Abstract
ε-poly-l-lysine (ε-PL) is a homo-poly-amino acid of l-lysine which is used as a safe food preservative. The productivity of ε-PL in currently reported wild type strains is low. This study was aimed at finding novel ε-PL producing strains with higher productivity and new fermentative characters. An improved detection method was employed using methylene blue as an ε-PL secretion indicator. 137 strains forming transparent circles were isolated. The best one was identified as Streptomyces griseofuscus according to the morphological characteristics and the comparison of internal transcribed spacer (ITS) ribosomal DNA (rDNA) gene sequences. The fermentative behavior of S. griseofuscus was investigated, and the ε-PL production was enhanced to 2.3 g/L in 5-L bioreactor by a pH control strategy. The yield of ε-PL reached 7.5 g/L in the fed-batch process. Compared with the reported wild type strains, S. griseofuscus produced relatively higher amounts of ε-PL, and might be a promising Streptomyces for ε-PL production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
ε-poly-l-lysine (ε-PL) is a homo-poly-amino acid where l-lysine monomers are linked by peptide bonds between the carboxyl and the epsilon-amino groups. Because it is water soluble, biodegradable, edible and non-toxic toward humans and the environment, ε-PL and its derivatives have been of interest for a broad range of industrial applications such as food preservatives, emulsifying agents, dietary agents, biodegradable fibers, highly water absorbable hydrogels, drug carriers, etc. [1, 2]. Therefore, the commercial production of ε-PL is considered highly promising.
The first microbe found to produce ε-PL was S. albulus [3]. While the original ε-PL-producing strain has been bred to increase its productivity, natural microbial resources have been explored to find other strains with new properties. One of the key techniques in isolating ε-PL producing strains is the direct visual observation of a Petri dish. The major developed methods depend on the haloes formed due to the interaction between charged groups of secreted polypeptides and basic or acidic dyes such as methylene blue, Poly R-478 [4]. However, the disadvantage of such methods is the toxicity of dyes to a single microbial cell; therefore some of the ε-PL producing strains might be overlooked. In this paper, a modified method was designed to avoid the problem of using toxic methylene blue as an indicator during screening. Using this method, a novel ε-PL producing S. griseofuscus strain was isolated, and its ε-PL fermentation performance was investigated. To our knowledge, this is the first report that S. griseofuscus was used in ε-PL production.
Materials and methods
Soil samples, culture media and actinomycete strain
Soil samples were collected from different provinces of China, such as Shandong Province, Shanxi Province, Jiangsu Province and Inner Mongolia Autonomous Region.
Solid medium consisted of glucose (10 g), yeast extract (2 g), peptone (4 g), agar (20 g) per liter deionized water. pH was adjusted to 7.2 before sterilization.
Screening medium A was the same as the solid medium but contained K2Cr2O7 (30 mg), norfloxacin (3 mg), penicillin (2 mg) and nystatin (80 mg) per liter to inhibit the growth of bacteria and fungi.
Screening medium B was the same as the solid medium but contained methylene blue (50 mg) per liter.
Dragendorff reagent is a bismuth potassium iodide solution which forms a red sediment with alkaline substances including ε-PL [7]. Salt-starch agar, oat meal agar and emperor gao agar were employed to detect whether the obtained strains could grow well on them according to Bergey’s Manual of Determinative Bacteriology [5].
Liquid medium consisted of glucose (50 g), (NH4)2SO4 (10 g), yeast extract (5 g), MgSO4·7H2O (0.5 g), K2HPO4 (0.8 g), KH2PO4 (1.4 g), ZnSO4·7H2O (0.04 g), FeSO4·7H2O (0.03 g) per liter deionized water. pH was adjusted at 6.8 with NaOH (1 N) before sterilization.
An actinomycete strain, referred to as S. griseofuscus LS-H1, was isolated from a soil sample collected in Wuxi City, China. It was deposited at the China Center for Type Culture Collection as CCTCC M 209211.
Screening method
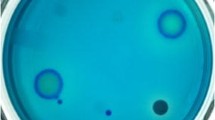
The soil samples were suspended and serially diluted in sterile saline. The aliquots were spread on screening medium A. The plates were incubated for 7 days at 30°C. The whole agar of medium A was then removed from the Petri dish and placed on screening medium B, forming a sandwich. The Petri dish with the above two layers of agar was put into the incubator for 2 h at 40°C to observe whether haloes would appear, indicating that alkaline substances in the top agar A layer had penetrated into the agar B underlayer. The colonies which exhibited the haloes were selected and streaked on additional new plates until homogenous colonies were observed.
