Abstract
Synthetic polymers, commonly named plastics, are among the most widespread anthropogenic pollutants of marine, limnic and terrestrial ecosystems. Disruptive effects of plastics are known to threaten wildlife and exert effects on natural food webs, but signs for and knowledge on plastic biodegradation are limited. Microorganisms are the most promising candidates for an eventual bioremediation of environmental plastics. Laboratory studies have reported various effects of microorganisms on many types of polymers, usually by enzymatic hydrolysis or oxidation. However, most common plastics have proved to be highly recalcitrant even under conditions known to favour microbial degradation. Knowledge on environmental degradation is yet scarcer. With this review, we provide a comprehensive overview of the current knowledge on microbiological degradation of several of the most common plastic types. Furthermore, we illustrate the analytical challenges concerning the evaluation of plastic biodegradation as well as constraints likely standing against the evolution of effective biodegradation pathways.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastic materials occupy a specific position in the spectrum of anthropogenic chemicals of environmental concern. Commonly, the (eco-)toxicological hazard associated with a chemical is approximated as the product of a measure of exposure and a measure of its undesired effects. Extensive exposure of organisms and ecosystems to plastic materials is obvious these days. Large-scale pollution has been reported for terrestrial and marine environments (Castaneda et al. 2014; Jambeck et al. 2015) and is a result of the mass production of synthetic polymers that began in the last century and the fact that plastics contribute roughly 10 % of all (properly and improperly) deposited waste (Barnes et al. 2009), in concert with their deliberate longevity. A recent study estimated an annual input of 4.8 to 12.7 million metric tons (MMT) of plastic waste into the ocean (Jambeck et al. 2015), thereby contributing 60 to 80 % of marine macro- and mega debris (Gregory and Ryan 1997). The lack of direct toxicity of polymeric substances, on the other hand, may explain the thoughtlessness with which synthetic polymers have been wasted and the serenity with which plastic pollution, e.g. of the marine environment, has been accepted for decades. This attitude has been changing in the recent past, triggered by reports about widespread killing of marine wildlife by plastic debris (Derraik 2002). For plastic materials, it appears more obvious than for any other class of pollutants that our planet is not in balance regarding their environmental input and removal. It has even been suggested that all plastic, except that incinerated, may still be around (Thompson et al. 2005), a view that we cautiously contest in this review. The present situation of a global plastic pollution that is already regarded as unacceptable in concert with unabatedly increasing production rates (PlasticsEurope 2015b) demands stricter regulation of the use and handling of synthetic polymers. Consequently, possibilities for the ban or higher taxation of plastic bags in the European Union have recently been agreed on (EU 2014).
In this context, we examine the extant literature for indications of biological degradation of recalcitrant plastic materials in the aquatic and terrestrial environment. Whereas physical weathering of plastic due to mechanical stress, sometimes preceded by the leaching of chemical plasticisers is well documented (Cozar et al. 2014), information about biodegradation of synthetic polymers is still scarce. We will thus also reflect about principle obstacles for the biochemical breakdown of plastic and prospects based on theory-driven considerations. This review will briefly address the problems associated with environmental pollution with conventional plastic materials (chemical structures depicted in Fig. 1), consider the physico-chemical and biochemical reasons of their recalcitrance, give a systematic overview about recent advances regarding biodegradation of major classes of plastics and propose options for the management of plastic pollution. We will address the microbial degradation of the so-called bioplastics only shortly, due to the focus of this review on persistent conventional plastics and in regard of their comparatively little economic importance. For more in-depth discussion of the biodegradation of bioplastics, readers are referred to reviews by Shah et al. (2008) and Tokiwa et al. (2009). Due to the lack of literature information and the low promise of degradability, we will furthermore exclude silicon-based polymers and polyfluorinated polymers.
Overview on plastics in the environment
Major types and amounts of plastics and their ways into the environment
Most of the plastic types in use today entered large-scale production around the middle of the 20th century. Industrial production of polystyrene (PS) and polyvinyl chloride (PVC) began around 1930, polyamides, polyurethane (PUR) and polyethylene (PE) later in the 1930s and polyethylene terephthalate (PET) and polypropylene (PP) followed during the 1940s and 1950s (Andrady and Neal 2009; Howard 2002). Ever since, plastics manufacturing has been a steadily growing industry, reaching a production volume of 299 MMT in 2013 (PlasticsEurope 2015b) with PE, PP, PVC, PS, PET, PUR and polyamides contributing 82, 57, 39, 18, 18, 14 and 3 MMT, respectively (PlasticsEurope 2015a). So-called bioplastics (the term does not inevitably imply biodegradability but indicates a partly or fully biomass-based origin (Vert et al. 2012)) play a rather negligible role with production volumes of just 1–2 MMT. About 60 % of them are non-biodegradable polymers (European Bioplastics 2013; OECD 2013; Storz and Vorlop 2013).
After having fulfilled its intended purpose, a plastic item will follow either of three pre-determined paths. Recycling, i.e. reuse of the material for the production of new plastic items is the most environmentally benign path. Another path is burning to recover its energy content. The remaining plastic waste goes to landfills, where it is permanently buried. The fractions of all plastics following each path vary substantially between different countries and regions. In Europe, in 2012 approximately 26 % of plastic wastes were recycled, 36 % burned for energy recovery and 38 % deposited (PlasticsEurope 2015b), whereas in the USA in the same year, only 9 % were recycled (no distinct value for energy recovery available) (EPA 2014).
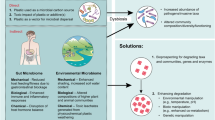
Unfortunately, plastic materials get lost at all steps of their life cycle and enter the environment. Large amounts end up in the open oceans, either being dumped there directly or being washed or blown from the land into the sea. There, multiple processes act on plastic items. Combinations of weathering by light, seawater, mechanical action and possibly biota cause the fragmentation of larger plastic items to smaller scales eventually giving rise to so-called microplastics smaller than 5 mm (Arthur et al. 2009; Cooper and Corcoran 2010; O’Brine and Thompson 2010; Thompson et al. 2004). Although most plastics are buoyant, colonisation by marine organisms may eventually cause them to sink deeper into the ocean, affecting the submarine environment and compounding efforts to quantify marine plastic pollution (Song and Andrady 1991). Eventual degradation of these materials by microorganisms has been suggested (Zettler et al. 2013). However, the efficacy of plastic biodegradation is a largely unsolved question. Virtually all types of plastics have already been identified in marine debris, such as polystyrene production beads, polyethylene microplastics and polyester fibres (Colton et al. 1974; Ng and Obbard 2006; Woodall et al. 2014). Most of these plastics are thought to be highly resistant to biodegradation (see ‘Biodegradation of hydrolysable plastics’ and ‘Biodegradation of non-hydrolysable plastics’) resulting in lifetimes of decades or even centuries (Derraik 2002; Ryan and Moloney 1993).
Knowledge on the total amount and distribution of plastics in the marine environment is extremely limited. Thompson et al. (2004) accordingly asked ‘Where is all the plastic?’ and pointed to microplastics that previously had received less attention than larger plastic debris. However, questions about the quantities of plastic debris in the oceans have mostly been addressed in recent years. For the eastern Pacific Ocean, Law et al. (2014) calculated at least 21,000 t of microplastics in surface waters, based on plankton sampling from 2001 to 2012. Cozar et al. (2014) calculated an amount of 10,000–40,000 t of plastic debris afloat in the oceans, based on data from sampling at 141 sites in the oceans in 2010–2011 and literature data. Compared with other estimates, this amount appears to be extremely low, but it highlights the large uncertainty about this issue. At the same time, Eriksen et al. (2014) reported approximately 270,000 t of macro- and microplastics in the open oceans, stating this to be a conservative estimate. Still, even this estimate appears to be rather low, given the cumulative production of several billion tons of plastics during the last decades and the fact that only a minor proportion had been burned or recycled. Recently, Jambeck et al. (2015) calculated that a range of 4.8–12.7 MMT of plastics enters the oceans annually, which is almost two orders of magnitude above the cumulative estimate by Eriksen et al. (2014). Consequently, the fate of the vast majority of the annual plastic input to the oceans is currently unknown. The probably most-important sink for the missing plastics is the deep sea (Goldberg 1997), which has indeed been reported to be littered by macro- and microplastic debris (Van Cauwenberghe et al. 2013; Woodall et al. 2014). The final fate of plastic particles in the deep sea is unknown, but the observation that most conventional plastics are extremely recalcitrant to biodegradation even under optimised laboratory conditions (see ‘Biodegradation of hydrolysable plastics’ and ‘Biodegradation of non-hydrolysable plastics’ and Table 1 for those rates of microbial degradation of plastics to be considered as comparatively high) makes it appear unlikely that considerable biodegradation takes place in deep sea sediments. Most likely, plastic debris is there to stay for a long time.
