Abstract
A fungus, designated as strain SS2 able to degrade aliphatic polyesters, poly(3-hydroxybutyrate) (PHB) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), was isolated from soil. Strain SS2 was identified through rDNA gene sequencing and showed maximum closeness to Penicillium oxalicum. The newly isolated P. oxalicum strain SS2 had completely degraded PHB and PHBV both in emulsion and films form within 36–48 h at 30 °C. Furthermore, P. oxalicum SS2 degraded PHB and PHBV films in soil environment in lab-built soil microcosms within 1 week. The polymer films were evaluated for changes after degradation through scanning electron microscopy (SEM), nuclear magnetic resonance (NMR), differential scanning calorimetry (DSC) and Fourier Transform Infrared spectroscopy (FTIR). The PHBV depolymerase enzyme was purified to homogeneity through column chromatography and molecular mass was found approximately 36 kDa. The depolymerase was stable over a wide range of temperature (15–60 °C) and pH (3.0–8.0) with optimum 40 °C and pH 5.0. The enzyme activity was significantly affected by various metal ions and surfactants. The enzyme activity was strongly enhanced in the presence of divalent cationic metal Cu2+ while inhibited by Zn2+ and non-polar detergents Tween 20 and Tween 60. Finally, it is concluded that P. oxalicum strain SS2 has profound degradation capabilities, and can be applied for the treatment of plastic-contaminated environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The environmental burden due to conventional plastics and decrease in oil resources has led to the development of biodegradable plastics. Among the biodegradable plastics, some partially degradable polymers result in diffusion of micro- and nano-particles of plastics into the environment thus contaminating soil and water resources. In this regard, bio-based plastics that are completely biodegradable in a reasonable time frame under natural environmental conditions are of great interest most importantly in short-term application such as packaging and agriculture. Poly(hydroxyalcanoates) (PHAs) are among the widely studied and used bio-based polymers as they are completely biodegradable, biocompatible, and their properties are comparable to the commercial synthetic counterparts such as polypropylene and polyethylene. Poly(3-hydroxybutyrate) (PHB) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) are among the leading biodegradable polyesters used as replacement of conventional fossil fuel-based polymers [1]. PHB and PHBV are used in agriculture and construction, automotive industry, electrical devices, sanitary products, packaging, biomedical industry, covering films, shopping bags and compost bags. With these wide spread commercial applications, it is mandatory to study the fate of these polymers in the environment and identify the potent PHB/PHBV-degrading microbial strains from relevant habitats and determine the nature and properties of their depolymerases. As most of these applications are intended to be used and disposed off in mesophilic environment particularly in soil; therefore, it is necessary to study the process of their degradation in soil under mesophilic conditions. Most of the PHBV degrading microorganisms grow better at mesophilic temperature [2]. The fate of any pollutant in soil is affected by different factors including properties of pollutant, precipitation and soil characteristics such as temperature, pH, moisture, organic matter and additional carbon sources [3,4,5,6,7,8].
Biodegradation is a complex phenomenon including both biotic and abiotic factors and needs more exploration using the high-throughput approaches [9]. Co-metabolic biotransformation is considered as the future bioremediation strategy to cope with the plastic waste but present information on potential degraders (bacteria and fungi) is insufficient and needs more exploration [10]. Many researchers have isolated PHB- and PHBV-degrading microorganisms from various habitats such as Bacillus, Streptomyces, Pseudomonas mendocina, Acidovorax facilis and Variovorax paradoxus, Aspergillus fumigatus and Penicillium sp. from soil [11, 12]. Previously, PHBV-degrading Bacillus sp. AF3 was isolated from sewage sludge in the author’s laboratory [13]. In the current study, a fungal strain Penicillium oxallicum strain SS2 was isolated from the dumping site of Islamabad, Pakistan and its degradation potential against PHB/PHBV in soil microcosms under mesophilic conditions was evaluated. The physico-chemical changes occurred in polymeric materials as a result of degradation activity by the fungal strain SS2 were analyzed through various analytical techniques. Moreover, production of depolymerase enzyme, purification and characterization was also done. Reports on bacterial/fungal species completely degrading PHB/PHBV and the potential depolymerases isolated from plastic-contaminated sites are scarce, and the current study reports complete biodegradation of PHB/PHBV by Penicillium oxallicum strain SS2 leaving no disintegrated particles.
Materials and Methods
PHB (Mw 3.5 × 102 kDa), PHBV (Mw 2.3 × 102 kDa) and Sabouraud Dextrose Agar (SDA) were purchased from Sigma-Aldrich, Germany, and Chloroform (CHCl3) was obtained from Panreac Quimica, SA. All the other chemicals and reagents were commercial products of highest grade available.
Isolation of Aliphatic Polyester-Degrading Fungi
Polymer emulsions and films were prepared by the previously described method [14]. Soil sample collected from waste dumping site of Islamabad, Pakistan, was screened for isolation of polyester-degrading fungi. The samples were serially diluted in normal saline before spreading onto PHB- and PHBV-emulsified mineral salt medium (MSM) g/L; K2HPO4 0.5, KH2PO4 0.04, NaCl 0.1, CaCl2·2H2O 0.002, (NH4)2SO4 0.2, MgSO4.7H2O 0.02 and FeSO4 0.001, pH adjusted to 7.0. After inoculation, the plates were incubated at 37 °C for 5 days. Fungal isolates showing zones of hydrolysis around their growth on polymer-emulsified MSM agar plates were purified and identified. Isolated fungal strains were stored in the form of spore suspensions at 4 °C for further use.
Characterization of the Isolates
Morphological and Microscopic Examination
The fungal isolate, designated as SS2, was characterized by colony morphology and microscopic examination. Colony morphology was observed on SDA and compared with the descriptions provided in Advances in Penicillium and Aspergillus Systematics [15]. Lactophenol cotton blue staining of fungal mycelia and spores was performed for microscopic examination.
