the γ-PGA broth was centrifuged at 12000 rpm for 30 min using Eppendorf centrifuge 5810 R. The supernatant comprising γ-PGA was passed through 500 kDa filter membrane. This helped in the removal of the lower molecular weight impurities. The concentrated broth was then mixed with two volumes of absolute ethanol. Precipitates obtained out of the γ-PGA containing broth were dried using lyophilizer Vir 2K BTXL-75. An aqueous solution of the dried pellets was prepared and further analyzed using UV-Vis spectrophotometer and UHPLC.
Analytical Methods
Qualitative Analysis of γ-PGA Using UV-Vis Spectrophotometer
Standard solutions of 0.1 g/L γ-PGA and 0.1 g/L glutamic acid were analyzed using Varian Cary 5000 UV-Vis-NIR spectrophotometer. Differentiation between the monomeric and polymeric forms of glutamic acid was attempted using various dyes like methylene blue (Himedia), bromo-cresol green (SRL), methyl orange (SRL), acridine orange (Himedia), neutral red (Fischer Scientific), and phenol red (Himedia) at a concentration of 25 μM. Scanning of the spectra was performed between 200 to 800 nm.
Quantification of γ-PGA by UHPLC
Microorganisms selected from methylene blue-containing agar plate were further examined for γ-PGA production using Thermo Scientific Dionex Ultimate 3000 UHPLC system combined with WPS 3000 RS auto sampler, LPG 3400 RS Pump, TCC 3000 RS column compartment, VWD 340 RS variable wavelength detector. A Waters Size exclusion column (BEH 450 SEC 4.6 × 450 mm, 2.5 μm) with a mobile phase of 0.1 M sodium phosphate buffer, pH 6.8, and at a flow rate of 0.3 mL/min was used. The UHPLC analysis was done to quantify the polymer production. The standard for the experiment was γ-PGA sodium salt powder procured from Hebei Fulong Import and Export Co. Ltd. China, (more than 1000 kDa molecular weight). A calibration curve was made using 1 g/L γ-PGA solution prepared in 0.1 M sodium phosphate buffer, pH 6.8. The range used was 0.1 to 0.5 μg of the standard.
Confirmation of γ-PGA by Chemical and Enzymatic Hydrolysis
0.1 g/L standard γ-PGA sodium salt, 1 g/L L-glutamic acid sodium salt and 0.1 g/L culture precipitate of the broth obtained after ethanol purification were dissolved in 0.1 M sodium phosphate buffer. This was followed by 8 M HCl treatment for chemical hydrolysis. The sample and standards were incubated at 37 °C for 24 h. The reaction was terminated by the addition of 8 M NaOH. The final pH of the samples was adjusted to 7 before injecting in UHPLC.
Enzymatic hydrolysis of test sample and standards were carried out with 0.001 g of carboxypeptidase G (5 Units) from Pseudomonas sp. (Sigma-Aldrich). The enzyme was dissolved in 300 μL of 0.2 M Tris-HCl buffer pH 7.5 containing 0.001 M zinc chloride. The test sample was prepared by mixing 0.96 mL of 0.01 M sodium phosphate buffer (pH 6.8) to the 10 μL culture precipitate and standards. Thirty microliter of enzyme carboxypeptidase G (0.1 Unit) was added to the above reaction mixture and incubated at 30 °C for 24 h followed by the UHPLC analysis.
Results and Discussion
Screening γ-PGA Producers
γ-PGA producing microbes were screened from various soil samples on differential agar media containing methylene blue using serial dilution spread plate method. The best optimum workable concentration of the dye for identifying γ-PGA producers from the crowded mix of colonies was observed to be 0.04 g/L as it was sufficient to produce a zone of clearance with or without a prominent zone of condensed dye around the colony within its surrounding on the agar plate as shown in Fig. 1.These results are comparable to similar studies conducted on the screening of a cationic polyamino acid, ɛ-poly-l-lysine which was carried out using an acidic dye [19]. Based on the similar principle, methylene blue was incorporated into the agar medium wherein probable electrostatic interactions could take place between γ-PGA produced by the microbe and dye. Binding of methylene blue on agar surface is a chemical adsorption process [20]. γ-PGA being an anionic biopolymer promotes chemical adsorption of cationic dyes. Electrostatic interaction of negatively charged carboxylate groups of the polymer with a positive charge of the dye molecules may lead to the chemical adsorption process. Methylene blue, a basic dye, can exist in either monomeric or dimeric form through adsorption process [21]. We observed that the aqueous solution of methylene blue exhibited the existence of above mentioned phenomenon of monomer and dimer form using UV-Vis spectral studies. In our studies, we later examined the formation of more of dimer and trimer species of methylene blue on binding with γ-PGA in solution as evidenced from blue shift in the absorption spectra. Changes occurring in the dye at the molecular level due to γ-PGA stimulated our studies to use the dye in solid media for screening γ-PGA producers.