The purified strains were inoculated into liquid media and incubated at 30°C on a rotating shaker. After 72 h of incubation, the culture broth was centrifuged, and the supernatant was mixed with Dragendorff and methyl orange (MeO) solution. Supernatant which formed sediment with the above reagents was hydrolyzed with hydrochloric acid (6 mol/L) followed by thin layer chromatography (TLC) analysis with ninhydrin spray for amino acids detection. Silica gel G thin layer plate was adopted with a mixed solvent of n-butanol-glacial acetic acid-pyridine-water (4:1:1:2) as the mobile phase. Lysine purchased from Sigma (USA) and ε-PL from Zhejiang Silver Elephant Bioengineering Inc. (China) were used as standards. The strains which excreted polylysine into the culture broth were thus selected and preserved.
Purification and characterization of ε-PL
Extraction and purification of polylysine was carried out based on the method of Hirohara et al. [6]. The culture filtrate was adjusted to pH 2.5 with an HCl solution and saturated with a methanol/acetone (3:1) mixture. After incubation for 3 h, the resulting precipitate was dissolved in deionized water and purified by ion-exchange chromatography on a TSKgel CM-5PW column (Tosoh, Japan) equilibrated with 50 mM sodium phosphate buffer (pH 7.6). Elution was performed with a linear gradient of NaCl in the buffer. Finally, the purified polylysine was precipitated by acetone and dried as a white powder.
The characterization of ε-PL was performed by determination of the linkage pattern of lysine residues according to the method of Shima and Sakai [7].
The concentration of ε-PL in various solutions was measured according to the method of Itzhaki [8] and Shima [9] with modifications. 0.5 mL of sample solution was mixed with 2 mL methyl orange (MeO) solution (1 mM MeO and 50 mM sodium phosphate, pH 7.0) and vortexed. After incubation for 30 min at 30°C, mixtures were centrifuged at 5,000 rpm, and the resulting supernatants were diluted tenfold. Absorbance of the resulting dilutions was measured at 465 nm.
Identification and phylogenetic analysis of isolate H1
The phylogenetic position of the strain which produced the highest amounts of ε-PL of all isolates (designated H1) was determined by classical and molecular taxonomy. Physiological, structural and biochemical characteristics used for classification were based on Bergey’s Manual of Determinative Bacteriology [5]. Morphological properties were examined under a scanning electron microscope Quanta 200 (Fei, Eindhoven, Netherlands) with an accelerating voltage of 12.5 kV.
Sequence analysis of the 16S rDNA was performed by amplifying the 16S rDNA of the strain H1 with PCR (forward primers: 5′-CGTGCTTAACACATGCAAGTCGAAC-3′; reverse primers: 5′-AGCAATGCTGATCTGCGATTACTAG-3′). The obtained 16S rDNA sequence was aligned to sequences in GenBank using the BLAST program. The nucleotide sequence of 16S rDNA gene reported in this paper was assigned to the GenBank with the accession no.GU138373.
Cell growth and ε-PL production in fermentation
A 5-liter automatic fermentor (Shanghai Baoxing Bio-engineering Equipment Co., Ltd., China) containing 2,760 mL of liquid medium was inoculated with 240 mL of 28 h old seed culture. The initial pH was adjusted to 6.8 with NaOH (1 N). The dissolved oxygen (DO) was kept at 10% by control of the stirrer speed. The culture broth was sampled every 3 h, centrifuged, and the resulting supernatant was used for measurement of the ε-PL and residual glucose concentrations. The change of pH during cultivation was detected by a pH electrode. The biomass was determined by harvesting a culture sample, filtering, washing the mycelia twice with distilled water, and drying until a constant weight was achieved at 95°C. The glucose concentration was determined by biosensor analyzer SBA-40B (Shandong Academy of Sciences).
To maintain the pH at 3.5, 3.65, 3.8, 4.0 respectively, NH4OH solution was automatically added to the culture broth.