Phylogenetic Analysis
Fungal DNA was extracted according to the method described by Zhang et al. [16] with slight modification and internal transcribed spacer (ITS) regions were amplified using primers ITS1 having sequence 5′-TCCGTAGGTGAACCTGCGG-3′ and ITS4 having sequence 5′-TCCTCCGCTTATTGATATGC-3′. ITS1 and ITS4 primers were used to amplify the rDNA sequence that included partial 18S and 28S regions, and complete ITS1, 5.8S and ITS2 regions. The standard PCR of 50 µL contained 25 µL PCR master mix (Platinum super mix 2X, Invitrogen), 5 µL each primer (25 pmol/µL), 6 µL template DNA and 9 µL PCR water to a final volume of 50 µL. The PCR conditions were set as primary denaturation at 96ºC for 5 min, 30 cycles of denaturation were repeated at 94 °C for 30 s, annealing was done at 55ºC for 45 s and extension at 72 °C for 45 s, followed by a final extension at 72 °C for 10 min. PCR product was purified with PCR clean up kit to remove any unincorporated primers and dNTPs, and then confirmed by agarose gel electrophoresis. Sequencing was performed using Big Dye Terminator cycle sequencing kit v.3.1 (Applied Bio-Systems, USA) and the product was resolved on an Applied Bio-Systems model 3100 automated DNA sequencing system (Applied Bio-Systems, USA) at the Macrogen, Inc., Seoul, Korea. Sequences were analyzed through DNA baser and further evaluated by comparing the nucleotide sequences available in NCBI (National Center for Biotechnology Information) database by BLAST (Basic Local Alignment Search Tool) search analysis. Phylogenetic tree was constructed from rDNA sequences using the neighbor-joining method by ClustalW software as implemented in MEGA 6 (Molecular Evolutionary Genetics Analysis) [17]. A total of 632 aligned bases were included in the analysis, and the dataset was bootstrapped to 1000 replicates.
Biodegradation of Aliphatic Polyester
Degradation activity of P. oxalicum SS2 against PHB and PHBV was determined using clear zone method, emulsion assay and weight loss technique.
Plate Assay (Zone of Hydrolysis)
Polymer-emulsified turbid MSM plates were prepared and inoculated with strain SS2 using point inoculation method followed by incubation at 30 °C. The polymer agar plates contained PHB and PHBV at 0.2% and 0.3% concentration, respectively.
Broth Assay (Decrease in Turbidity)
The degradation potential of strain SS2 was also determined by observing its capacity to reduce turbidity of the polymer emulsion. The emulsions were prepared by mixing PHB (0.2%) and PHBV (0.3%) powder in MSM broth. The emulsions were inoculated with 5% spore suspension and incubated at 30 °C in shaker incubator at 150 rpm.
Weight Loss (Polymer Film)
Weight loss method previously described by Shah et al. [18] was used to determine the hydrolyzing capability of P. oxalicum SS2 against polymer films. Films were prepared using solvent cast method; PHB and PHBV powder at a concentration of 0.2% and 0.3%, respectively, were dissolved in 100 mL chloroform and poured in Petri plates. The plates were placed in vacuum desiccator at room temperature and solvent was allowed to evaporate slowly. The polymer films (~ 0.2 mm in thickness) were sterilized using 70% ethanol and exposed to UV radiation for 5 min (UV dosage 10,771 J/m2) followed by washing with sterilized distilled water prior to adding to the liquid MSM in Erlenmeyer flask inoculated with strain SS2 for 48 h at 30 °C in shaker incubator at 150 rpm. The experiment was run in triplicates while un-inoculated flask was used as a negative control. Degradation of plastic film was monitored by measuring its weight loss every 8 h for a period of 48 h.
Effect of Extra Carbon Source on the Rate of Polyesters Biodegradation
The effect of extra carbon source on degradation rate was determined by growing P. oxalicum SS2 in 500 mL Erlenmeyer flask containing 200 mL MSM with 200 mg of PHB films (1.5 cm × 1.5 cm, 50 mg) each, and supplemented with different carbon sources (0.2%). Another flask was setup, having the nutrient-rich medium, LB (Luria–Bertani) medium, supplemented with polyester films and inoculated with fungus. The composition of LB medium is g/l: tryptone 10; yeast extract 5.0; NaCl 5.0. The flasks were then incubated at 30 °C for 05 days at 100 rpm and were observed on a daily basis. At the end of experiment, the films were recovered and medium was centrifuged at 6000×g for 10 min at 4 °C. The recovered cells were freeze-dried and weighed. The hydrolysis of polymer film as a result of fungal activity was depicted through weight loss. All experiments were conducted in triplicate.
Degradation of Polyesters in Soil Microcosms by Penicillium oxalicum SS2
The soil has been collected from a garden located in Horticulture Section of Quaid-i-Azam University, Islamabad, and used for degradation of polyesters (Table 1). Both un-sterilized and sterilized garden soil were used in the experiment. Chemical and physical characteristics of the soil were analyzed based on the ISO-described standards [19, 20]. The PHB and PHBV films (50 mg) were first weighed and then sterilized in 70% ethanol and exposed to UV radiation for 5 min followed by rinsing with sterilized distilled water. These films were separately buried in sterilized soil (30 g) taken in pots and inoculated with 10 mL of P. oxalicum SS2 in MSM under aseptic conditions and incubated at 30 °C for 2 weeks. Negative controls were setup with plastic films in sterilized soil inoculated with MSM without the fungal strain. A similar set of experiment was also established with un-sterilized soil. The samples of PHB and PHBV films were recovered on a daily basis to observe their weight loss. Besides this, films were analyzed for their degradation after 1 week of incubation using different analytical techniques. Every treatment had three replicates.
The soil microbiota was determined by measuring total viable count (CFU/mL) as described previously [21] with slight modification. Nystatin (33 μg/mL) containing nutrient agar was used in case of bacteria, whereas malt extract medium supplemented with chloramphenicol (40 μg/mL) was used for fungi. Chloramphenicol containing plates were incubated at 30 ̊C for 72 h while nystatin plates at 37 ̊C for 24 h.