Environmental, health and aesthetic problems associated with plastic pollution
Reports about effects of environmental pollution with plastic materials have been skyrocketing in the last few years. Several categories of effects on organisms and ecosystems can be distinguished. (i) Aesthetic problems resulting from macroscopic items have been recognised for the longest time and are commonly considered as a nuisance rather than a hazard. (ii) Large plastic objects may also threaten the life of animals in several ways. Entanglement of animals in fishnets or plastic foil restricts their mobility, which in an extreme case keeps marine mammals and turtles from breathing at the surface and leads to drowning. Ingested plastics may also remain in the digestive tract of animals, reducing the quantity of food that can be taken up and thereby reducing animal fitness (Derraik 2002). Plastic items may furthermore lead to choking when they occlude the respiratory system. (iii) More recently, hazards arising from microscopic remnants of plastic objects have been recognised. Leaching of plasticisers and ultraviolet (UV) irradiation render synthetic polymers brittle and the action of waves or grinding on beaches lead to their fragmentation to small and minute particles (Barnes et al. 2009). These particles have often been found in the intestinal tracts of birds, fish and various invertebrates. Various negative effects have been associated with microplastics taken up by animals, but many effects are still poorly understood (Cole et al. 2011). Even smaller particles, such as microsized abrasives or microplastics used as ingredients in cosmetic products may enter the food chain at earlier stages and be transferred to higher trophic levels (Setala et al. 2014). (iv) Danger may also arise from additives such as plasticisers, which leach out from plastics thereby becoming bioavailable and exerting their toxicity to target organisms (Rochman et al. 2014). Similarly, many synthetic polymers are potent sorbents of hydrophobic environmental chemicals, which they may strongly accumulate, transport and release to recipient organisms, e.g. after being ingested (Bakir et al. 2014; Chua et al. 2014). (v) Plastic materials have also been recognised as substratum and vector of microbial biofilms constituted of microbial assemblages differing from those in the surrounding ocean. The possible involvement of plastics in the long-distance transport of pathogens and invasive species has been suggested (McCormick et al. 2014; Zettler et al. 2013).
Microbial degradation of plastics—limitations, options and prospects
Analytical challenges
Plastics are usually solids, often containing biochemically inert structures reducing the observable rates of transformation or degradation. Secondly, the bioavailability of the densely cross-linked polymers is quite limited as its accessibility for microbes or enzymes (Tables 1 and 2) is restricted to the outermost layer of the plastic items. Finally, the solid nature poses difficulties as to the unambiguous detection of their biological attack, which has to rely on either the determination of mass loss, the identification of surface modifications, the appearance of metabolites or the observation of microbial growth at the expense of the polymer.
Colourimetry and even simple visual observation can be applied to detect colour changes of biochemically altered plastics (Ali et al. 2014; Pastorelli et al. 2014). Surface deteriorations are monitored using scanning electron (SEM) and sometimes also atomic force microscopy (AFM) (Harrison et al. 2014; Santana et al. 2012; Yang et al. 2014). However, such rather rough morphological characterisations are of limited explanatory power.
Various quantitative methods for the assessment of biodegradation of plastics based on standardised tests conducted with different types of inocula (e.g. activated sludge, compost, marine samples, single microbial strains, microbial consortia) have been reviewed before (Eubeler et al. 2009). However, frequently monitored parameters such as CO2 (and sometimes also CH4) evolution and O2 (or dissolved oxygen) consumption are not specific. They further may remain meaningless if productive degradation processes are very slow and in case of cometabolic biotransformations (compare following section). Analysing the biodegradation of radiolabelled plastics (Eubeler et al. 2009) is compound specific, but complicated by only rarely available plastics with a radioactive label and the related requirements for analytical instrumentation and safety measures. Quantitative insights into alterations of plastics further can be retrieved from analyses of various properties of the parent plastics, which will be briefly introduced in the following. These methods require the removal of potentially interfering microbial biomass, which is frequently observed to form close associations with plastic materials (Ali et al. 2014; Yang et al. 2014). Gravimetric determinations of weight losses are often performed to gather first evidence for biodegradation (Agamuthu and Faizura 2005; Arkatkar et al. 2009; Eubeler et al. 2009). Gravimetry, however, may not be sensitive enough if biodegradation is slow and would overlook biochemical alterations not leading to mass losses. Moreover, gravimetry may lead to false-positive results caused by biodegradation or leaching of additives like, e.g. plasticisers, which are not part of the polymer network, and in case of fragmentation of parent plastics into smaller pieces, which then may not completely be recovered from the respective test system, e.g. compost systems (Eubeler et al. 2009), prior to weight determination. Changes in physical properties such as tensile strength and relative elongation are also used to monitor the degradation of plastics (Agamuthu and Faizura 2005; Eubeler et al. 2009; O’Brine and Thompson 2010; Tosin et al. 2012). Thermogravimetric analysis (TGA) may indicate altered thermal stability resulting from biological activity (Jeyakumar et al. 2013), whereas increased surface hydrophilicity resulting from microbial oxidation can be inferred from contact angle measurements (Santana et al. 2012). Decreases in molecular mass indicating depolymerisation reactions are frequently recorded using gel permeation chromatography (GPC) and viscosity measurements (Ali et al. 2014; Klun et al. 2003), and changes in the degree of crystallinity of plastics can be followed with differential scanning calorimetry (DSC) and X-ray diffraction (XRD) analysis (Klun et al. 2003; Volke-Sepulveda et al. 2002).
A more detailed information with respect to biocatalytically induced alterations of the chemical structures of parent plastics and their possible breakdown products can be derived from, e.g. Fourier transform infrared (FTIR) spectroscopy, microattenuated total reflectance/Fourier transform infrared (micro-ATR/FTIR) imaging, X-ray photoelectron spectroscopy (XPS), nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry mass (MS)-based detection methods (Biffinger et al. 2014; Deguchi et al. 1998; Jeon and Kim 2013; Yang et al. 2014). For instance, XPS, micro-ATR/FTIR imaging and FTIR spectroscopy all can detect the formation of oxygen-containing functional (carbonyl) groups resulting from, e.g. bacterial oxidation of PE films (Jeon and Kim 2013; Yang et al. 2014). FTIR spectroscopy can also be used to monitor microbial dehydrogenation reactions, e.g. of PE (Jeon and Kim 2013) and to substantiate the mode of biodegradation of e.g. the polyester PUs, where a preferential hydrolysis of the ester component was inferred from an increase in the NH to carbonyl peak ratio of the remaining solid polymer (Biffinger et al. 2014). NMR analysis of fungal nylon-6,6 degradation yielded breakdown products with end groups like –CH3, −NHCHO and –CHO, which are indicative of the oxidative cleavage of C–C and C–N bonds (Deguchi et al. 1998). NMR also detected γ-aminobutyric acid (GABA) oligomers and GABA as products of the bacterial hydrolysis of amide bonds in nylon-4 (Yamano et al. 2008). Water-soluble products of microbial PE degradation were recorded using MS (Yang et al. 2014).
Known drawbacks of the currently applied methodology for assessment of plastics biodegradation, in particular those related to a very low reactivity of the insoluble polymers and only little analytical specificity and sensitivity (Eubeler et al. 2009), would have to be overcome in the future. Sophisticated and sensitive analytical methods suitable to combine the quantification of the elemental composition of plastics surfaces with related structural information, e.g. XPS, enable the quantification of rates of biochemical reactions involving polymeric structures of plastics, even if such reactions proceed very slowly and do not immediately result in substantial amounts of easily detectable metabolites or mass losses. Non-destructive and chemically selective analytical techniques such as Raman spectroscopy, which enables to investigate the cellular uptake and intracellular fate of xenobiotics and has for instance been used to quantify plastic particles of different size classes in aquatic sediments (Imhof et al. 2013, 2012; Kann et al. 2015), may be applied to track biodegradation (and also other degradative processes).