When the pH was at 3.5, fed-batch fermentation was carried out by adding a mixture of glucose and ammonium sulfate whose proportion was the same as the initial liquid medium. When glucose concentration decreased to 0.5%, the mixture was manually added to the broth until the final glucose concentration reached 1.5%. The process was then repeated until no further consumption of glucose was found.
Results
Two-layer agar screening method
Methylene blue has been employed as an indicator by a majority of screening methods because it is a positively charged dye which can reject alkaline substances, including ε-PL, to form haloes around its producing strains through electrostatic interaction (Fig. 1). In this article, we removed the whole agar medium A and placed it on solid medium B, containing the methylene blue, to form a sandwich, which avoided the toxicity problem caused by the indicator dyes. This modified method proved effective, and 137 strains were found to produce alkaline substances.
Identification of ε-PL product
In order to further confirm that the alkaline substance produced by the above 137 strains was ε-PL, their culture broths were reacted with Dragendorff reagent [7]. The culture broths of 50 strains which formed red sediment with the reagent were selected for further detection.
The chemical nature of the Dragendorff-positive substances produced by the 50 strains was investigated. The products were extracted from the culture broth and purified by ion-exchange chromatography. The purified products were then hydrolyzed by hydrochloric acid (6 mol/L). The hydrolytes were analyzed by thin layer chromatography (TLC). Figure 2a was the TLC profile of amino acids in three representative hydrolytes. It showed that only lysine existed as an amino acid in the hydrolytes, which suggested the product was a polymer polymerized by lysine. In total, 42 isolates were detected to have polylysine synthetic ability. Among them, the isolate designated as H1 exhibited the highest polylysine production.
a TLC profile of the amino acids. 1: non hydrolysed ε-PL standard; 2: hydrolysed ε-PL standard; 3: non hydrolysed ε-PL from an extract; 4–6: hydrolysed ε-PL from three extracts. The purple strips showed the amino acids reacted with ninhydrin. b TLC profile of ε-PL and the basic product secreted by strain H1. 1: Nε-2,4-DNP-l-lysine; 2: Nα,Nε-di (2, 4-DNP)-l-lysine; 3: Nα-2,4-DNP-l-lysine; 4: hydrolysate of DNP-protected ε-PL standard; 5: hydrolysate of DNP-protected ε-PL produced by H1; 6: lysine standard
To define its structure, the polylysine secreted by H1 was analyzed by the TLC with ε-PL standard as control. The linkage pattern of lysine residues was examined according to Shima [7] and Nishikawa [4]. Free amino groups of polylysine were protected by 2, 4-dinitrophenol (DNP) and then the obtained polymer was completely hydrolyzed by HCl (6 mol/L). The TLC result (Fig. 2b) showed that the main bands of strain H1 product hydrolyte were completely consistent with those of ε-PL. Thus, the basic substance secreted by strain H1 was identified as polylysine linked by the ε-amide bond, namely ε-PL.
Identification of strain H1
The strain H1 is a good ε-PL producer with unique morphology which is quite different from other strains. Therefore it might be a novel strain for ε-PL production. The cultural and morphological properties suggested that H1 was a strain of actinomycete. Sporulation emerged on standard media such as inorganic salt-starch agar and oatmeal agar rather than emperor gao agar. The aerial spore on these media was gray in color. Melanin pigments were produced on peptone yeast extract iron agar. H1 formed substrate mycelium and aerial hyphae that were white in color, which turned black as the culture aged. The spore chains were composed of spherical to oval-shaped spores, with smooth surfaces. H1 was able to use glucose, d-fructose, mannitol, d-xylose, l-rhamnose, d-galactose, glycerol, maltose, lactose or mannose as sole carbon source, but was not able to use inositol or cellulose. Nearly complete 16S rDNA gene sequence (1,484 bp) of H1 was obtained and was found to be most similar to those of Streptomyces griseofuscus, Streptomyces microsporus, Streptomyces misawanensis, with sequence identities of 99.7, 98.2, 98.2%, respectively. The sequence identity to the previously described ε-PL producing strain S. albulus was only 93.4%, suggesting that they were different strains. The properties of culture, morphology and carbon source utilization of strain H1 were consistent with those of Streptomyces griseofuscus as described by Bergey’s Manual of Determinative Bacteriology [5]. Combined with 16S rDNA sequence analysis, strain H1 was identified as S. griseofuscus.