During screening, the plating of the respective soil samples yielded 350 colonies, out of which 34 colonies were sub-cultured based on difference in their morphologies. These 34 cultures were later transferred to the methylene blue containing agar plate for screening the biopolymer production. The screening on the solid media with methylene blue resulted in 25 presumably γ-PGA producing colonies (Table 1). Two colonies were found to give a false negative test where the culture cells did not produce a zone of clearance in the given media but were found to be positive when analyzed using UHPLC. Four cultures gave a false positive test where the cultures produced a zone of clearance but were found to be negative for the product under UHPLC analysis. The chance of losing potent cultures using methylene blue was calculated to be 5.9%. The false positive test might be due to the production of other types of polymers by the bacteria which carry a similar type of properties as that of γ-PGA. Based on the γ-PGA production, Bacilli and E. coli were respectively tested as positive and negative control cultures on the plate. Out of the 34 cultures that were screened, we took our studies forward with the colony that was observed to produce maximum γ-PGA amongst all the cultures i.e. IC2 (1.5 g/L). IC2 was isolated from the soil in the vicinity of chilly plant (Capsicum annuum) roots collected from the agricultural field of Pirangut, Pune, India and was identified as B. methylotrophicus by 16S rRNA sequencing. The culture was later deposited at National Collection of Industrial Microorganisms (NCIM), Pune, India where it was deposited as Bacillus sp. NCIM 5536.
Development of Spectrophotometric Method for Rapid Detection of γ-PGA in Fermentation Broth
Methylene blue was used for screening of γ-PGA producing microorganisms on agar plate after studying various dyes. The bacteria that were positive for the zone of clearance on agar plate were used for γ-PGA production in the basal media. The traditional method of analyzing the final product involves precipitation of the biopolymer using absolute alcohol followed by drying. The dried pellet is then dissolved in water, digested by strong acids and later analyzed by TLC or HPLC methods. To avoid a tedious and time-consuming method, in the present study, we have tried to reduce the number of steps involved in the process and thereby also to reduce the total time required for the experiment. Here we report, a method of analyzing the intact form of γ-PGA produced in the fermented broth by directly subjecting the broth to UV-Vis spectrophotometric analysis. The fermentation media contains both glutamic acid and γ-PGA. Absorption maxima of aqueous solution of glutamic acid and γ-PGA have been reported at 215 [22] and 216 nm [23] wavelengths, respectively. It was thus earlier difficult to differentiate between the monomer and polymer of glutamic acid in aqueous media by directly subjecting it to UV-Vis spectrophotometer due to similar wavelength absorption patterns. To develop a quick screening method for γ-PGA production, we screened several dyes, namely bromo cresol green, phenol red, neutral red, acridine orange, and methylene blue for UV-Vis spectral studies. Amongst various dyes that were tested, methylene blue clearly stood out in the UV-Vis spectral profile on binding to the γ-PGA, whereas the absorbance pattern of methylene blue with glutamic acid remained unchanged. The screening of the γ-PGA producing culture makes it imperative to differentiate the monomer from the polymer as during fermentation, a monomer of glutamic acid may serve as a substrate, and hence the proper selection of dye was crucial for developing the analytical method. Methylene Blue is a cationic dye with a planar structure and has been known to bind with proteins lacking tryptophan and tyrosine groups [24]. The dye by itself exists in various forms such as monomer, dimer, trimer, tetramer, and aggregate depending upon the physico-chemical conditions [25, 26]. In aqueous solution, the dye is present as a combination of its monomeric and dimeric forms. However, in the presence of γ-PGA, the spectral profile of methylene blue undergoes change. With increasing concentrations of γ-PGA, there is a decrease in the monomer peak with a simultaneous increase in the dimer and the appearance of trimer peak at around 571 nm as shown in Fig. 2. This change in the monomeric form of methylene blue to trimer formation in the presence of γ-PGA suggests a phenomenon of self-aggregation exhibited by the dye. Methylene blue was observed to specifically bind with γ-PGA standard as well as test sample, respectively. The abovementioned dye did not show any affinity for glutamic acid using UV- Vis Spectrophotometer. The interaction of methylene blue with the other components of the media such as BSA, yeast extract, and peptone was also tested. These proteins showed no change in the absorption pattern indicating the absence of their interaction with the dye. The complexity of monomer to dimer conversion involves hydrogen bonding, hydrophobic forces, and electrostatic interactions [27]. Spectroscopic studies in our experiments suggested that the dye undergoes oligomerization on binding with γ-PGA as evident from the occurrence of the absorption peak at 571 nm. Hence, the dye was found to be suitable for primary screening. Methylene blue was found to be sensitive to the presence of γ-PGA in microgram level (3 μg/mL). Therefore, the method is quick and sensitive for analyzing the fermentation broth for the production of γ-PGA as observed in the change of spectral profile in Fig. 2. The affinity of the dye for the bio-polymer offered a new approach for it to be used in agar plates to differentiate the positive γ-PGA producers from the negative ones.