Analysis of Biodegradation
Scanning Electron Microscopy (SEM)
PHB and PHBV films recovered at the end of soil microcosms experiment were analyzed for surface changes through scanning electron microscopy (SEM) (Hitachi SU 1500, Japan). Samples were washed thoroughly with distilled water and mounted on copper stubs with gold paint. Gold plating was carried out in vacuum through evaporation to enhance the conductivity of samples. The control samples were also analyzed along with the test for comparison.
Fourier Transform Infrared Spectroscopy (FTIR)
PHB and PHBV films were subjected to FTIR (L160000A, Perkin Elmer, USA) to detect changes in functional groups as a result of degradation. For this purpose, films were placed on FTIR sample plate and a spectrum of 500–4000 wavenumbers/cm was taken for each sample in duplicate. Abiotic control was also analyzed for comparison.
Differential Scanning Calorimetry (DSC) for Thermal Analysis
Polyester films were subjected to thermal analysis using DSC technique to observe changes in thermal properties of the films. For this purpose, a Mettler oscillating differential scanning calorimeter (DSC 822e, STARe Software, USA) was used. The treated films were placed in a sealed aluminum pan and an empty pan was used as a reference. Both the pans were sealed in such a way so as to avoid the evaporation of water during the process of scanning. The instrument was calibrated with indium and then samples were scanned at heating rate of 10ºC/min from − 100 to 200 °C. After scanning at high temperature, the samples were then cooled down at a cooling rate of 10ºC/min to − 100 °C. Melting temperature (Tm), the temperature when polymer changes from crystalline or semi crystalline form to amorphous form, was observed at the onset of the melting peak. A non-isothermal crystallization temperature (Tc), at which polymer changes from rubbery amorphous form to glassy solid amorphous form, was obtained from the cooling process.
Proton-Nuclear Magnetic Resonance (1H-NMR) Spectroscopy
To determine changes in the chemical structure, H-NMR spectra of the treated PHB and PHBV films were taken using Bruker Avance 400 NMR spectrometer (400 MHz). Polyester films were harvested after incubation with P. oxalicum SS2. The treated films were then dissolved in deuterated chloroform (CDCl3). This solution was then filtered through 0.47-μm PTFE syringe filter (25 mm diameter, Alpha Analytical) and then shifted to NMR tube to perform H-NMR experiment. The residual protons of solvent, i.e., chloroform-d were used as an internal standard to express chemical shifts of the polymer that were stated as parts per million.
PHBV Depolymerase Assay
PHBV depolymerase activity by Penicillium oxalicum strain SS2 was evaluated using method as described by Park et al. with some modifications [22]. Stock solution of substrate, p-nitrophenyl butyrate (pNPB), was prepared in acetonitrile. About 0.25 mL of culture supernatant was added to 2.5 mL buffer along with 2 µL pNBP and 10 µL ethanol. Reaction mixture was incubated for 20 min at 37 °C and optical density was calculated at 410 nm. The depolymerase assay was performed for 120 h and sample was withdrawn every 24 h. All the experiments were performed in duplicates and a negative control was also run. The amount of enzyme required to release 1 µmol of product (p-nitrophenol) in 1 min was defined as one unit of enzyme.
Optimization of Fermentation Conditions for PHBV Depolymerase Production
The effects of various physical parameters such as temperature (25–50 °C), pH (3–8), substrate concentration (0.01–0.3%), size of inoculum (2–10% v/v; approximately 105 spores/mL), and supplementary carbon source (sucrose, dextrose and glucose) on PHBV depolymerase production were evaluated for maximum enzyme production. The depolymerase activity was performed every 24 h.
Production of PHBV Depolymerase Under Optimized Conditions
In an Erlenmeyer flask, about 500 mL MSM (pH 6.0) containing 0.2% (w/v) PHBV emulsion was taken and inoculated with about 10 mL of freshly grown spores of Penicillium oxalicum strain SS2. The flask was incubated at 30° C and 120 rpm for 72 h. By the end of production, the crude enzyme was harvested from the culture medium by centrifugation being performed at 10,000×g for 10 min at 4 °C and supernatant was collected for further purification.
Purification of PHBV Depolymerase
Acetone Precipitation
The purification steps were performed at room temperature. About 450 mL of cell-free supernatant was collected after centrifugation procedure as the crude enzyme extract. Precipitation of PHBV depolymerase from the above-mentioned crude enzyme extract was performed using acetone precipitation method. Varying amounts (10–80%) of cold solvent was added to the crude extract to determine the optimum amount of acetone required for protein precipitation. To the 450 mL of cell-free supernatant acetone (80% saturation) was added slowly at 4 °C with continuous mixing. The precooled solvent was used to avoid formation of locally high concentration of organic solvent. After complete addition of solvent, the medium was kept undisturbed and allowed to stand for 15 min to attain equilibration followed by centrifugation at 10,000×g at 4 °C for 20 min. the precipitate was collected and suspended in 100 mM Tris–HCl.
Gel Filtration Chromatography
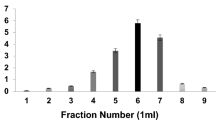
The crude enzyme extract was first dialyzed overnight using 100 mM Tris–HCl buffer (pH 6.0). The dialysate was further purified through size exclusion chromatography where a gel column (1.8 × 44 cm) packed with sephadex G-75 was used. Elution of sample was performed using 100 mM Tris–HCL buffer pH 6.0 at a flow rate 3.0 mL/5 min. About 23 fractions with 2 mL each were collected. Fractions showing maximum depolymerase activity were pooled and stored at – 80 °C for further use.
Molecular Weight Determination
Molecular weight determination of purified depolymerase was done by Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on 12% gel according Laemmli [23] using the standard protein marker (Bio-Rad, USA). Coomassie brilliant blue R-250 was utilized to mark gel.
Characterization of Purified PHBV Depolymerase
Effect of Temperature and pH on Enzyme Activity and Stability
The influence of temperature on enzyme activity was evaluated for temperature range (15 − `60 °C). The purified enzyme was pre-incubated in 100 mM Tris–HCL buffer (pH 6.0) at various temperature 15 − 60 °C for 2 h to determine thermostability and residual activity was determined. The influence of pH on enzyme activity was calculated over various pH range (3.0–8.0). The purified enzyme was incubated with different buffer systems at 30 °C for 2 h to determine pH stability and residual activity was calculated. The following buffer systems (0.1 mM) were employed: sodium phosphate (pH 6.0–7.0), sodium acetate (pH 3.0–5.0), and glycine–NaOH buffer (pH 8.0–9.0).