Reasons for the poor biodegradability of plastics and prospects for microbial adaptation
The solid nature of plastics leads to extremely low bioavailability, since only a minute fraction of the polymer is exposed to potential degrader organisms. Plastics hence are poor growth substrates, including even those types possessing well biodegradable structural elements such as amide and ester bonds (Biffinger et al. 2014; Loredo-Trevino et al. 2012; Negoro et al. 2012) and despite their high-energy content and favourable electron donor quality. Poor bioavailability is not only an obstacle for biodegradation but can also be regarded as unfavourable for the evolution of productive degradation pathways. This view is supported by the general observation that productive degradation pathways are rare also for other groups of rarely bioavailable pollutants. Examples include the so-called micropollutants (or emerging contaminants), a chemically heterogeneous group of chemicals comprising industrial chemicals, pesticides, pharmaceuticals and components of personal care products (PPCPs). Their quality as driver of biochemical evolution is likely restricted by the typical minute concentrations occurring in the aquatic environment (ng/l to the lower μg/l range) (Kümmerer 2011; Murray et al. 2010). As a consequence, micropollutants are insufficiently degraded in wastewater treatment plants, and microbes capable of utilising them as growth substrates appear to be rare (Harms et al. 2011; Kümmerer 2011; Lapworth et al. 2012; Silva et al. 2012). Also, only a few microbes grow on poorly bioavailable high molecular mass polycyclic aromatic hydrocarbons (PAHs) with five or more aromatic rings (Harms et al. 2011). Notwithstanding, PAHs, many micropollutants and notably most plastics are good potential energy sources.
Unfavourable (i.e. too high) C/N ratios may also potentially limit the utilisation of plastics, an influence known from composting of natural organic matter (Khalil et al. 2008; Prahl et al. 1994). It remains to be elucidated whether the nitrogen from the moderately biodegradable PUs and polyamides (Fig. 1) could be assimilated by potential degraders, i.e. in analogy to the microbial utilisation of certain pesticides and other environmental pollutants as sole sources of nitrogen (Cai et al. 2014; Guo et al. 2014; Wang et al. 2011). Both fungi and bacteria have been found to attack plastics (Tokiwa et al. 2009), and representatives of either group have been reported to grow on certain of the comparatively more easily degradable polymers such as, e.g. polyester PURs and PE (Russell et al. 2011; Yang et al. 2014).
An alternative to productive biodegradation might be cometabolic biotransformation. Cometabolism dominates the alteration of xenobiotics in fungi and is also found among bacteria. It is commonly less compound specific than the productive degradation of xenobiotics (Harms et al. 2011; Solé and Schlosser 2014). Presently, the available information is too limited to judge if cometabolic or productive degradation of plastics prevails.
Another obstacle to microbial degradation of plastics comes from their macromolecular structure, which necessitates the extracellular initiation of breakdown into smaller products suitable for cellular uptake and further metabolisation. Hydrolysable plastics such as PURs, polyamides and PET, which possess ester or amide bonds, can be attacked by various extracellular hydrolases, which are related to the lifestyle of their microbial producers (i.e. feeding on natural macromolecules such as cellulose and proteins) (Loredo-Trevino et al. 2012; Negoro et al. 2012; Roth et al. 2014). Extracellular microbial attack appears more complicated when non-hydrolysable synthetic polymers such as PE, PP, PS and PVC are concerned. While in principle PE could be oxidised and then further degraded analogously to the ß-oxidation of n-alkanes between 10 and 50 C atoms, the involved enzymes are intracellular, thus requiring initial molecular mass reduction prior to cellular uptake and intracellular oxidation (Restrepo-Florez et al. 2014). Reported reductions in the molecular masses of PE catalysed by bacterial as well as fungal extracellular laccases (Fujisawa et al. 2001; Santo et al. 2013) are thus of high interest.
With respect to particularly inert structural elements of certain plastics such as C–C or C–Cl bonds, two particular ecophysiological groups of higher fungi (basidiomycetes) causing the so-called white-rot and brown-rot decay types of wood and other lignocellulosic materials are of particular interest. To access the lignocellulose polysaccharides, white-rot basidiomycetes employ an array of extracellular lignin-modifying enzymes including manganese peroxidase (MnP), versatile peroxidase (VP), lignin peroxidase (LiP) and the multi-copper oxidase laccase to initiate the cometabolic decomposition of lignin and its mineralisation into CO2 and H2O (Gutierrez et al. 2012; Hofrichter et al. 2010; Ruiz-Duenas and Martinez 2009). Lignin resembles certain plastics in not being hydrolysable and possessing structural elements such as non-phenolic aromatic rings, C–C and various ether bonds (compare Fig. 1), which are oxidised and cleaved during lignin degradation (Kim et al. 2011; Ruiz-Duenas and Martinez 2009). Such structural similarities might enable lignin-modifying enzymes of white-rot fungi like laccase and MnP to degrade the C–C bond-based PE and PP (Fujisawa et al. 2001; Iiyoshi et al. 1998; Jeyakumar et al. 2013). White-rot fungi have also been shown to be active on comparatively more easily biodegradable synthetic polymers such as polyamides (nylon), polyacrylates, polyacrylamides, polyvinyl alcohol and phenolic resins (Gusse et al. 2006; Larking et al. 1999; Stahl et al. 2000). Brown-rot fungi are thought to use hydroxyl radicals, which are produced during extracellular Fenton-type reactions maintained through redox cycling of certain Fe(III)-reducing fungal hydroquinones and catechols, as oxidants for the decomposition and acquisition of especially the crystalline parts of cellulose. Hereby, also aromatic lignin structures are attacked at different positions, finally resulting in some extent of lignin modification (Arantes et al. 2012; Daniel et al. 2008; Yelle et al. 2008, 2011). Moreover, such extracellular Fenton reactions have been implicated in the fungal dehalogenation and hydroxylation of chloro- and fluorophenols (Kramer et al. 2004; Schlosser et al. 2000), in the dechlorination of further chloroaromatics and chloroaliphatic compounds (Marco-Urrea et al. 2009) and in the depolymerisation of the recalcitrant ether polyethylene glycol (Kerem et al. 1999). Hence, they may also enable the oxidation of recalcitrant structures of plastics. Our own previous results have demonstrated the depolymerisation of the polystyrene analogue polystyrene sulfonate by hydroquinone-driven Fenton reactions operative in brown-rot fungi (Krueger et al. 2015).
Biodegradation of hydrolysable plastics
Natural polymers like cellulose, chitin or proteins are typically depolymerised via hydrolytic cleavage of the bonds linking their subunits. Therefore, one might expect man-made polymers with hydrolysable backbone structures to be easily biodegradable by hydrolytic means (Fig. 2; see Table 2 for corresponding hydrolases). For many of the so-called bioplastics, this is the case, leading to complete biodegradation in the environment within timescales ranging from days to a few months (Tokiwa et al. 2009). However, there are some notable exceptions, which are unfortunately the most widespread of the theoretically hydrolysable plastics: polyamides, most notable among them nylon, polyethylene terephthalate and polyurethanes.
Pathways of polymer biodegradation. Polymer hydrolysis is contrasted with oxidative degradation, which can transform both hydrolysable and non-hydrolysable polymers. Green lines, functional groups, including hydrolysable bonds inside hydrolysable polymers. Question mark so-far unknown enzymes Abbreviations: Cut cutinase, Nyl nylon hydrolase, AlkB alkane hydroxylase, Lac laccase, MnP manganese peroxidase
Polyamides
Synthetic polyamides consist of repeating units linked by amide bonds, which closely resemble the bonds in natural proteins. When their constituents are aliphatic, polyamides are commonly called nylons, but there are also semi-aromatic or aromatic polyamides like the high tensile strength para-aramid Kevlar™. Polyamides are typically synthesised by condensation of a diamine with a dicarboxylic acid or the self-condensation of a lactam or an aminocarboxylic acid. Hydrogen bonds between the polyamide bonds of different strands stabilise the polymers, whereas the chain length of the constituents, reflected by the nomenclature, determines the bulk properties. The most important polyamides are nylon-6,6, the polymer of hexamethylene diamine and adipic acid, and nylon-6, polycaprolactam (Acmite 2014). For nylon, two pathways of biodegradation, i.e. hydrolysis or by oxidative cleavage of the polyamide bond have been reported.
Nylon hydrolysis has been studied extensively using oligomers. Enzymes catalysing the hydrolysis are found in Flavobacterium, Pseudomonas, Arthrobacter and Agromyces strains (Negoro 2000; Ohki et al. 2005; Yasuhira et al. 2007). In Flavobacterium, the three genes nylA, nylB and nylC were found to encode two nylon dimer hydrolases and an endo-type nylon oligomer hydrolase (Negoro 2000). Negoro et al. (2012) resolved the crystal structure of the oligomer hydrolase NylC from Agromyces and demonstrated that a quadruple mutant was able to catalyse the depolymerisation of polymeric nylon. However, it remains to be seen whether nylon hydrolases can cause substantial degradation of polymeric nylon within a reasonable timeframe.