Absorption pattern of methylene blue exhibits monomer peak at 664 nm and shoulder of dimer at 614 nm. Monosodium glutamate and 0 h fermentation media caused inconsequential changes with methylene blue when bound with it. Fermentation broth at 96 h when bound with methylene blue changed the structure of the dye causing the absorption of UV light to shift. PGA standard 0.0008% on binding with the dye triggered its trimerization indicating the presence of the biopolymer
Validation of γ-PGA Production Using UHPLC
Analysis Using Size Exclusion Chromatography
Process for γ-PGA standardization was carried out using UHPLC. The standard was observed to elute at the retention time (RT) of 2.9 min as shown in Fig. 3 with chromatogram labeled as 1 and was compared with monosodium glutamate labeled as 2.
Conventional methods of γ-PGA analysis included digestion of polymer into monomeric form and their analysis using either thin layer chromatography (TLC) or HPLC. These methods were quite laborious, time-consuming, and indirect. We could analyze the biopolymer production using UHPLC with precision directly in the broth without hydrolyzing it to determine its concentration. In our method, we had used UV detector which narrowed down the possibility of elution of amino acids only. Elution of high molecular weight γ-PGA was carried out on size exclusion column. Culture precipitate containing majority of γ-PGA was observed to elute at the same time as that of standard (chromatogram labeled as 3) along with few low molecular impurities. Thus, this method can be used for quick and accurate quantification of the final product.
Chemical Hydrolysis of Culture Precipitates
To rule out the possibility of any other high molecular weight entity eluting at the same position of γ-PGA, hydrolysis experiments were carried out where the sample was treated with 8 M HCl. γ-PGA standard and fermented broth obtained from B. methylotrophicus showed hydrolysis of γ-PGA to glutamic acid as shown in Fig. 3. with chromatogram labeled as 4. HCl was observed to have broken down the complex form of this poly amino acid to its simpler form as analyzed using UHPLC. HCl caused acidic hydrolysis of peptide bonds between the two consecutive glutamic acid residues in γ- PGA. Broadness of the peak between 6 to 7 min RT suggests the elution of oligomers, as well as monomers of the biopolymer.
Confirmation of γ-PGA Using Carboxypeptidase G
The presence of γ-PGA was further checked using the enzyme Carboxypeptidase G. This enzyme is an exopeptidase that cleaves the peptide bond between the γ-carboxyl group and α-amino group attached with the next L-glutamic acid moiety in γ-PGA [28]. This enzyme specifically breaks γ-PGA into its simpler forms. The presence of the biopolymer was confirmed after the treatment with Carboxypeptidase G followed by detection of the hydrolyzed product through UHPLC as shown in Fig. 3 with chromatogram labeled as 5. Simpler products of the biopolymer were detected between 6 to 7 min RT with monomers being eluted at 6.9 RT where glutamate elution was also observed.
Process Optimization at Shake Flask Level
After the analysis of γ-PGA production, optimization of fermentation conditions with respect to media components, pH, and aeration was attempted. Process optimization was carried out to increase the concentration of the desired final product. Effect of varying sources of carbon and nitrogen were assesed. The γ-PGA production was therefore increased from 1.5 to 36 g/L through media optimization studies.
Effect of Aeration, Agitation, and pH on γ-PGA Production
γ-PGA production by Bacilli is known to be an aerobic process where the requirement of oxygen is more during the growth of the microorganisms. In the final hours of the product formation, the broth tends to become viscous restricting the supply of oxygen and nutrients to the organisms. Hence, the agitation plays a crucial role for adequate mass transfer. In our studies, we observed a decreasing trend in the γ-PGA production on increasing the volume ratio of fermentation broth to flask from 0.1 to 0.4 as shown Fig. 4. This indicated the requirement of more aeration with less volume of the media in the flasks. The gradual increase in the volume of media proved to be deterrent for microbial growth and γ-PGA production as the 500 mL flask containing 50 mL of media showed the maximum γ-PGA production at a concentration of 36 g/L compared to the flask containing 200 mL media where the production dipped to 5 g/L. Maximum growth of the culture cells (OD600nm 17.8) was observed in the flasks containing a minimum amount of the media, hence indicating the significance of aeration for the bacteria to grow and multiply. Apart from aeration, agitation and pH also had a significant impact on biomass growth as well as γ-PGA production. In our studies, we have observed that flasks at 80 rpm supported less growth with the OD600 of 6.1 at 96 h whereas agitation with 120, 150, and 200 rpm exhibited the OD600 of 11.7, 10.9, and 16.4, respectively, indicating the requirement of optimum friction amongst the cells for multiplication. Highest production of biopolymer was observed at 150 and 200 rpm with 34.9 g/L and 35.8 g/L γ-PGA. Lowest was recorded at 80 rpm with 16.4 g/L. The results indicated the significance of proper mass transfer of nutrition in the later stages of biopolymer accumulation, where the rise in viscosity leads to declined nutrient distribution to the cells. Therefore, γ-PGA production by B. methylotrophicus was observed to be highly impacted by the conditions of agitation and aeration in our studies. High aeration was recorded to be beneficial for the growth of the microbes and high agitation was observed to show positive effect on the biopolymer production. Another physical parameter evaluated was pH. pH 3 and 4 did not support the growth of the bacteria, hence no conversion was expected. Biomass growth was the highest in the media with pH 5 but the product formation was minimal. In our studies, pH 6.5 before steam sterilization was found to be optimum for the fermentation as shown Fig. 5.