Effect of Metal Ions and Surfactants on Enzyme Activity
Effect of metal ions on the enzyme activity was calculated by incubating the purified enzyme with metal ions at various concentrations (10 mM and 20 mM); [Hg2+, Cu2+, Zn2+, Ni2+, Fe3+, Co2+, Cr3+, Na1+ and As3+] using salts [HgCl2, CuSO2, NiCl2, FeCl3, CoCl2, CrCl3, NaCl, AsCl3] at 30 °C for 2 h and the residual activity was recorded. The effect of surfactants (5 mM) [Tween 20, Tween 60, sodium dodecyl sulfate (SDS), Polyethylene glycol (EDTA) and Triton X-100] on the activity of purified enzyme was evaluated. The purified enzyme was incubated with different surfactants for 1 h at 30 °C and residual activity was calculated.
Statistical Analysis
Data were analyzed for statistical significance using the IBM-SPSS software using analysis of variance (one-way ANOVA), Tukey’s HSD (honestly significant difference) test. p value was set at < 0.05 as threshold for the level of significance.
Results
Screening for Polyester-Degrading Microorganisms
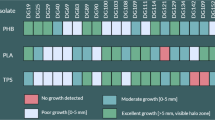
Soil samples were screened for polyester-degrading microorganisms. Initially, a total of 56 potential degraders including fungi, bacteria and actinomycetes were isolated but the fungal strain designated as SS2 was selected for further study based upon its maximum polyester-degrading ability at 30 °C within 48 h (Fig. 1).
Characterization of Fungal Strain SS2
The colony of strain SS2 was initially white in color, which gradually turned dark green after 5 days of incubation at 30 °C, while the aerial mycelia remained white. Fungal colony observed from lower side of agar plate was off white to pale yellow in color. Microscopic examination revealed that its hyphae were 7 µm in diameter, whereas conidia were 2–3 mm, smooth and globulus. The fungal strain SS2 was found to grow at wide temperature range (20–55 °C) with an optimal temperature of 30 °C that represents its mesophilic nature. The degradation potential of the fungus against aliphatic polyesters was checked using PHB and PHBV. Zone of hydrolysis was observed at different temperatures between 20 and 50 °C indicating its activity at wide range of temperatures.
rDNA sequencing results were analyzed by comparing the nucleotide sequences available in NCBI database through BLAST search analysis. Based on sequence homology obtained from NCBI database, the strain SS2 showed close homology to genus Penicillium having 100% similarity with P. oxalicum strain QTYC27 (KM103315.1) (Fig. 2). Thus, strain SS2 was identified as Penicillium oxalicum and the nucleotide sequence reported here can be obtained from NCBI nucleotide sequence database under accession number KY962009.
Degradation Activity Against Aliphatic Polyesters
Degradation Activity on Polymer Emulsions
The ability of P. oxalicum SS2 to degrade PHB and PHBV emulsions as well as films was investigated. P. oxalicum SS2 can efficiently degrade PHB and PHBV and turned both the emulsions completely transparent within 36 h (Fig. 3a and b). The degradation ability of strain SS2 was also investigated against PHB and PHBV films and the rate of degradation was examined after different time intervals by measuring weight loss. P. oxalicum SS2 was found to degrade PHB and PHBV films very efficiently and almost 100% of PHB and PHBV film was degraded within 48 h (Fig. 3c–e).
Degradation of PHB and PHBV emulsion and film by Penicillium oxalicum strain SS2. The turbidity of PHB (a) and PHBV (b) emulsions disappeared after treatment with strain SS2 within 36 h. PHB (c) and PHBV (e) films were also degraded by strain SS2 in 48 h, as indicated in d PHB and f PHBV. Vertical bars represent standard deviation
Effect of Extra Carbon Source on Rate of Polyester Degradation by Penicillium oxalicum SS2
The relationship between growth of P. oxalicum SS2 in various media and rate of degradation (hydrolytic activity) against polyesters is given in Table 2. The fungus grew well on all media. It was observed that the rate of degradation rate was highest in MSM where PHB was the only carbon source for strain SS2 while presence of extra carbon sources had negative effect on hydrolytic activity. Strain SS2 showed sufficient growth in minimal medium supplemented with extra carbon sources like glucose, sucrose, maltose, lactose and dextrose as well as in LB medium but the degradation rate against PHB was not appreciable. There was a significant difference in rate of degradation in medium supplemented with PHB as a sole source of carbon (p < 0.05) as compared to the medium with extra carbon sources along with PHB. Moreover, no significant change in the hydrolytic activity was observed in presence of a detergent, Tween 20. Experimental data were fitted to a linear reaction with an equation type W = a + k × t. Here, W (mg) is the weight of the film at time t (s), a the intercept at t = 0, and k the rate of reaction for a zero-order reaction.
Biodegradation of Polyesters in Soil Microcosm by Penicillium oxalicum Strain SS2
Lab-scale soil burial experiment was carried out to determine the role of P. oxalicum strain SS2 in degradation of aliphatic polyesters, as a simulation of an actual soil environment. The garden soil was thoroughly characterized and its constituents are presented in Table 1. The experiment was performed by burying the polyester films (PHB and PHBV) separately in sterilized and un-sterilized soil, both inoculated with P. oxalicum SS2. Separate controls were run for both sterile and non-sterile soil with polyester films but without the fungal strain. The rate of degradation of PHB and PHBV films in both sterile and non-sterile soils was determined after every 24 h. Almost 99% of PHB and PHBV films were degraded on 6th and 7th day of incubation, respectively, as indicated by the remnants of the films. In case of controls, the films remained intact and no weight loss was observed (Fig. 4). CFU of soil sample was calculated for both bacteria and fungi. Based on the results, both the bacterial and fungal population were calculated as 1.7 × 107 CFU/g and 5 × 103 CFU/g, respectively, while our test strain SS2 was not considered as part of the indigenous fungal population of the soil.