Oxidative attack on nylon-6,6 was first reported by Deguchi et al. (1997) for the white-rot fungi IZU-154, Trametes versicolor and Phanaerochaete chrysosporium. Via NMR spectroscopy, the authors identified several products of oxidative degradation in nylon samples incubated with the fungi, while they were unable to detect hydrolysis products. The observation that manganese improved degradation pointed to the lignin-modifying enzymatic system of the fungi, especially MnP, as the agents responsible for nylon biodegradation. This was confirmed by a subsequent report (Deguchi et al. 1998), where a MnP from IZU-154 was purified and demonstrated to be responsible for the degradation, although by a mechanism distinct from standard MnP action. Later, degradation of nylon-6 by the white-rot fungi P. chrysosporium and Bjerkandera adusta as well as the possible utilisation of the polymer as sole source of nitrogen for mycelial growth was reported (Friedrich et al. 2007; Klun et al. 2003).
In contrast to nylon-6 and nylon-6,6, biodegradation of nylon-4 (poly-γ-aminobutyric acid) has been shown to be a rather fast and simple process in soil and activated sludge (Hashimoto et al. 1994; Kawasaki et al. 2005). Pseudomonas strains producing hydrolytic exoenzymes have been implicated in this biodegradation (Yamano et al. 2008). These reports demonstrate that biodegradation of polyamide is strongly dependent on its exact composition and physico-chemical properties.
Polyethylene terephthalate
PET is an aromatic polyester of terephthalic acid with ethylene glycol. It is mainly used for the production of synthetic fibres, e.g. in the textile industry, but probably better known for its utilisation in plastic bottles, which also accounts for a substantial part of the annual PET production.
For a long time, PET was regarded as inert to biological degradation (Muller et al. 2001), which was attributed to the high melting point of aromatic polyesters resulting in low mobility of the polymer chains (Marten et al. 2003, 2005). Only in 2005, a hydrolase from the thermophilic actinomycete Thermobifida fusca was reported to cause weight losses of low-crystallinity PET films of approximately 50 % within 3 weeks at 55 °C (Muller et al. 2005). Since then, published reports mostly focused on potential industrial applications, e.g. in recycling or fibre modification. Ronkvist et al. (2009) reported that a cutinase from the soft-rot fungus Humicola insolens was capable of degrading 97 % of low-crystallinity PET films within 96 h at 70 °C. A comparison of these results was used to illustrate the importance of degradation temperatures close to the glass transition temperature of the substrate, stated to be 75 °C in both publications. However, this observation also indicates large problems for environmental PET biodegradation, as typical ambient temperatures are much lower. When interpreting degradation rates, it should be noted that time needed for the biodegradation of solids strongly depends on the exposed surface to mass ratio.
Cutinase enzymes have frequently been implicated in PET hydrolysis (Chen et al. 2010; Kawai et al. 2014; Muller et al. 2005; Ronkvist et al. 2009). Their natural substrate cutin, a main constituent of the plant cuticle, is a hydrophobic aliphatic polyester of omega hydroxy acids (Purdy and Kolattuk 1973). Therefore, it is not surprising that cutinases are capable of depolymerising the hydrophobic PET, at least to a certain extent.
Hydrolytic PET biodegradation shows some parallels to the depolymerisation of other, natural polymers, such as cellulose. Crystallinity of the polymer has been shown to be a crucial determinant for biodegradation, as highly crystalline regions are resistant to hydrolysis, while more amorphous regions are substantially more susceptible both in PET and cellulose (Horn et al. 2012; Marten et al. 2003, 2005). This effect has been attributed to the improved mobility of amorphous polymer chains in aqueous medium facilitating enzymatic action. Furthermore, cellulose-degrading enzymes have long been known to be inhibited by the products of cellulose depolymerisation, such as cellobiose and glucose (Gan et al. 2003). Similarly, Barth et al. (2015) recently demonstrated that PET hydrolysis by the cutinase TfCut2 is inhibited competitively by the PET hydrolysis intermediates mono- and bis-2-hydroxyethyl terephthalate. Drawing an analogy to the multi-component cellulose degradation system, the authors suggested that these limitations could be overcome in biotechnological processes by the application of a second enzyme with high affinity for the intermediate, which, however, is not an option for environmental PET degradation.
Polyurethane
PUR is a general designation for polymers composed of polyol and polyisocyanate subunits linked by carbamate/urethane bonds. PUR are best known as the material of a wide variety of hard and soft foams. Depending on their components, PUR can have vastly different properties, qualifying them as either non-melting thermosets or thermoplastic polymers. As PUR biodegradation has been reviewed extensively (Cregut et al. 2013), we will cover only the most relevant points and more recent findings here.
The polyol moiety appears to control PUR biodegradation. Whereas polyether polyols make PUR highly recalcitrant, polyester polyols result in high vulnerability to microbiological activity, as already observed by Darby and Kaplan (1968). Esterases and proteases are mainly involved in ester cleavage (Howard 2002); whereas the urethane bonds appear to be more stable against enzymatic hydrolysis, they have also been found to be degraded enzymatically (Akutsu-Shigeno et al. 2006).
Despite the vast number of PUR formulations, the anionic polyester polyurethane colloidal dispersion Impranil DLN™ from Bayer Material Science has found widespread application as a screening tool for PUR biodegradation activity (Crabbe et al. 1994; Peng et al. 2014; Russell et al. 2011). Its colloidal nature in aqueous solution permits use in agar plate zone clearing assays. As an alternative, a novel plate-based assay for the evaluation of biodegradability of PUR coatings on ZnSe coupons by infrared spectroscopy has recently been developed (Biffinger et al. 2014). Besides providing a useful tool for biodegradability evaluations, the authors also demonstrated that the typical Impranil assays are not universally valid for PUR biodegradation, as neither a different type of polyester PUR coating nor a polyether PUR coating were affected by Pseudomonas protegens, which easily degraded Impranil.
Biodegradation of non-hydrolysable plastics
In contrast to the polymers described before, those polymers with the highest production amounts lack one chemical feature that would allow a comparatively easy attack: hydrolysable bonds. Instead, they feature a backbone purely made of C–C bonds with no reactive groups attached to it, leaving (possibly radicalic) redox reactions as the only option to break the polymer into smaller molecules that might be assimilated or mineralised by microorganisms (Fig. 2).
Macromolecules inert to hydrolysis are quite common in nature. In fact, lignin, a central component of the plant cell wall, is considered as the second-most abundant natural polymer. Various peroxidases, including most prominently MnP, VP and LiP, multi-copper oxidases of the laccase group, as well as Fenton processes driven by hydroquinone redox cycling have been implicated in lignin biodegradation (Lundell et al. 2010; Martinez et al. 2005; Ruiz-Duenas and Martinez 2009; Suzuki et al. 2006), and MnP can also attack even more recalcitrant, non-hydrolysable compounds like humic substances and brown coal (Hofrichter 2002). Some of the aforementioned oxidative agents have also been reported to be involved in plastics biodegradation (Table 2). However, lignin is considerably easier to attack than plastic polymers, as its partially oxidised constituents make it far more hydrophilic and lower the redox potential required to successfully attack parts of the macromolecule through the creation of more uneven distributions of electron density. Bonds between subunits of lignin usually have bond dissociation energies (BDE) between 160 and 300 kJ/mol in case of C–O ether bonds or typically 240–425 kJ/mol (exceptions going up to 500 kJ/mol) for C–C bonds (Elder 2013, 2014; Kim et al. 2011; Younker et al. 2012). Synthetic plastics like PE, PP, PS and PVC, on the contrary, have BDE in the range of 330–370 kJ/mol in the C–C bonds of their backbones and 350–470 kJ/mol for C–H bonds (Cheremisinoff 1989; Dick 2014; Knyazev 2007; Luo 2007; Valko et al. 1972). As the weaker bonds are likely more susceptible to cleavage, these BDE values show that synthetic plastics are considerably harder to attack than lignin.
Polyethylene
Polyethylene is the most widespread synthetic polymer nowadays. Basically, it consists of long hydrocarbon chains with varying degrees of branching depending on its desired properties. Most prominently, PE is utilised for packaging, making it especially prone to ending up in the environment when not discarded properly. PE is regarded as highly resistant to degradation, due to its unreactive C–C and C–H bonds, its hydrophobic nature, its high molecular weight and the lack of vulnerable chemical groups (Gautam et al. 2007). Nevertheless, the amount of literature published on the issue suggests that PE is still more easily degradable than the other prevalent hydrocarbon polymers.