Carbon and Nitrogen Source Optimization for γ-PGA Production
Media optimization relating to carbon and nitrogen sources plays an important role in increasing the γ-PGA production. Requirement of glutamic acid also differs from strain to strain. The composition of the media directly impacts the production, yield, and productivity of the final desired product as shown in Table 2. γ-PGA producing microorganisms can be classified into either glutamic acid dependent or independent bacteria on the basis of their substrate requirement. Additionally, the presence of organic and inorganic nitrogenous source also has been observed to impact the survival of the microorganisms and the biopolymer production. In the previous literatures, B. licheniformis ATCC9945 has been reported to produce 23 g/L γ-PGA under the influence of L-glutamic acid and inorganic salt NH4Cl along with other media components [29]. Another strain of B. licheniformis CICC10099 produced 16.9 g/L after mutation using L-glutamate and inorganic nitrogenous source [35]. B. licheniformis P-104 is the highest γ-PGA producing bacteria amongst the strains of B. licheniformis with 44.7 g/L of γ-PGA production and 1.49 g/ (Lh) productivity [34]. B. subtilis ZJU- 7 has been reported to be highest producer of the biopolymer amongst all the strains of B. subtilis [37] and other Bacillus species under the influence of L-glutamate, sucrose, and tryptone as shown in Table 2.
In our studies, B. methylotrophicus exhibited a significant dependency on the carbon and nitrogen sources for γ-PGA production. This particular species required glutamic acid for increased production of the biopolymer but could not survive in inorganic nitrogen sources. Unlike B. methylotrophicus SK19.001, although our isolate could produce γ-PGA in the absence of glutamic acid but significant increase in the biosynthesis of γ-PGA could take place only in presence of glutamic acid as the bacteria was observed to be dependent on substrate for accumulation of long polymerized chain of the abovementioned amino acid. Water soluble sugars that were assessed at shake flask level for γ-PGA production were preferred on the basis of the Hi-Carbo kit test results which indicated the utilization of xylose, maltose, fructose, glucose, galactose, sucrose, l-arabinose, glycerol, sorbitol, and mannitol.
Amongst all the sugars that were tried, simple sugars, i.e., glucose, lactose, and maltose, did not support the growth of the cells at the concentration of 50 g/L and therefore no γ-PGA production was observed. With the other carbon sources at 50 g/L of concentration each, fructose, sucrose, glycerol, sorbitol, and mannitol supported the cell growth but sucrose and mannitol were observed to be the preferred carbon source for γ-PGA production and yielded 26 and 36 g/L γ-PGA respectively as shown in Fig. 6a. Concentration of glucose was observed to be an important factor in biomass development. Addition of glucose with more than 20 g/L prevented the growth of the cells. Apart from B. methylotrophicus, the pattern of carbon source utilization was also evaluated for B. subtilis and B. licheniformis. Both the microorganisms have been reported to produce γ-PGA mostly using C sources like glucose, glycerol, and sucrose [32, 38,39,40,41] during fermentation. Although few γ-PGA producing bacteria can utilize mannitol, very less amount of γ-PGA production has been reported till now using the abovementioned carbon source. B. methylotrophicus could not grow well in the media containing glucose above 20 g/L. Biomass growth and biopolymer production were assessed in glucose, fructose, and combination of the glucose and fructose as shown in Fig. 6b to understand the inclination of the culture towards carbohydrate sources. Media containing fructose supported the cell biomass, but γ-PGA production was observed to be minimal. The combination of the two simple sugars of fructose and glucose also did not support the biomass development. At the concentration of 50 g/L glucose and fructose proved to be deterrent for microbial growth, but mannitol and sucrose respectively supported the growth at the same concentrations. Fructose and glucose when used at lower concentrations also could not improve γ-PGA concentration in fermentation broth indicating the inclination of the bacteria towards either disaccharides or polyols. Here, we report mannitol dependent γ-PGA production for the first time where it has been used as the carbon and energy source.