Time profile of ε-PL production in batch culture
Figure 3 shows that the yield of ε-PL was 0.8 g/L, and the dry weight of biomass was about 6 g/L in batch culture, which was equivalent to those in the shake flask. Although the ε-PL was much higher than those of the wild type strains of S. albus, Kitasatospora, S. gniseoaurantiacus [10–12], strategies for further improving production are needed. Based on the previous reports of the effects of pH and glucose on ε-PL production fermented by other Streptomyces strains [13], fed-batch culture and pH control methods should be taken into account.
Effect of pH on cell growth and ε-PL production
According to Kahar et al. [13] and Kito [14], an ε-PL-degrading enzyme exists on a membrane of ε-PL producing strains. This degrading enzyme shows high activity when pH is higher than 5 and it can degrade the synthesized ε-PL immediately. But when pH is around 4, the activity of this enzyme is inhibited and ε-PL will dramatically accumulate. Thus, there must be a suitable pH for strains to produce ε-PL. It was indeed obvious that the pH influenced the ε-PL production of S. griseofuscus (Fig. 4). When the pH was controlled as 4.0, it hardly generated ε-PL whereas the biomass reached 17 g/L. For pH 3.8, the yield of ε-PL was 1.2 g/L and biomass was 14 g/L. When pH dropped to 3.5, ε-PL accumulated to 2.4 g/L with a biomass of 8 g/L. Therefore, the optimal pH for ε-PL production of S. griseofuscus was at 3.5, which was different from that of Kitasatospora and S. albulus whose optimal pH values are both at 4.0.
Cell growth and ε-PL production in fed-batch fermentation
The fermentation time could be extended to 120 h if a mixture of glucose and ammonium sulfate were added into fermentor under pH 3.5 (Fig. 5). Finally the output of ε-PL accumulated to 7.5 g/L, while the biomass was 17 g/L. The interesting phenomenon was that ε-PL was still increasing even though biomass began to decline. It seemed that the production of ε-PL in S. griseofuscus was not dependent upon cell density, in contrast to that of S. albulus [13] and Kitasatospora [11], where the ε-PL was secreted only when biomass was increasing.
Discussion
After S. albulus was found to produce ε-PL in 1977 by Shima [3], great efforts have been made to screen ε-PL producing strains. Methylene blue has been employed as an indicator by a majority of screening methods. However, the addition of methylene blue into agar medium has brought the problem of inhibition of actinomyces growth due to the toxicity of the dye. Duan Shan [10] modified this screening method by spraying methylene blue solution onto the surface of the agar medium. However in practice, methylene blue absorbs water from the medium and makes it quickly dry. What’s more, the spray also causes a liquid flow on the agar surface resulting in the mixture of colonies to be selected. In this article, we removed the whole agar medium and covered it on another solid medium containing the methylene blue to form two-layer agar, which solved the problems of direct spay.
By using this newly developed method, we carried out an exhaustive screening of soil microflora. Surprisingly, the amount of ε-PL producers was as many as 42. Besides S. griseofuscus, there were also some other species, such as S. padanus, S. graminearus and S. hygroscopicus. According to Nishikawa and Ogawa [4], ε-PL producers were quite limited among bacteria in the family Streptomycetaceae and ergot fungi, among which the relevant genes might transfer horizontally. It is very interesting to note that these two evolutionally distant groups, bacteria and fungi, both produce ε-PL. To prove the possibility of transkingdom genetic transfer, further investigations are required.
The yields of ε-PL with the 42 screened strains differed greatly. The lowest output in the shake flask was 0.2 g/L, whereas the highest production was 0.7 g/L, such as strain H1 (S. griseofuscus). It is reported that the yields of Kitasatospora, S. albulus and S. gniseoaurantiacus are 0.41, 0.24, 0.2 g/L, respectively [10–12]. In addition, wild type Streptomyces strains Kitasatospora and S. albulus produced ε-PL 6–7 g/L [11] and 4–5 g/L [9] in a fed-batch process. Compared with those strains, the S. griseofuscus (7.5 g/L) showed a higher potential to produce ε-PL. However, very recently Zhang et al. [15] described a mutant named Kitasatospora sp. MY 5-36 which seemed to be able to produce up to 22 g/L in liquid culture and 34 g/L with immobilized cells. Early in 2001, a mutant of S. albulus no. 410 yielded ε-PL as many as 48.3 g/L in fed-batch fermentation [13], which was the highest output of which we are aware.