Biodegradation of PE has recently been reviewed by Restrepo-Florez et al. (2014), so we will be brief on PE biodegradation. In many cases, abiotic pre-oxidation of the polymer by UV light or heat has been suggested to play a crucial role in the initiation of PE biodegradation (Albertsson et al. 1987; Gilan et al. 2004; Hadad et al. 2005). However, there are also reports of substantial biodegradation without prior oxidation, such as by a Pseudomonas species degrading 5 % of untreated PE within 45 days (Tribedi and Sil 2013). Very recently, a highly interesting report on the isolation of two novel PE degraders from the Bacillus and Enterobacter genera was published (Yang et al. 2014). The authors isolated these organisms from a rather inventive source, namely the guts of plastic-eating waxworms, and they demonstrated substantial biodegradation of virgin PE of up to 6.1 and 10.7 % weight loss within 60 days for the Enterobacter and the Bacillus strains, respectively. Other comparably effective degraders under laboratory settings are Rhodococcus ruber and Brevibacillus borstelensis (Gilan et al. 2004; Hadad et al. 2005).
Insight into the biological mechanisms underlying PE biodegradation is extremely limited, since most studies published so far remained on the observational level. The few exceptions we are aware of concern a Pseudomonas strain, for which it was shown that its alkane hydroxylase gene alkB was involved in the degradation of low-molecular weight PE (Yoon et al. 2012). AlkB is usually involved in the biodegradation of alkanes, although at much shorter chain lengths than those present in PE. Nevertheless, it does not appear unlikely that enzyme variants could also attack PE. Another enzyme shown to be involved in PE biodegradation is the bacterial laccase of R. ruber, which was shown to cause oxidation and molecular weight reduction of UV pre-irradiated PE films (Santo et al. 2013; Sivan 2011). Interestingly, PE is structurally vastly different from the canonical substrates of laccases, namely phenolics (Giardina et al. 2010), and Santo et al. (2013) also reported that laccase from T. versicolor, a typical model enzyme, had no effect on PE. However, bacterial laccases are less well studied than fungal ones, so it may be possible that the R. ruber laccase has special properties enabling it to attack PE directly. Otherwise, it is also possible that R. ruber employs a so-far unknown redox mediator, as it is well known that these small molecules can greatly enhance the substrate spectrum of laccases (Giardina et al. 2010). For instance, it has been demonstrated that a system made of a fungal laccase and a synthetic redox mediator can successfully attack PE membranes (Fujisawa et al. 2001). More detailed characterisation of the R. ruber laccase and its actions on PE will be needed to elucidate how the enzyme contributes to PE biodegradation. Fungal manganese peroxidase has also been implicated in PE membrane degradation (Iiyoshi et al. 1998).
However, in real environmental settings, biodegradation rates observed were significantly lower than under laboratory conditions, ranging from 0.1 to 0.4 % weight loss within 800 days in soil to 1.6–1.9 % weight loss after 1 year in seawater off the Indian coast (Albertsson 1980; Artham et al. 2009).
As abiotic oxidation has been shown to play an important role in PE biodegradation, ‘degradable’ formulations of PE have been introduced and advertised as more environmentally friendly alternatives. Typically, these polymers contain either weak sites in the polymer backbone (Bremer 1982) or additives that act as pro-oxidants (David et al. 1992), leading to enhanced fragmentation and degradation of the plastics. However, a review on the state of the art of these polymers found little evidence that complete degradation was substantially enhanced under conditions prevailing in the environment (Roy et al. 2011). Consequently, the environmental impact of the so-called degradable PE remains questionable.
Polypropylene
Polypropylene, the second-widespread synthetic plastic worldwide, is an aliphatic hydrocarbon like PE, but differs by the presence of a methyl group on every subunit of the polymer backbone. Like PE, its use for packaging is widespread, but PP biodegradation has received considerable less attention. Cacciari et al. (1993) first reported tentative biodegradation of PP, observing that extractable fractions of low molecular weight compounds increased during 5 months of incubation with an enrichment culture. After 1 year in seawater, Artham et al. (2009) found 0.65 % weight loss and minor changes on the molecular level for their PP samples. Similarly, only 0.4 % weight loss were observed after 12 months in soil for untreated PP, but 10.7 % weight loss for thermally pre-treated PP, indicating that pre-oxidation, as for PE, plays an important role in PP biodegradation (Arkatkar et al. 2009). A strain of Bacillus flexus was isolated from these PP samples. Arkatkar et al. (2010) reported in vitro weight losses of up to 2.5 % after 1 year in minimal medium when UV-pre-treated PP films were incubated with several Bacillus and Pseudomonas strains, including B. flexus. However, without pre-treatment, the PP showed little changes. Jeyakumar et al. (2013) used the fungi P. chrysosporium and Engyodontium album to investigate the effects of pre-treatment and metal ion pro-oxidant blending of PP. They reported weight losses of up to 18.8 % in case of UV-pre-treated metal-blended PP and 10 % for UV-treated pure PP after 1 year, while samples without pre-treatment lost about 5 % weight. Thermal pre-treatment was found to have relatively little effect.
One strategy that has been explored to improve the biodegradability of PP materials is grafting with biodegradable polymeric compounds. Mikulasova et al. (2001) produced grafts with 10–30 % lignin content and investigated biodegradation by the fungus P. chrysosporium. Evidence of partial PP degradation in lignin-containing grafts was found via the extraction of low molecular weight fractions of PP, which were absent in abiotic controls and pure PP, but without being able to quantify the extent of biodegradation of the PP part. Jeyakumar et al. (2013) explored the effect of blending starch into the PP and reported approximately 10 % weight loss after 1 year incubation with fungi, compared with 5 % for pure PP.
Polystyrene
Polystyrene is a hydrocarbon polymer that has a phenyl ring linked to every second carbon atom of its backbone chain. It is well known for its application as styrofoam. Reports on PS biodegradability are scarce and indicate that it is almost undegradable. Kaplan et al. (1979) investigated PS biodegradation by fungi and mixed microbial cultures and found weight losses of only up to 0.57 % within 11 weeks, with degradation practically plateauing during the last 7 weeks. Similarly, R. ruber was found to be capable of forming biofilms and reducing the weight of PS films by 0.8 % within 8 weeks when PS represented the sole source of carbon in the medium (Mor and Sivan 2008). Sielicki et al. (1978) reported that 1.5–3 % of 14C-labelled polystyrene was mineralised when placed in different soils for 16 weeks. In our research, we found that the brown-rot fungus Gloeophyllum trabeum substantially degraded the water-soluble PS analogue polystyrene sulfonate (Krueger et al. 2015), but it appears incapable of attacking solid PS with any reasonable efficiency (Krueger and Schlosser, unpublished data). Grafting of polystyrene with biodegradable compounds, like lignin or starch, has also been explored, but biodegradation experiments revealed that in such cases, only the non-PS component of the graft was degraded (Milstein et al. 1992; Pushpadass et al. 2010). Enzymatic degradation of PS has been reported with a hydroquinone peroxidase from Azotobacter beijerinckii but only in an organic-aqueous two-phase system of little environmental relevance (Nakamiya et al. 1997).
Polyvinyl chloride
Polyvinyl chloride differs from other non-hydrolysable polymers due to the presence of a chlorine atom on every second carbon of the backbone. It is mainly used for construction purposes like piping, but it can also be used in applications requiring greater flexibility when combined with suitable plasticisers, which pose issues of their own (Erythropel et al. 2014). The presence of the heteroatom chlorine is the most likely reason for its extremely low biodegradability. Otake et al. (1995) found that no apparent biodegradation had happened to a PVC sample buried in soil for 32 years. Likewise, Ali et al. (2014) found little evidence for biodegradation of PVC films either buried in soil for 10 months or exposed to several fungal isolates in liquid culture for 4 weeks. Slight reductions in the average molecular weight and small changes in FTIR and NMR spectra were the only indications of biodegradation. Also, Santana et al. (2012) did not report substantial changes of PVC films subjected to biodegradation trials in soil.
Uncertainties associated with reported plastic biodegradation
It could reasonably be argued that usually only minor biochemical alterations of plastics can be expected, which hampers their experimental reproducibility. Here, particular difficulties are certainly related to those microbial processes not leading to substantial mass losses or molecular mass reductions within the observed time span, where sufficiently sensitive analytical methods enabling the detection of biochemical alterations beyond the aforementioned level would have to be applied (please also refer to the section dealing with “Analytical challenges” related to the microbial degradation of plastics). Even in cases where weight loss or molecular mass reduction have been observed, frequently very low reaction rates reported (Table 1) illustrate the difficulty of a sound, and particularly, a quantitative reproduction of the effects. Nevertheless, microbial degradation of both hydrolysable as well as non-hydrolysable plastics sometimes has been reproduced and thus established. Polyamides (nylon-6,6 and nylon-6) have consistently been reported to be attacked by MnP from ligninolytic fungi in studies from different working groups, with P. chrysosporium commonly being applied as a white-rot fungal species in different laboratories (Deguchi et al. 1997; Deguchi et al. 1998; Friedrich et al. 2007; Klun et al. 2003). Moreover, the versatile nature the radical-based enzymatic attack of MnP on plastics was also expanded to non-hydrolysable PE (Iiyoshi et al. 1998). PE biodegradation using R. ruber represents an example where the same laboratory has demonstrated reproducibility by obtaining consistent results applying a range of different methods and approaches in consecutive studies (Gilan et al. 2004; Santo et al. 2013; Sivan 2011). Nevertheless, sometimes microbial degradation of plastics, claimed in studies going less into the analytical depth, remains arguable, and the necessity of more in-depth studies to prove biodegradation cannot be ignored.