In another study, individual components of the media were analyzed for their role in fermentation. B. methylotrophicus was found to be a glutamic acid-independent bacteria, which could make its own γ-PGA but the improvement in production of the biopolymer was observed on inclusion of exogenously supplied monosodium glutamate in the media, increasing the γ-PGA production from 11 to 36 g/L. Additionally, we observed that peptone served as the best nitrogen source for the growth of the bacteria and γ-PGA production. The presence of mannitol along with peptone was observed to be essential for biomass as well as γ-PGA production. Without mannitol the γ-PGA production did not take place. Mannitol alone could not support the growth of microorganisms. The abovementioned result indicated the utilization of mannitol in biosynthesis of endogenous glutamic acid via TCA cycle. Though the bacteria could grow in the absence of mannitol but the biopolymer production initiated only in its presence. The flask containing MSG and peptone supported the growth (OD600 10.9) but γ-PGA production did not take place indicating the absence of trigger for the product formation. Therefore, we could conclude that mannitol plays a very important role in γ-PGA production as it presumably impacts the TCA cycle. B. methylotrophicus is a glutamic acid-independent bacterium which makes its own glutamic acid and then internally polymerizes the substrate. Mannitol is broken down to fructose and mannose. Certain pathways may get triggered in its presence that may have a direct or indirect impact on γ-PGA production. Mannitol and MSG without peptone did not support the growth of the cells which indicated the requirement of amino acids for their survival. Peptone contained requisite amino acids for the bacteria to grow and it supported the multiplication of cells. Mannitol was observed to have triggered the biopolymer production followed by polymerization of extra as well as intra cellular glutamic acid into γ-PGA. The absence of any of the individual component (mannitol/peptone/monosodiumglutamate) had an adverse effect on the biopolymer production as shown in Fig. 6c. Biosynthesis of γ-PGA has been elucidated through the process of glycolysis followed by TCA cycle where endogenous L-glutamic acid is produced which on racemization and polymerization produces γ-PGA [42]. However, glutamic acid-dependent bacteria have to rely on exogenously provided L-glutamic acid. Biosynthetic pathway studies on γ-PGA production are limited to B. subtilis, B. licheniformis, and B. anthracis. In our study, B. subtilis NCIM 5537, isolated from Japanese dish and B. methylotrophicus NCIM 5536 were compared to check their capability to utilize mannitol for γ-PGA production. B. methylotrophicus NCIM 5536 produced 36 g/l γ-PGA in the fermentation media whereas B. subtilis was unable to utilize mannitol for cell growth and γ-PGA production and was observed for its dependency on glucose. This can be inferred from an unchanged concentration of mannitol in the media after fermentation. Mannitol concentrations before and after fermentation were analyzed using UHPLC. Mannitol is a sugar alcohol which is broken down into fructose and mannose followed by its utilization in the metabolic process of the cells. Mannitol was observed to be consumed in 72 h of fermentation. The breakdown products of mannose and fructose were not detected under the given conditions of UHPLC, indicating the intracellular production and utilization of the simple sugars. B. methylotrophicus was observed to be a glutamic acid-independent microorganism, but the γ-PGA production increased to a large extent in the presence of monosodium glutamate. This could be due to the presence of an enzyme mannitol-1-phosphate 5-dehydrogenase in B. methylotrophicus NCIM 5536. Various Bacillus sp. have been studied for biosynthesis of γ-PGA from endogenous as well as exogenous glutamic acid. The α-ketoglutaric acid which is considered as one of the precursors in biopolymer production leads to the intracellular formation of L-glutamic acid. D-glutamic acid is formed from L-glutamic acid in a three-step process [43] but Peng et al. 2005 [18] suggested a one-step reaction where L-glutamate is isomerized to D-glutamate by Glutamate racemase (GLR) in glutamate-independent strain B. methylotrophicus. In our studies, we have observed that though the bacteria were able to produce γ-PGA in the absence of the substrate, exogenous supply of L-glutamic acid led to more production of γ-PGA as shown in Fig. 7.γ-PGA production was observed to start at the log phase of the cells and reached its peak by 96 h. These results indicated that exogenous supply of glutamic acid along with mannitol had a greater impact on γ-PGA production. Extracellular L-glutamic acid gets converted to L-glutamine by L-glutamine synthetase (GS) in most of the Bacillus sp. When glutamine is available, glutamine-2-oxo-glutarate aminotransferase (GOGAT) gets activated to form L-glutamic acid from α - ketoglutaric acid and glutamine [32]. Biosynthesis of L and D form of glutamic acid further leads to polymerization form γ-PGA. γ-PGA production depends on the presence of glutamate which in turn is dependent on the source of nitrogen.
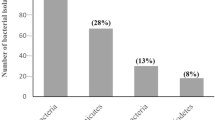
The most studied γ-PGA producing microorganisms, namely B. subtilis and B. licheniformis have been reported to utilize inorganic sources of nitrogen [29, 44]. Hence, media containing inorganic sources of nitrogen such as ammonium chloride, ammonium nitrate, potassium nitrate, sodium nitrate, and ammonium sulfate were tried but they were not able to support the growth of B. methylotrophicus NCIM 5536 showing the dependency of this bacterium on rich media for its survival shown in Fig. 8. Later, peptone was found to be a better nitrogenous source as compared to others for γ-PGA production. We observed an increase of 2.6-fold in γ-PGA concentration using peptone over tryptone. Similar pattern of nitrogen source utilization was observed in the studies conducted by Peng et al. [31].