The S. griseofuscus has the potential to produce ε-PL, and it is just a wild type strain. In the future, the mass of work should focus on strain breeding. A primary aim is to improve the potential of S. griseofuscus no matter what methods must be employed, including traditional mutation, genetic engineering and genome shuffling. In addition, optimum conditions promoting this culture would also be taken into account. Inspection of Figs. 4 and 5 makes it clear that ε-PL appears to be produced only in stationary phase cultures, and that growth of biomass is most rapid at pH 4, whereas ε-PL production is best at pH 3.65–3.5. An obvious way to improve ε-PL production in the future would be to employ fermentation conditions that use pH 4 or higher to achieve high biomass concentration followed by a second stage of fermentation at pH 3.65–3.5 to stimulate the production of ε-PL. It is also possible that a fed-batch fermentation strategy could be developed to employ a nutrient limitation to induce ε-PL production even during the log-phase of growth. In any case, the fermentation performance of S. griseofuscus differs from the reported strains in such aspects as different optimal pH and independence of cell density, which suggests that it was an ε- PL producing strain with new characters. This strain might be a promising one which is worth exploiting in industrial applications as well as a Streptomyces for ε-PL synthetic mechanism study.
References
Hiraki J, Ichikawa T, Ninomiya S (2003) Use of ADME studies to confirm the safety of polylysine as a preservative in food. Regul Toxicol Pharmacol 37:328–340
Shih IL, Shen MH (2006) Microbial synthesis of poly (ε-lysine) and its various applications. Bioresour Technol 97:1148–1159
Shima S, Sakai H (1977) Polylysine produced by Streptomyces. Agric Biol Chem 41:1807–1809
Nishikawa M, Ogawa K (2002) Distribution of microbes producing antimicrobial ε-Poly-l-Lysine polymers in soil microflora determined by a novel method. Appl Environ Microbiol 68:3575–3581
Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST (1994) Bergey’s manual of determinative bacteriology, 9th edn. The Williams and Wilkins Company, Baltimore
Hirohara H, Takehara M, Saimura M, Masayuki A, Miyamoto M (2006) Biosynthesis of poly(ɛ-l-lysine)s in two newly isolated strains of Streptomyces sp. Appl Microbiol Biotechnol 73:321–331
Shima S, Sakai H (1981) Poly-l-lysine Produced by Streptomyces. Part III. Chemical studies. Agric Biol Chem 45:2497–2502
Itzhaki RF (1972) Colorimetric method for estimating poly-lysine and polyarginine. Anal Biochem 50:569–574
Shima S, Sakai H (1981) Poly-l-lysine Produced by Streptomyces. Part II. Taxonomy and fermentation studies. Agric Biol Chem 45:2503–2508
Duan S, Zhu WS (2007) Isolating of a ε-Polylysine-producing strain. Food Ferment Ind 33:14–17
Zhu HY, Xu H, Wu Q (2005) Screening and identification of ε-PL producing strain. Microbiol 32:127–130
Zhang C, Zhang DR (2006) A Simple and sensitive method for screening ε-PL producing strains from soils. J Shandong Univ 44:1104–1107
Kahar P, lwata T, Hiraki J (2001) Enhancement of ε-poly-l-lysine production by Streptomyces albulus strain 410 using pH control. J Biosci Bioeng 91:190–194
Kito M, Takimoto R, Yoshida T, Nagasawa T (2002) Purification and characterization of ε-poly-l-lysine-degrading enzyme from an ε-poly-l-lysine-producing strain Streptomyces albulus. Arch Microbiol 178:325–330
Zhang Y et al (2010) ε-Poly-l-Lysine production by immobilized cells of Kitasatospora sp. MY 5-36 in repeated fed-batch cultures. Bioresour Technol. doi:10,1016/j.biortech,2010.02.021
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, S., Tang, L., Chen, X. et al. Isolation and characterization of a novel ε-poly-l-lysine producing strain: Streptomyces griseofuscus . J Ind Microbiol Biotechnol 38, 557–563 (2011). https://doi.org/10.1007/s10295-010-0803-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0803-9