Biodegradation potential of so-far unexplored biodiversity
Based on the published literature, we estimate that there are good chances to identify novel degraders of conventional plastics among so-far unexplored biodiversity, especially for hydrolysable polymers. Most natural polymers (with lignin and lignocellulose-derived recalcitrant macromolecules such as humic/fulvic substances and coals as the only notable exceptions) are susceptible to hydrolysis, which makes this degradation pathway a ubiquitous process. Consequently, it is highly likely that some of the enzymes responsible for natural polymer degradation also show activity against hydrolysable synthetic polymers. Russell et al. (2011) demonstrated these impressively isolating PUR-degrading endophytic fungi from the Ecuadorian rainforest, which were able to utilise PUR as the sole source of carbon when grown anaerobically. Furthermore, the known organisms and enzymes capable of degrading nylon, PET and PUR originate from diverse environments like factories, soils and compost (Chen et al. 2010; Crabbe et al. 1994; Negoro 2000), so it is highly likely that more interesting and efficient degraders can be isolated from so-far unexplored environments.
In contrast to hydrolysable plastics, we estimate that the chances of isolating promising degraders of hydrocarbon plastics from novel sources are lower. Although environments hosting such organisms exist, as Yang et al. (2014) demonstrated isolating PE degraders from waxworm guts, it remains a fact that considerable obstacles need to be overcome for efficient degradation of non-hydrolysable plastics. Such organisms need to expose their extracellular enzymes directly to the extremely hydrophobic plastics. More demandingly, they must have redox potentials high enough to permit electron abstraction from unreactive C–H or C–C bonds that are even more inert than those found in lignin (see ‘Biodegradation of non-hydrolysable plastics’). A sufficiently high redox potential is likely to be the largest obstacle to efficient polymer degradation, especially for rather generalist enzymes, as we could demonstrate for the synthetic polymer polystyrene sulfonate (Krueger et al. 2015). Although this polymer is water-soluble and correspondingly bioavailable, white-rot fungi were found to be incapable of causing substantial depolymerisation despite having a considerable array of extracellular oxidative enzymes. Other publications on the degradation of plastics by white-rot enzymes reported some degree of degradation, usually determined via mechanical properties or reduced polymer molecular weight, but none of them could report sufficient degradation to become evident as weight loss (Fujisawa et al. 2001; Iiyoshi et al. 1998). It appears likely that the observed degradative effects were caused by internal defects in the polymers which allowed enzymes to attack these weak points.
Taking into account the dearth of reports on substantial biodegradation of hydrocarbon plastics, especially PP, PS and PVC, chances of finding highly efficient degraders for these polymers appear rather small.
Indirect ways of microbes/enzymes to act on plastics
Weathering of plastics is a long-term process (i.e. in the range of years) driven by UV irradiation, elevated temperatures, mechanical action, loss of additives such as plasticisers and other disintegrating influences. As a result, the polymers become brittle and finally may be disaggregated into smaller particles eventually down to microplastics (Bejgarn et al. 2015; Cooper and Corcoran 2010). The related increases in surface areas would potentially improve the bioavailability to degrading microorganisms. Such physico-chemical and mechanical breakdown processes are especially relevant for marine and beach environments and less important in soil (Corcoran et al. 2009).
Photooxidation particularly by UV light is considered to be the most important weathering process (McKeen 2013). It has been shown to increase surface oxidation and hydrophilicity, to cause bond scissions in the polymer backbone and may enhance the biodegradability of plastics (Arkatkar et al. 2010; Cooper and Corcoran 2010; David et al. 1992; Restrepo-Florez et al. 2014). Similar effects are observed upon exposure of plastics to elevated temperatures (Arkatkar et al. 2009; Berdahl et al. 2008) and Fenton processes (Arkatkar et al. 2010; Huang et al. 2002). In order to accelerate the physico-chemical degradability of plastics, oxo-biodegradable synthetic polymers have been developed. They contain any of a choice of pro-oxidant additives, which are commonly based on metals like, e.g. Co, Mn, Fe or Ti and form radicals when exposed to UV light or high temperatures. Follow-up reactions with atmospheric oxygen result in polymer chain scission and the formation of compounds that can be degraded by microorganisms (da Luz et al. 2013; Koutny et al. 2006; Ojeda et al. 2009). In this context, the possible hydroxylation of recalcitrant non-phenolic aromatic rings in PS (Fig. 1) by highly reactive hydroxyl radicals could make such structures accessible to those fungal lignin-modifying enzymes preferably acting on phenolic aromatics (Harms et al. 2011; Ruiz-Duenas and Martinez 2009). The biodegradability-enhancing effects of physico-chemical pre-oxidations however cannot be generalised. Depending on the respective type of polymer, oxidation process and pro-oxidant used, even more recalcitrant polymer structures (e.g. new cross-links, recalcitrant oxidised oligomers) may be formed, and also toxicity and biodegradability issues may arise (Costa et al. 2015; David et al. 1992; Fontanella et al. 2013; Stloukal et al. 2012).
Plasticisers are additives embedded between the chains of synthetic polymers, in order to render them soft, flexible and durable. Phthalate esters are widely used for such purposes, with di(2-ethylhexyl) phthalate (DEHP) used in PVC probably representing one of the most prominent examples. Plasticisers frequently evaporate and may be leached from polymers, making them more brittle and susceptible to disintegration processes. Certain plasticisers such as DEHP are persistent and environmentally ubiquitous and have gained much attention due to concerns related to endocrine-disrupting activities especially of their breakdown products (Erythropel et al. 2014; Zolfaghari et al. 2014). Accordingly, the biodegradation of plasticisers for bioremediation purposes has been investigated intensively (Barnabé et al. 2008; Chatterjee and Karlovsky 2010; de Moura Carrara et al. 2011), and both fungi (Luo et al. 2012; Pradeep et al. 2013) as well as bacteria (Ogawa et al. 2009; Pradeep et al. 2015) are known to degrade such compounds. Owing to environmental and human health concerns related to especially phthalate ester plasticisers, more ‘benign’ and better biodegradable plasticisers based on diverse organic acids, e.g. succinate and benzoate, have now been developed (Kastner et al. 2012; Kermanshahi pour et al. 2009; Pradeep et al. 2013). Beyond bioremediation of plasticisers themselves, the removal of such compounds from their parent plastics via degradation by biofilm-forming microbes colonising surfaces of plastics (Wang et al. 2004; Webb et al. 2000) would be expected to accelerate the disintegration of plastics. Such processes may be of particular relevance for marine systems combining various physico-chemical and mechanical weathering processes with the colonisation of plastic marine debris by microbial biofilm communities composed of diverse bacteria, cyanobacteria and eukaryotic organisms (Harrison et al. 2014; Oberbeckmann et al. 2014; Zettler et al. 2013).
Outlook
Regarding the intended, and globally observed persistence of present-day synthetic polymers, it appears quite obvious that biological degradation cannot counteract continued environmental pollution with plastic materials. The observed increase of the environmental load of plastic materials (Derraik 2002) testifies that chemical and photochemical degradation do not keep up with the rate of environmental input. Only a drastic reduction of careless and deliberate contamination, preferably to the unavoidable minimum, will lead to lowered environmental plastic loads. Recovery, such as advocated for instance by The Ocean Cleanup (http://www.theoceancleanup.com/), might be an option for severely contaminated or extraordinarily precious environments. Routine cleanup operations at beaches in touristic destinations are present examples for such practice (see, for instance, http://5gyres.org/how_to_get_involved/projects/plastic_beach_project/). Regarding the standing stock of plastic materials, there is however hope that ongoing weathering and fragmentation, slow biodegradation, spreading and relocation into less critical environments will eventually reduce the magnitude of local plastic contamination to subcritical levels. As to the biological degradation, we advocate and encourage the publication of negative results to complete the picture as a basis for decision making by environmental authorities. Our long-standing experience with biodegradation studies is telling us that published research is strongly biased to successes obtained under optimised conditions, thus painting an overly optimistic picture of limited transferability to real environments.