Effects of different C and N sources were evaluated to optimize the media (Supplementary Fig. 1). Apart from the abovementioned C and N sources, Tween 20 was used to increase permeability of the cells for proper uptake of glutamic acid and to release the high molecular weight, γ-PGA (Supplementary Fig. 2).
In our further attempts to recover pure form of γ-PGA from the fermentation broth, we used a 500-kDa cutoff membrane filter to reduce the low molecular weight impurities in the sample. γ-PGA obtained in our study ranged between ~ 1800 to ~ 2300 kDa (Supplementary Fig. 3a, b). Hence, separation of less than 500 kDa proteins and other impurities ensured the purification of γ-PGA. The broth was concentrated to ten times its original volume. γ-PGA was then precipitated using two volumes of absolute ethanol. Higher concentration of γ-PGA warranted ten times lesser requirement of ethanol for the product precipitation. Lyophilization was then carried out to obtain the powder form of the final product.
Conclusion
A rapid qualitative method was developed for monitoring γ-PGA production in liquid media using methylene blue by UV-Vis spectral studies. This is the first study reporting the use of methylene blue to differentiate the monomeric and the polymeric forms of glutamic acid. We have also made an attempt to optimize the process with respect to the carbon and nitrogen sources used. The work proposes B. methylotrophicus as an organism which can be further exploited and studied for the γ-PGA production.
References
Shih, I. L., Wu, P. J., & Shieh, C. J. (2005). Microbial production of a microbiology’ derivative by Bacillus subtilis. Process Biochemistry, 40, 2827–2832.
Richard, A., & Margaritis, A. (2003). Rheology, oxygen transfer, and molecular weight characteristics of poly (glutamic acid) fermentation by Bacillus subtilis. Biotechnology and Bioengineering, 82, 299–305.
Kubota, H., Nambu, Y., & Endo, T. (1996). Alkaline hydrolysis of poly (γ-glutamic acid) produced by microorganism. Journal of Polymer Science Part A: Polymer Chemistry, 34, 1347–1351.
Shih, I. L., Van, Y. T., & Shen, M. H. (2004). Biomedical applications of chemically and microbiologically synthesized poly (glutamic acid) and poly (lysine). Mini Reviews in Medicinal Chemistry, 4, 179–188.
Park, C., Choi, J. C., Choi, Y. H., Nakamura, H., Shimanouchi, K., Horiuchi, T., Misono, H., Sewaki, T., Soda, K., Ashiuchi, M., & Sun, M. H. (2005). Synthesis of super- high-molecular weight poly-γ-glutamic acid by Bacillus subtilis subsp.chungkookjung. Journal of Molecular Catalysis B: Enzymatic, 3, 128–133.
Hezayen, F. F., Rehm, B. H., Tindall, B. J., & Steinbüchel, A. (2001). Transfer of Natrialba asiatica B1T to Natrialba taiwanensis sp. Nov. and description of Natrialba aegyptiaca sp. Nov., a novel extremely halophilic, aerobic, non-pigmented member of archea from Egypt that produces extracellular poly (glutamic acid). International Journal of Systamatic and Evolutionary Microbiology, 51, 1133–1142.
Niemetz, R., Kärcher, U., Kandler, O., Tindall, B. J., & König, H. (1997). The cell wall polymer of the extremely halophilic archaeon Natronococcus occultus. European Journal of Biochemistry, 249, 905–911.
Thorne, C. B., Gomez, C. G., Blind, G. R., & Housewright, R. D. (1952). Synthesis of glutamic acid and glutamyl polypeptide by Bacillus anthracis. III. Factors affecting peptide production in synthetic liquid media. Journal of Bacteriology, 65, 472–478.
Kimura, K., Tran, L. S., Uchida, I., & Itoh, Y. (2004). Characterization of Bacillus subtilis γ-glutamyltransferase and its involvement in the degradation of capsule poly-gamma-glutamate. Microbiology, 150, 4115–4123.
Tanaka, T., Hiruta, O., Futamura, T., Uotani, K., & Satoh, A. (1993). Purification and characterization of poly (γ-glutamic acid) hydrolase from a filamentous fungus, Myrothecium sp. TM-4222. Bioscience Biotechnology and Biochemistry, 57, 2148–2153.
Volcani, B. E., & Margalith, P. (1957). A new species (Flavobacterium polyglutamicum) which hydrolyzes the gamma-L-glutamyl bond in polypeptides. Journal of Bacteriology, 74, 646–655.
Candela, T., Moya, M., Haustant, M., & Fouet, A. (2009). Fusobacterium nucleatum, the first Gram-negative bacterium demonstrated to produce polyglutamate. Canadian Journal of Microbiology, 55, 627–632.