Time course of degradation of PHB (a) and PHBV (b) by Penicillium oxalicum strain SS2 in soil microcosms. Both PHB and PHBV films were completely broken down within 06 and 07 days, respectively, by strain SS2 as indicated by approximately 100% weight loss. Each soil microcosm contained 30 g soil medium inoculated with 50 mg PHB or PHBV films and 10 mL MSM to adjust the moisture content
Analysis of Degradation of Polyester Films
Fourier Transform Infrared (FT-IR) Spectroscopy
The changes in functional groups of PHB and PHBV during degradation were examined through FTIR spectroscopy (Fig. 5). For PHB, a peak at 1743 cm−1 representing C=O decreased in intensity and appeared at 1737 cm−1 which is not only indicative of decrease in carbonyl functionality but also a decrease in the amorphous phase due to preferential degradation leading to relevant increase in crystalline phase. Intense peak at 1032 cm−1 representing C–O of the ester linkage greatly decreased in intensity and appeared as multiple small peaks at 1032 cm−1 and 1054 cm−1 indicating breakdown of ester linkages by microbial esterases. In case of PHBV, a peak at 3026 cm−1 represents C–H (SP2) stretching, disappeared in the treated PHBV films. Moreover, a new sharp peak emerged at 1723 cm−1 representing carboxylic functionality. These observations represent hydrolysis of the polymer chains resulting in formation of carboxylic acids. The decrease in intensity of peaks in the area 2868 cm−1 to 2979 cm−1 indicates that intensity of C–H (SP3) stretching has been decreased by conjugation. Peak at 1032 cm−1 represents C–O of ester functional group in the untreated control and its disappearance in the treated PHBV sample indicates hydrolysis of ester bonds in the PHBV films exposed to microbial treatment.
FTIR spectra of PHB (a) and PHBV (b) films pieces recovered from soil inoculated with Penicillium oxalicum strain SS2. a Pink line: control; green line: test. b Blue line: control; pink line: test. The changes in functional groups of PHB and PHBV after degradation could be observed in FTIR spectra at different wavenumbers (Color figure online)
Scanning Electron Microscopy (SEM)
The progression of degradation was confirmed by SEM micrographs. SEM of PHB and PHBV films recovered from soil after 7 days was performed to observe morphological changes on the surface of film and compared to that of untreated control. Appearance of pits, cracks and holes in the test sample after microbial treatment, as compared to untreated control, can be observed (Fig. 6).
SEM images of both PHB (a–c) and PHBV (d–f) films recovered from soil inoculated with Penicillium oxalicum strain SS2. The films were washed with sterilized distilled water to remove dirt and fungal growth. The micrographs clearly indicate appearance of changes in the form of pits and cracks on surface of both PHB (b and c) and PHBV (e and f) films in comparison to controls (a and d)
Proton-Nuclear Magnetic Resonance (1H-NMR) Spectroscopy
The 1H NMR spectra of both test and control samples for PHB as well as PHBV were compared and a clear difference was observed. In case of PHB, the methyl protons [–CH3 (1)] of the 3-HB side chain corresponded to the doublet at 1.01 ppm were shifted to 0.84 ppm whereas the methines [–CH (2)] of the 3-HB bulk structure chain corresponding to two main multiplets were observed at about 5.5 ppm in case of the test sample but not in control (Fig. 7). While in case of PHBV control, the methyl protons [–CH3 (5)] of the 3-HV side chain represented by the triplet at 0.9 ppm disappeared in test sample and the methylene protons [–CH2 (6)] of the 3-HV side chain appeared in test sample as multiplet at 1.6 ppm.
NMR spectra of PHB (above) and PHBV (below) films recovered from soil inoculated with Penicillium oxalicum strain SS2 after 7 days. ‘C’ represents for control while ‘T’ for test sample. Change in the intensity of resonance could be observed in test samples of both PHB and PHBV in comparison to control, as shown by changes in the integration values
Differential Scanning Calorimetry (DSC) for Thermal Analysis
A DSC curve taken for PHB and PHBV films before and after enzymatic degradation showed remarkable changes in thermal properties of the polymers. In case of PHB, the melting temperature Tm of microbially treated film increased up to 172 °C from 168 °C in control. In case of PHBV, melting temperature Tm decreased from 172 °C to 158 °C (Fig. 8).
DSC spectra of PHB (a) and PHBV (b) films recovered from soil inoculated with Penicillium oxalicum strain SS2, after 7 days. C represents for control while T for test sample. A DSC curve taken for both PHB and PHBV films before and after enzymatic degradation showed remarkable changes in melting temperature Tm of polymers. Vertical axis represents heat flow (Mw/mg) for both exothermic and endothermic curves
Optimization of Culture Conditions for PHBV Depolymerase Production
The effect of various physical and chemical parameters on PHBV depolymerase production was evaluated using specific activity calculation of depolymerase from Penicillium oxalicum strain SS2. The maximum specific activity was found at 30 °C (p < 0.05), pH 6.0 (p < 0.05), 0.2% substrate concentration (p < 0.05) and 2% inoculum (p < 0.05) after 72 h of incubation with PHBV as substrate with no supplementary carbon source.
Production and Purification of PHBV Depolymerase
About 500 mL of production medium (pH 6.0) containing PHBV as substrate (0.2%) was inoculated with Penicillium oxalicum strain SS2 and incubated at 30 °C in shaker incubator at 120 rpm for 72 h for depolymerase production. The enzyme from cell-free supernatant was precipitated using acetone precipitation method at 80% solvent concentration. The precipitated enzyme was further purified to homogeneity through column chromatography using sephadex G-75 resin. The average molecular weight of the purified enzyme was found to be approximately 36 kDa (Fig. 9). Table 3 describes various steps of purification for enzyme; a 6.12-fold increase in purification of enzyme was achieved.