The replacement of biochemically inert polymers by more readily degradable ones might somewhat ease the tension. Many of the so-called bioplastics, like, e.g. polyhydroxy alkanoates or polylactide, have been designed with the specific aim of improved biodegradability. However, it should be considered that biodegradability is circumstantial as it requires the presence of active microbes and appropriate conditions. These effects are demonstrated by the observation that some nominally biodegradable plastics are degraded in the marine environment, while others are not (Thellen et al. 2008; Tsuji and Suzuyoshi 2002; Volova et al. 2011). This behaviour parallels that of many other chemicals which are biodegraded fairly well under more or less optimal laboratory conditions but are highly persistent under given environmental conditions. Although the demand for bioplastics is projected to increase substantially (European Bioplastics 2013), they will likely continue to play a niche role in the global plastic markets. Obstacles to a more widespread application of bioplastics are mainly of financial nature, because conventional plastics are still substantially cheaper to produce (OECD 2013). Additionally, there have also been concerns about the true impact of bioplastics as estimated by life cycle assessments (Tabone et al. 2010; Yates and Barlow 2013). Nevertheless, the improved environmental impact of many bioplastics at the end of their life cycle constitutes a compelling argument to accept some other trade-offs associated with bioplastics.
However, as long as conventional synthetic polymers dominate the plastic market, any reduction of the environmental load will have to rely on regulatory measures such as bans and monetary incentives, all in concert with educational efforts increasing the awareness of the ecological impact of plastic materials in the environment as already suggested by GESAMP (2015).
References
Acmite (2014) Global polyamide market. Acmite Market Intelligence, Ratingen
Agamuthu P, Faizura PN (2005) Biodegradability of degradable plastic waste. Waste Manag Res 23:95–100
Akutsu-Shigeno Y, Adachi Y, Yamada C, Toyoshima K, Nomura N, Uchiyama H, Nakajima-Kambe T (2006) Isolation of a bacterium that degrades urethane compounds and characterization of its urethane hydrolase. Appl Microbiol Biotechnol 70:422–429
Akutsu Y, Nakajima-Kambe T, Nomura N, Nakahara T (1998) Purification and properties of a polyester polyurethane-degrading enzyme from Comamonas acidovorans TB-35. Appl Environ Microbiol 64:62–67
Albertsson AC (1980) The shape of the biodegradation curve for low and high-density polyethenes in prolonged series of experiments. Eur Polym J 16:623–630
Albertsson AC, Andersson SO, Karlsson S (1987) The mechanism of biodegradation of polyethylene. Polym Degrad Stab 18:73–87
Ali MI, Ahmed S, Robson G, Javed I, Ali N, Atiq N, Hameed A (2014) Isolation and molecular characterization of polyvinyl chloride (PVC) plastic degrading fungal isolates. J Basic Microbiol 54:18–27
Andrady AL, Neal MA (2009) Applications and societal benefits of plastics. Philos Trans R Soc B 364:1977–1984
Arantes V, Jellison J, Goodell B (2012) Peculiarities of brown-rot fungi and biochemical Fenton reaction with regard to their potential as a model for bioprocessing biomass. Appl Microbiol Biotechnol 94:323–338
Arkatkar A, Arutchelvi J, Bhaduri S, Uppara PV, Doble M (2009) Degradation of unpretreated and thermally pretreated polypropylene by soil consortia. Int Biodeterior Biodegrad 63:106–111
Arkatkar A, Juwarkar AA, Bhaduri S, Uppara PV, Doble M (2010) Growth of Pseudomonas and Bacillus biofilms on pretreated polypropylene surface. Int Biodeterior Biodegrad 64:530–536
Artham T, Sudhakar M, Venkatesan R, Nair CM, Murty KVGK, Doble M (2009) Biofouling and stability of synthetic polymers in sea water. Int Biodeterior Biodegrad 63:884–890
Arthur C, Baker J, Bamford H (2009) Proc. International Research Workshop on the occurrence, effects and fate of microplastic marine debris, 9-11 September 2008. NOAA Technical Memorandum NOS-OR&R30, US NOAA Marine Debris Division, Silver Spring
Bakir A, Rowland SJ, Thompson RC (2014) Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ Pollut 185:16–23
Barnabé S, Beauchesne I, Cooper DG, Nicell JA (2008) Plasticizers and their degradation products in the process streams of a large urban physicochemical sewage treatment plant. Water Res 42:153–162
Barnes DKA, Galgani F, Thompson RC, Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Philos Trans R Soc B 364:1985–1998
Barth M, Oeser T, Wei R, Then J, Schmidt J, Zimmermann W (2015) Effect of hydrolysis products on the enzymatic degradation of polyethylene terephthalate nanoparticles by a polyester hydrolase from Thermobifida fusca. Biochem Eng J 93:222–228
Bejgarn S, MacLeod M, Bogdal C, Breitholtz M (2015) Toxicity of leachate from weathering plastics: an exploratory screening study with Nitocra spinipes. Chemosphere 132:114–119
Berdahl P, Akbari H, Levinson R, Miller WA (2008) Weathering of roofing materials—an overview. Constr Build Mater 22:423–433
Biffinger JC, Barlow DE, Pirlo RK, Babson DM, Fitzgerald LA, Zingarelli S, Nadeau LJ, Crookes-Goodson WJ, Russell JN (2014) A direct quantitative agar-plate based assay for analysis of Pseudomonas protegens Pf-5 degradation of polyurethane films. Int Biodeterior Biodegrad 95:311–319
Bremer WP (1982) Photodegradable polyethylene. Polym-Plast Technol Eng 18:137–148
Cacciari I, Quatrini P, Zirletta G, Mincione E, Vinciguerra V, Lupattelli P, Sermanni GG (1993) Isotactic polypropylene biodegradation by a microbial community—physicochemical characterization of metabolites produced. Appl Environ Microbiol 59:3695–3700
Cai S, Cai T, Liu S, Yang Q, He J, Chen L, Hu J (2014) Biodegradation of N-methylpyrrolidone by Paracoccus sp. NMD-4 and its degradation pathway. Int Biodeterior Biodegrad 93:70–77
Castaneda RA, Avlijas S, Simard MA, Ricciardi A (2014) Microplastic pollution in St. Lawrence River sediments. Can J Fish Aquat Sci 71:1767–1771
Chatterjee S, Karlovsky P (2010) Removal of the endocrine disrupter butyl benzyl phthalate from the environment. Appl Microbiol Biotechnol 87:61–73
Chen S, Su LQ, Billig S, Zimmermann W, Chen J, Wu J (2010) Biochemical characterization of the cutinases from Thermobifida fusca. J Mol Catal B Enzym 63:121–127
Cheremisinoff NP (1989) Handbook of polymer science and technology. M. Dekker, New York
Chua EM, Shimeta J, Nugegoda D, Morrison PD, Clarke BO (2014) Assimilation of polybrominated diphenyl ethers from microplastics by the marine amphipod, Allorchestes compressa. Environ Sci Technol 48:8127–8134
Cole M, Lindeque P, Halsband C, Galloway TS (2011) Microplastics as contaminants in the marine environment: a review. Mar Pollut Bull 62:2588–2597
Colton JB, Knapp FD, Burns BR (1974) Plastic particles in surface waters of the northwestern Atlantic. Science 185:491–497
Cooper DA, Corcoran PL (2010) Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai, Hawaii. Mar Pollut Bull 60:650–654
Corcoran PL, Biesinger MC, Grifi M (2009) Plastics and beaches: a degrading relationship. Mar Pollut Bull 58:80–84
Costa P, Ribeiro S, Botelho G, Machado AV, Lanceros Mendez S (2015) Effect of butadiene/styrene ratio, block structure and carbon nanotube content on the mechanical and electrical properties of thermoplastic elastomers after UV ageing. Polym Test 42:225–233
Cozar A, Echevarria F, Gonzalez-Gordillo JI, Irigoien X, Ubeda B, Hernandez-Leon S, Palma AT, Navarro S, Garcia-de-Lomas J, Ruiz A, Fernandez-de-Puelles ML, Duarte CM (2014) Plastic debris in the open ocean. Proc Natl Acad Sci U S A 111:10239–10210244
Crabbe JR, Campbell JR, Thompson L, Walz SL, Schultz WW (1994) Biodegradation of a colloidal ester-based polyurethane by soil fungi. Int Biodeterior Biodegrad 33:103–113