Bang, B. H., Rhee, M. S., Kim, K. P., & Yi, D. H. (2012). Influences of culture medium components on the production poly (γ-glutamic acid) by Bacillus subtilis GS-2 isolated Chungkookjang. The Korean Journal of Food and Nutrition, 25, 677–684.
Zeng, W., Lin, Y., Qi, Z., He, Y., Wang, D., Chen, G., & Liang, Z. Q. (2013). An integrated high-throughput strategy for rapid screening of poly (gamma-glutamic acid)-producing bacteria. Applied Microbiology and Biotechnology, 97, 2163–2172.
Inbaraj, B. S., Chiu, C. P., Ho, G. H., Yang, J., & Chen, B. H. (2006). Removal of cationic dyes from aqueous solution using an anionic poly-γ-glutamic acid based adsorbent. Journal of Hazardous Materials, 137, 226–234.
Ashiuchi, M. (2013). Microbial production and chemical transformation of poly-γ-glutamate. Microbial Biotechnology, 6, 664–674.
Moraes, L. P., Brito, P. N., & Alegre, R. M. (2013). Existing studies on biosynthesis of poly (−ɣ-glutamic acid) by fermentation. Food and Public Health, 3, 28–36.
Peng, Y., Zhang, T., Mu, W., Miaoa, M., & Jianga, B. (2016). Intracellular synthesis of glutamic acid in Bacillus methylotrophicus SK19.001, a glutamate-independent poly (γ-glutamic acid)-producing strain. Journal of the Science of Food and Agriculture, 96, 66–72.
Nishikawa, M., & Ogawa, K. (2002). Distribution of microbes producing antimicrobial ε-poly-L-lysine polymers in soil microflora determined by a novel method. Applied and Environmental Microbiology, 68, 3575–3581.
Samiey, B., & Ashoori, F. (2012). Adsorptive removal of methylene blue by agar: effects of NaCl and ethanol. Chemistry Central Journal, 6, 14.
Avena, M. J., Valenti, L. E., Pfaffen, V., & De pauli, C. P. (2001). Methylene blue dimerization does not interfere in surface-area measurements of kaolinite and soils. Clays and Clay Minerals, 49, 168–173.
Robinson, JW. (1991) Practical handbook of spectroscopy. CRC press.
Zeng, W., Chen, G., Zhang, Y., Wu, K., & Liang, Z. (2012). Studies on the UV spectrum of poly (γ-glutamic acid) based on development of a simple quantitative method. International Journal of Biological Macromolecules, 5, 83–90.
Xiaoyu, L., Xia, W., & Jinghe, Y. (2010). Protein determination using methylene blue in a synchronous fluorescence technique. Talanta, 81, 760–765.
Zhao, Z., & Malinowski, E. R. (1999). Determination of the hydration of methylene blue aggregates and their dissociation constants using visible spectroscopy. Applied Spectroscopy, 53, 1567–1574.
Blandamer, M. J., Brivati, J. A., Fox, M. F., Symons, M. C. R., & Verma, G. S. P. (1967). Water induced dimerization of various dye and related paramagnetic ions studied by optical and electron spin resonance spectroscopy. Transactions of the Faraday Society, 63, 1850–1857.
Heger, D., Jirkovsky, J., & Kla’n, P. (2005). Aggregation of methylene blue in frozen aqueous solutions studied by absorption spectroscopy. Journal of Physical Chemistry A., 109, 6702–6709.
Nishikawa, M., & Kobayashi, K. (2009). Streptomyces roseoverticillatus produces two different poly (amino acid) s: lariat-shaped gamma-poly (L-glutamic acid) and epsilon-poly (L-lysine). Microbiology, 155, 2988–2993.
Cromwick, A. M., Birrer, G. A., & Gross, R. A. (1996). Effects of pH and aeration on gamma-poly(glutamic acid) formation by Bacillus licheniformis in controlled batch fermentor cultures. Biotechnology and Bioengineering, 50, 222–227.
Kunioka, M., & Goto, A. (1994). Biosynthesis of poly(γ-glutamic acid) from l-glutamic acid, citric acid, and ammonium sulfate in Bacillus subtilis IFO3335. Applied Microbiology and Biotechnology, 40, 867–872.
Peng, Y., Jiang, B., Zhang, T., Mu, W., Miao, M., & Hua, Y. (2015). High-level production of poly (γ- -glutamic acid) by a newly isolated glutamate-independent strain Bacillus methylotrophicus. Process Biochemistry., 50, 329–335.
Bajaj, I. B., Lele, S. S., & Singhal, R. S. (2009). Sequential optimization approach for enhanced production of poly (у-glutamic acid) from newly isolated Bacillus subtilis. Food Technology and Biotechnology, 47, 313–322.
Zhang, H., Zhu, J., Zhu, X., Cai, J., Zhang, A., Hong, Y., Huang, J., Huang, L., & Xu, Z. (2012). High-level exogenous glutamic acid-independent production of poly-(c-glutamic acid) with organic acid addition in a new isolated Bacillus subtilis C10. Bioresource Technology, 116, 241–246.