Characterization of Purified PHBV Depolymerase
Effect of Temperature and pH on Enzyme Activity
The effect of temperature on purified PHBV depolymerase was observed at various temperatures (15–60 °C) for 2 h. The purified esterase from Penicillium oxalicum strain SS2 showed high activity between 30 and 55 °C, with optimum at 40 °C (p < 0.05). The enzyme retained 99% of its activity at 40 °C up to 120 min (p < 0.05), while above 80% between 30 and 55 °C for 120 min (p < 0.05). The effect of pH on purified PHBV depolymerase was observed by incubating the enzyme at various pH (3.0–8.0) for 2 h, then enzyme activity was calculated. The purified depolymerase showed activity over a wide pH range, with maximum at pH 5.0 (p < 0.05). Purified enzyme was highly stable within pH range 3.0–7.0, and retained more than 80% activity at pH 5.0 for 120 min (p < 0.05).
Effect of Metal Ions and Surfactants on Enzyme Activity
The effect of various monovalent, divalent and trivalent metal ions on esterase activity was evaluated at two different concentrations (10 mM and 20 mM). Cu2+ strongly enhanced enzyme activity at 20 mM (p < 0.05), while As3+, Co2+, Fe3+, Na1+, Hg2+, Ni2+ and Cr3+ did not affect the enzyme activity much (p < 0.05); Zn2+ inhibited the enzyme activity at both concentrations (p < 0.05) and the relative activity was dropped to less than 10%. The effect of surfactants on specific activity of purified esterase from Penicillium oxalicum strain SS2 was determined by incubating the enzyme with two different concentrations of each surfactant, i.e., 1% and 5%. A decline in the enzyme activity was observed after treatment with surfactants. Maximum inhibitory effect was shown by triton X 100 at 5% concentration as purified depolymerase retained only 6.4% of its residual activity (p < 0.05). Tween 20 at both concentrations exhibited inhibitory effect on enzyme activity followed by Tween 60 (p < 0.05). EDTA and SDS also greatly decreased the enzyme activity at both concentrations (p < 0.05).
Discussion
The construction of simulated systems using potential microbial communities is future preferences among plastic waste management strategies, which involves comprehensive standardization and monitoring of biodegradation process as well. This requires more dedicated and robust search for the plastic-degrading microbial strains and highly efficient depolymerizing hydrolases. The incomplete biodegradation is hazardous as it releases plastic particles that contaminate ground water resources and often become part of our food web as well; therefore, complete biodegradation is highly desirable. The present study was intended to isolate potential polyester-degrading microorganisms from soil environment. There are limited reports on bacterial and fungal species as well as depolymerases that completely degrade polyester-based plastics. The current study reports complete biodegradation of PHB/PHBV by Penicillium oxallicum strain SS2 in liquid media as well as in lab-built soil microcosms leaving no disintegrated particles, which represent the novelty of the study. Penicillium oxalicum strain SS2 is a soil inhabiting fungus with dark green colonies having white smooth edges and pale yellow base. Penicillium species are well known for their role in PHB degradation from some previous studies [24, 25]. Newly isolated P. oxalicum strain SS2 efficiently degraded PHB and PHBV films and almost 99% degradation was achieved within 48 h. The degradation of aliphatic polyesters by P. oxalicum strain SS2 was also investigated in real soil environment using lab-scale microcosms built for this purpose. Degradation of PHB and PHBV films in soil microcosms upon augmentation with our fungus clearly indicates the degradation potential of our strain SS2 against polymer films and suggests that this strain could be implied in bioaugmentation strategies against other polyesters as well. The soil used in the experiment was loamy sand type and its texture could have provided good aeration and surface area for microbial colonization that facilitated biodegradation of polymer films. Other chemical properties of soil like organic content, C/N ratio, particle size and mineral levels may increase bioavailability and fungal attachment to polymer surface. The phenomenon of catabolite repression was observed while studying the effect of extra carbon sources on growth rate of strain SS2 and its hydrolytic activity against PHB. A profound fungal growth was found in the presence of extra carbon sources while a significant decrease in rate of degradation was observed as compared to the availability of PHB as the only carbon source, which promoted degradation activity. In a similar study, when poly(butylene adipate-co-terephthalate) (PBAT) degradation was investigated in actual soil environment using a fungus Isaria fumosorosea, strain NKCM1712, 40% of degradation rate was recorded against polymer films within 30 days of incubation and same effect of catabolite repression had been described [24].
Polyester degradation was monitored by evaluating changes in surface morphology and chemistry of the treated PHB and PHBV films through SEM and FTIR, respectively. Due to microbial treatment, FTIR spectra of partially degraded PHB and PHBV films clearly showed cleavage of polymer chain as well as appearance of new peaks. Major changes observed in the FTIR spectrum of PHB films indicated hydrolysis of ester functionality and preferential degradation of the amorphous regions leading to an increase in crystallinity with degradation. These results were also supported by DSC data where an increase in melting point of PHB film could easily be observed. Some changes were observed in FTIR spectrum of treated PHBV film in comparison to untreated control. Peaks representing C=O and C–O linkages of ester bond disappeared in treated films indicating ester hydrolysis; moreover, reduction in stretching of SP3 hybridized CH indicates appearance of double bonds. The microbially treated polyester films were further analyzed for their degradation by NMR and DSC techniques. Changes in intensity of the peaks in NMR spectra could be attributed to the structural changes going on in the polymer due to microbial treatment. In case of PHBV, change in intensity at 1.6 represents change in the carbanion group HV portion of polymer. This clearly shows that degradation