Zhang, Y. W., Wei, X., Hu, Z. B., & Liu, H. Z. (2012). Optimization of γ-polyglutamic acid production by bacillus licheniformis P-104. The Chinese Journal of Process Engineering, 12, 288–292.
Suo, C., Mei, L. H., Huang, J., & Sheng, Q. (2007). Selection of γ-poly glutamic acid high yield strain by ~(60)Co γ-irradiation and the optimization of its culture medium. Journal of Chemical Engineering of Chinese Universities, 21, 820–825 (In chinese).
Ashiuchi, M., Kamei, T., Baek, D. H., Shin, S. Y., Sung, M. H., Soda, K., Yagi, T., & Misono, H. (2001). Isolation of Bacillus subtilis (chungkookjang), a poly-gamma-glutamate producer with high genetic competence. Applied Microbiology and Biotechnology, 57, 764–769.
Shi, F., Xu, Z., & Cen, P. (2006). Optimization of γ-polyglutamic acid production by Bacillus subtilis ZJU-7 using a surface-response methodology. Biotechnology and Bioprocess Engineering, 11, 251–257.
Bhunia, B., Mukhopadhyay, D., Goswami, S., Mandal, T., & Dey, A. (2012). Improved production, characterization and flocculation properties of poly (γ)-glutamic acid produced from Bacillus subtilis. Journal of Biochemical Technology, 3, 389–394.
Birrer, G. A., Cromwick, A. M., & Gross, R. A. (1994). γ-Poly(glutamic acid) formation by Bacillus licheniformis 9945a: physiological and biochemical studies. International Journal of Biological Macromolecules, 16, 265–275.
Feng, S., Zhinan, X., & Peilin, C. (2006). Optimization of γ-polyglutamic acid production by Bacillus subtilis ZJU-7 using a surface-response methodology. Biotechnology and Bioprocess Engineering, 11, 251–257.
Najar, I. N., & Das, S. (2015). Poly-glutamic acid (PGA)—structure, synthesis, genomic organization and its application: a review. International Journal of Pharmaceutical Sciences and Research, 6, 2258–2280.
Shih, I. L., & Van, Y. T. (2001). The production of poly- (γ-glutamic acid) from microorganisms and its various applications. Bioresource Technology, 79, 207–225.
Yuanyuan, R., Huang, B., Meng, Y., Wei, L., & Zhang, C. (2015). Metabolic and phylogenetic analyses based on nitrogen in a new poly-γ-glutamic acid-producing strain of Bacillus subtilis. Biotechnology Letters, 3, 1221–1226.
Meissner, L., Kauffmann, K., Wengeler, T., Mitsunaga, H., Fukusaki, E., & Büchs, J. (2015). Influence of nitrogen source and pH value on undesired poly (γ-glutamic acid) formation of a protease producing Bacillus licheniformis strain. Journal of Industrial Microbiology and Biotechnology, 42, 1203–1215.
Shih, I. L., Van, Y. T., & Chang, Y. N. (2002). Application of statistical experimental methods to optimize production of poly(−glutamic acid) by Bacillus licheniformis CCRC 12826. Enzyme and Microbial Technology, 31, 213–220.
Yangyang Zhan, Y., Zhu, C., Sheng, B., Cai, D., Wang, Q., Wen, Z., & Chen, S. (2017). Improvement of glycerol catabolism in Bacillus licheniformis for production of poly-γ-glutamic acid. Applied Microbiology and Biotechnology, 101, 7155–7164.
Acknowledgements
This work has been financially supported by Tata Chemicals Limited, Pune, India. Authors would like to thank the Confederation of Indian Industry (CII) for supporting the research through Prime Minister Fellowship Scheme for Doctoral research, a public-private partnership between Science and Engineering Research Board (SERB) to P.M.C and D.P.T. Authors also thank Manipal University for registering P.M.C and D.P.T to pursue PhD research work on γ-polyglutamic acid.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors declared that they have no conflict of interest.
Electronic Supplementary Material
Supplementary Fig. 1
Effect of different concentration of MSG, Mannitol and Peptone on γ-PGA production (JPEG 166 kb)
Supplementary Fig. 2
Effect of Tween 20 (JPEG 49 kb)
Supplementary Fig. 3a
GPC chromatogram of final hour fermentation broth sample 1 (JPEG 62 kb)
Supplementary Fig. 3b
GPC chromatogram of final hour fermentation broth sample 2 (JPEG 94 kb)
Rights and permissions
About this article
Cite this article
Chatterjee, P.M., Datta, S., Tiwari, D.P. et al. Selection of an Effective Indicator for Rapid Detection of Microorganisms Producing γ-Polyglutamic Acid and Its Biosynthesis Under Submerged Fermentation Conditions Using Bacillus methylotrophicus . Appl Biochem Biotechnol 185, 270–288 (2018). https://doi.org/10.1007/s12010-017-2654-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2654-6