is going on in HV region preferably, also confirmed by FTIR results. Changes in the integration value of different signals indicate chemical shifting by proton extraction and addition leading to the structural modification [26]. Llauro-Darricades et al. [27] also reported change in intensity, integration values and chemical shifting of the signals in NMR analysis of plastics that undergoes biodegradation. Changes in thermal properties of polymer like melting temperature can be related to degradation of polymers as a result of microbial attack. In case of PHB, marked changes occurred in structure of polymer film after microbial treatment so Tg and Tm curves merged, showing complete deformity in structure of the polymer. It was observed from the DSC curves that PHB has been degraded preferably in the amorphous region that can be deduced from the rise of melting point. FTIR analysis of PHB also directed toward an increase in crystallinity of the polymer after exposure to microbial treatment. A decrease in Tm for PHBV was observed which depicts decrease in crystallinity of the film. Coelho et al. [28] and Osman et al. [29] also reported the degradation of polymer with changes in its thermal properties using DSC. Microbial enzymes are responsible for degradation of polymers both in soil as well as liquid medium. The solid substrates are difficult to depolymerize in comparison to water-soluble substrates, because plastic films have low contact efficacy with enzymes. Thus, the enzymes that represent degradation potential against solid substrates possess remarkable properties enabling them to adsorb to the surface of substrates. The production and activity of enzymes are influenced by different physico-chemical parameters. Penicillium oxalicum strain SS2 could produce maximum amount of PHBV depolymerase under optimized culture conditions. Shivakumar et al. [30] have reported a fungal strain Penicillium citrinum S2 that produced maximum amount of PHB depolymerase at 30 °C and 6.0 pH after 72 h of incubation. Substrate concentration is a key factor that could have direct impact on enzyme activity. Our strain expressed better enzyme activity at low substrate concentration; there are studies reporting low enzyme activity at higher substrate concentration (0.2%), which can be attributed to the saturation of the enzyme [31]. A PHBV-degrading Streptomyces sp. strain AF-111 has been reported where maximum amount of enzyme was produced in the presence of 0.2% PHBV concentration [32]. Both low and high inoculum size results in decreased enzyme activity as a result of low growth rate owing to inadequate utilization of nutrients and depletion of oxygen and nutrients, respectively [33]. In our case, maximum enzyme activity was reported from strain SS2 at 5% inoculum size. Supplementary carbon sources seemed to decrease enzyme activity which depicts catabolite repression thus confirming the results obtained in our study where extra carbon sources decreased degradation rate for PHB [34]. The depolymerase enzyme from Penicillium oxalicum strain SS2 was purified to homogeneity with a purification fold 6.12 and its molecular size was found to be approximately 36 kDa. Mao et al. [35] purified PHA depolymerase with molecular weight 33.8 kDa which could degrade PHB, PHBV, and P(3HB-co-4HB). The purified enzyme represented stability at acidic pH, which is desirable as hydrolysis of PHB/PHBV could release butyric acid and valaric acid in the medium making the environment slightly acidic; therefore, an enzyme with the ability to withstand acidic pH is ideal for de-polymerization of PHB/PHBV. In a previous study, Penicillium expansum PHB depolymerase showed better enzyme activity at pH values 4.0–6.0 with maximum activity detected on pH 5.0 and 50 °C [36]. Metal ions are very important for the structural stability and to maintain enzyme solubility and catalytic properties [37]. The enzyme activity was strongly enhanced in the presence of divalent cationic metal Cu2+ (p < 0.05) while Zn2+ inhibited the enzyme activity at both concentrations (p < 0.05) and the relative activity was dropped to less than 10% (p < 0.05). The surfactants inhibited the enzyme activity with maximum inhibitory effect shown by Tween 20 and Tween 60. Observation showed that SDS and EDTA also decreased the PHBV depolymerase activity. The suppression of enzyme activity by non-ionic surfactants represents the presence of hydrophobic region near the active site of enzyme [38].
Conclusion
The current study concludes that Penicillium oxalicum strain SS2, isolated from soil, has potential to completely biodegrade aliphatic polyesters (PHB and PHBV) within limited time period in liquid media as well as in soil microcosms at mesophilic temperature. The purified enzyme (36 kDa) from strain SS2 showed maximum activity and stability at wide range of temperature and pH. Degradation potential of the isolated fungal strain and physio-chemical properties of the depolymerase enzyme suggest that Penicillium oxalicum strain SS2 could be exploited in depolymerization of wide range of polyester-based biodegradable polymers and in development of sustainable plastic waste management strategies. Furthermore, the conditions for soil microcosms experiment could be further optimized to maximize the degradation rate.
References
Nadhman A, Hasan F, Shah Z, Hameed A, Shah AA (2012) Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) depolymerase from Aspergillus sp. NA-25. Appl Biochem Microbiol 48(5):531–536
Tokiwa Y, Calabia BP (2004) Degradation of microbial polyesters. Biotechnol Lett 26(15):1181–1189
Reid BJ, Jones KC, Semple KT (2000) Bioavailability of persistent organic pollutants in soils and sediments—a perspective on mechanisms, consequences and assessment. Environ Pollut 108(1):103–112
Singh R, Paul D, Jain RK (2006) Biofilms: implications in bioremediation. Trends Microbiol 14(9):389–397
Schroll R, Becher HH, Rfler UD, Gayler S, Grundmann S, Hartmann HP, Ruossandju R (2006) Quantifying the effect of soil moisture on the aerobic microbial mineralization of selected pesticides in different soils. Environ Sci Technol 40:3305–3312
Grundmann S, Fus R, Schmid M, Laschinger M, Ruth B, Schulin R, Munch JC, Schroll R (2007) Application of microbial hot spots enhances pesticide degradation in soils. Chemosphere 68:511–517
Fang H, Dong B, Yan H, Tang F, Yu Y (2010) Characterization of a bacterial strain capable of degrading DDT congeners and its use in bioremediation of contaminated soil. J Hazard Mater 184:281–289
Włóka D, Placek A, Rorat A, Smol M, Kacprzak M (2017) The evaluation of polycyclic aromatic hydrocarbons (PAHs) biodegradation kinetics in soil amended with organic fertilizers and bulking agents. Ecotoxicol Environ Saf 145:161–168
Megharaj M, Ramakrishnan B, Venkateswarlu K, Sethunathan N, Naidu R (2011) Bioremediation approaches for organic pollutants: a critical perspective. Environ Int 37:1362–1375
Krueger MC, Harms H, Schlosser D (2015) Prospects for microbiological solutions to environmental pollution with plastics. Appl Microbiol Biotechnol 99:8857–8874
Mergaert J, Webb A, Anderson C, Wouters A, Swings J (1993) Microbial degradation of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in soils. Appl Environ Microbiol 59:3233–3238
Liu H, Gao Z, Hu X, Wang Z, Su T, Yang L, Yan S (2017) Blending modification of PHBV/PCL and its biodegradation by Pseudomonas mendocina. J Polym Environ 25:156–164
Shah AA, Hasan F, Hameed A, Ahmed S (2007) Isolation and characterization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) degrading bacteria and purification of PHBV depolymerase from newly isolated Bacillus sp. AF3. Int Biodeterior Biodegrad 60:109–115
Shah AA, Hasan F, Hameed A (2010) Degradation of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) by a newly isolated Actinomadura sp. AF-555, from soil. Int Biodeterior Biodegrad 64:281–285
Rong IH, Baxter AP (2006) The South African National Collection of Fungi: celebrating a centenary 1905–2005. Stud Mycol 55:1–12
Zhang YJ, Zhang S, Liu XZ, Wen HA, Wang M (2010) A simple method of genomic DNA extraction suitable for analysis of bulk fungal strains. Lett Appl Microbiol 51:114–118
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Shah Z, Krumhulz L, Aktas DF, Hasan F, Khattak M, Shah AA (2013) Degradation of polyester polyurethane by newly isolated Pseudomonas aeruginosa strain MZA-85 and analysis of degradation products by GC–MS. Int Biodeterior Biodegrad 77:114–122
Maliszewska-Kordybach B, Smreczak B (2000) Ecotoxicological activity of soils polluted with polycyclic aromatic hydrocarbons (PAHs)-effect on plants. Environ Technol 21:1099–1110
Placek A, Grobelak A, Kacprzak M (2016) Improving the phytoremediation of heavy metals contaminated soil by use of sewage sludge. Int J Phytoremediat 18:605–618
Yamamoto-tamura K, Hiradate S, Watanabe T, Koitabashi M, Sameshima-yamashita Y, Yarimizu T, Kitamoto H (2015) Contribution of soil esterase to biodegradation of aliphatic polyester agricultural mulch film in cultivated soils. AMB Express 5:10
Park HJ, Jeon JH, Kang SG, Lee JH, Lee SA, Kim HK (2007) Functional expression and refolding of new alkaline esterase, EM2L8 from deep-sea sediment metagenome. Protein Expr Purif 52:340–347
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680
Kasuya KI, Ishii N, Inoue Y, Yazawa K, Tagaya T, Yotsumoto T, Nagai KJI (2009) Characterization of a mesophilic aliphatic–aromatic copolyester-degrading fungus. Polym Degrad Stab 94:1190–1196
Lee KM, Gimore DF, Huss MJ (2005) Fungal degradation of the bioplastic PHB (poly-3-hydroxy-butyric acid). J Polym Environ 13:213–219
Hoshino A, Tsuji M, Fukuda K, Nonagase M, Sawada H, Kimura M (2002) Changes in molecular structure of biodegradable plastics during degradation in soils estimated by FT-IR and NMR. Soil Sci Plant Nutr 48:469–473
Llauro-darricades MF, Bensemra N, Guyot A, Petiaud R (1989) 13C and 1H NMR analysis of structural defects in PVC: application to the production of defects at various polymerization stages. Makromol Chem Macromol Sym 29:171–184
Coelho A, Costa L, Marques MDM, Fonseca I, Lemos MA, Lemos F (2010) Using simultaneous DSC/TG to analyze the kinetics of polyethylene degradation—catalytic cracking using HY and HZSM-5 zeolites. React Kinet Mech Cat 99:5–15
Osman M, Satti SM, Luqman A, Hassan F, Shah Z, Shah AA (2017) Degradation of polyester polyurethane by Aspergillus sp. strain S45 isolated from soil. J Polym Environ 26:301–310
Shivakumar S, Jagadish SJ, Zatakia H, Dutta J (2011) Purification, characterization and kinetic studies of a novel poly (β) hydroxybutyrate (PHB) depolymerase PhaZ Pen from Penicillium citrinum S2. Appl Biochem Biotechnol 164:1225–1236
Elbanna K, Lutke-Eversloh T, Jendrossek D, Luftmann H, Steinbuchel A (2004) Studies on the biodegradability of polythioester copolymers and homopolymers by polyhydroxyalkanoate (PHA)-degrading bacteria and PHA depolymerases. Arch Microbiol 182:212–225
Akbar S, Hasan F, Nadhman A, Khan S, Shah AA (2013) Production and purification of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) degrading enzyme from Streptomyces sp. AF-111. J Polym Environ 21:1109–1116
Abusham RA, Zaliha NR, Rahman RA, Saleha AB, Basri M (2009) Optimization of physical factors affecting the production of thermo-stable organic solvent-tolerant protease from a newly isolated halo tolerant Bacillus subtilis strain Rand. Microb Cell Fact 8:20
Jablonski S, Krasowska A (2013) Characterisation of alkaline lipase from an arctic yeast strain Rhodosporidium babjevae BD19. Eur Sci J 9(21):17–34
Mao H, Jiang H, Su T, Wang Z (2013) Purification and characterization of two extracellular polyhydroxyalkanoate depolymerases from Pseudomonas mendocina. Biotechnol Lett 35:1919–1924
Gowda USV, Shivakumar S (2015) Poly(-β-hydroxybutyrate) (PHB) depolymerase PHAZ(Pen) from Penicillium expansum: purification, characterization and kinetic studies. 3 Biotech 5(6):901–909
Çolak A, Sisik D, Saglam N, Guner S, Canaksci S, Belduz AO (2005) Characterization of a thermoalkalophilic esterase from a novel thermophilic bacterium, Anoxybacillus gonensis G2. Bioresour Technol 96:625–631
Kim D, Rhee Y (2003) Biodegradation of microbial and synthetic polyesters by fungi. Appl Microbiol Biotechnol 61:300–308
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Satti, S.M., Shah, Z., Luqman, A. et al. Biodegradation of Poly(3-hydroxybutyrate) and Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Newly Isolated Penicillium oxalicum SS2 in Soil Microcosms and Partial Characterization of Extracellular Depolymerase. Curr Microbiol 77, 1622–1636 (2020). https://doi.org/10.1007/s00284-020-01968-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-01968-7