Cregut M, Bedas M, Durand MJ, Thouand G (2013) New insights into polyurethane biodegradation and realistic prospects for the development of a sustainable waste recycling process. Biotechnol Adv 31:1634–1647
da Luz JMR, Paes SA, Nunes MD, da Silva MDS, Kasuya MCM (2013) Degradation of oxo-biodegradable plastic by Pleurotus ostreatus. PLoS One 8:e69386. doi:10.1371/journal.pone.0069386
Daniel JY, John R, Fachuang L, Kenneth EH (2008) Evidence for cleavage of lignin by a brown rot basidiomycete. Environ Microbiol 10:1844–1849
Darby RT, Kaplan AM (1968) Fungal susceptibility of polyurethanes. Appl Microbiol 16:900–905
David C, Trojan M, Daro A, Demarteau W (1992) Photodegradation of polyethylene—comparison of various photoinitiators in natural weathering conditions. Polym Degrad Stab 37:233–245
de Moura Carrara SMC, Morita DM, Boscov MEG (2011) Biodegradation of di(2-ethylhexyl)phthalate in a typical tropical soil. J Hazard Mater 197:40–48
Deguchi T, Kakezawa M, Nishida T (1997) Nylon biodegradation by lignin-degrading fungi. Appl Environ Microbiol 63:329–331
Deguchi T, Kitaoka Y, Kakezawa M, Nishida T (1998) Purification and characterization of a nylon-degrading enzyme. Appl Environ Microbiol 64:1366–1371
Derraik JGB (2002) The pollution of the marine environment by plastic debris: a review. Mar Pollut Bull 44:842–852
Dick JS (2014) Rubber technology: compounding and testing for performance, 2nd edn. Hanser Publishers, Cincinnati
Elder T (2013) Bond dissociation enthalpies of a dibenzodioxocin lignin model compound. Energy Fuel 27:4785–4790
Elder T (2014) Bond dissociation enthalpies of a pinoresinol lignin model compound. Energy Fuel 28:1175–1182
EPA (2014) Municipal solid waste generation, recycling, and disposal in the United States: facts and figures for 2012. US Environmental Protection Agency, Washington
Eriksen M, Lebreton LC, Carson HS, Thiel M, Moore CJ, Borerro JC, Galgani F, Ryan PG, Reisser J (2014) Plastic pollution in the world’s oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS One 9:e111913. doi:10.1371/journal.pone.0111913
Erythropel HC, Maric M, Nicell JA, Leask RL, Yargeau V (2014) Leaching of the plasticizer di(2-ethylhexyl)phthalate (DEHP) from plastic containers and the question of human exposure. Appl Microbiol Biotechnol 98:9967–9981
EU (2014) Use of plastic bags: agreement on phasing down. Council of the European Union, Brussels
Eubeler JP, Bernhard M, Zok S, Knepper TP (2009) Environmental biodegradation of synthetic polymers I. Test methodologies and procedures. TrAC Trend Anal Chem 28:1057–1072
European Bioplastics (2013) Bioplastics—facts and figures. European Bioplastics, Berlin
Filip Z (1978) Decompostion of polyurethane in a garbage landfill leakage water and by soil-microorganisms. Eur J Appl Microbiol 5:225–231
Fontanella S, Bonhomme S, Brusson JM, Pitteri S, Samuel G, Pichon G, Lacoste J, Fromageot D, Lemaire J, Delort AM (2013) Comparison of biodegradability of various polypropylene films containing pro-oxidant additives based on Mn, Mn/Fe or Co. Polym Degrad Stab 98:875–884
Friedrich J, Zalar P, Mohorcic M, Klun U, Krzan A (2007) Ability of fungi to degrade synthetic polymer nylon-6. Chemosphere 67:2089–2095
Fujisawa M, Hirai H, Nishida T (2001) Degradation of polyethylene and nylon-66 by the laccase-mediator system. J Polym Environ 9:103–108
Gan Q, Allen SJ, Taylor G (2003) Kinetic dynamics in heterogeneous enzymatic hydrolysis of cellulose: an overview, an experimental study and mathematical modelling. Process Biochem 38:1003–1018
Gautam R, Bassi AS, Yanful EK (2007) A review of biodegradation of synthetic plastic and foams. Appl Biochem Biotechnol 141:85–108
GESAMP (2015) Sources, fate and effects of microplastics in the marine environment: a global assessment. Rep. Stud. GESAMP no. 90. International Maritime Organization, London
Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G (2010) Laccases: a never-ending story. Cell Mol Life Sci 67:369–385
Gilan I, Hadar Y, Sivan A (2004) Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber. Appl Microbiol Biotechnol 65:97–104
Goldberg ED (1997) Plasticizing the seafloor: an overview. Environ Technol 18:195–201
Gregory MR, Ryan PG (1997) Pelagic plastics and other seaborne persistent synthetic debris: a review of southern hemisphere perspectives. In: Coe J, Rogers D (eds) Marine debris. Springer Series on Environmental Management. Springer, New York, pp 49–66
Guo Q, Zhang J, Wan R, Xie S (2014) Impacts of carbon sources on simazine biodegradation by Arthrobacter strain SD3-25 in liquid culture and soil microcosm. Int Biodeterior Biodegrad 89:1–6
Gusse AC, Miller PD, Volk TJ (2006) White-rot fungi demonstrate first biodegradation of phenolic resin. Environ Sci Technol 40:4196–4199
Gutierrez A, Rencoret J, Cadena EM, Rico A, Barth D, del Rio JC, Martinez AT (2012) Demonstration of laccase-based removal of lignin from wood and non-wood plant feedstocks. Bioresour Technol 119:114–122
Hadad D, Geresh S, Sivan A (2005) Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis. J Appl Microbiol 98:1093–1100
Harms H, Schlosser D, Wick LY (2011) Untapped potential: exploiting fungi in bioremediation of hazardous chemicals. Nat Rev Microbiol 9:177–192
Harrison JP, Schratzberger M, Sapp M, Osborn AM (2014) Rapid bacterial colonization of low-density polyethylene microplastics in coastal sediment microcosms. BMC Microbiol 14:232
Hashimoto K, Hamano T, Okada M (1994) Degradation of several polyamides in soils. J Appl Polym Sci 54:1579–1583
Hofrichter M (2002) Review: lignin conversion by manganese peroxidase (MnP). Enzym Microb Technol 30:454–466
Hofrichter M, Ullrich R, Pecyna MJ, Liers C, Lundell T (2010) New and classic families of secreted fungal heme peroxidases. Appl Microbiol Biotechnol 87:871–897
Horn SJ, Vaaje-Kolstad G, Westereng B, Eijsink VGH (2012) Novel enzymes for the degradation of cellulose. Biotechnol Biofuels 5:45. doi:10.1186/1754-6834-5-45
Howard GT (2002) Biodegradation of polyurethane: a review. Int Biodeterior Biodegrad 49:245–252
Huang MH, Shih YP, Liu SM (2002) Biodegradation of polyvinyl alcohol by Phanerochaete chrysosporium after pretreatment with Fenton’s reagent. J Environ Sci Health A 37:29–41
Iiyoshi Y, Tsutsumi Y, Nishida T (1998) Polyethylene degradation by lignin-degrading fungi and manganese peroxidase. J Wood Sci 44:222–229
Imhof HK, Ivleva NP, Schmid J, Niessner R, Laforsch C (2013) Contamination of beach sediments of a subalpine lake with microplastic particles. Curr Biol 23:R867–R868
Imhof HK, Schmid J, Niessner R, Ivleva NP, Laforsch C (2012) A novel, highly efficient method for the separation and quantification of plastic particles in sediments of aquatic environments. Limnol Oceanogr Methods 10:524–537
Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL (2015) Plastic waste inputs from land into the ocean. Science 347:768–771
Jeon HJ, Kim MN (2013) Isolation of a thermophilic bacterium capable of low-molecular-weight polyethylene degradation. Biodegradation 24:89–98
Jeyakumar D, Chirsteen J, Doble M (2013) Synergistic effects of pretreatment and blending on fungi mediated biodegradation of polypropylenes. Bioresour Technol 148:78–85
Kann B, Offerhaus HL, Windbergs M, Otto C (2015) Raman microscopy for cellular investigations—from single cell imaging to drug carrier uptake visualization. Adv Drug Deliv Rev. doi:10.1016/j.addr.2015.02.006
Kaplan DL, Hartenstein R, Sutter J (1979) Biodegradation of polystyrene, poly(methyl methacrylate), and phenol formaldehyde. Appl Environ Microbiol 38:551–553
Kastner J, Cooper DG, Marić M, Dodd P, Yargeau V (2012) Aqueous leaching of di-2-ethylhexyl phthalate and “green” plasticizers from poly(vinyl chloride). Sci Total Environ 432:357–364
Kawai F, Oda M, Tamashiro T, Waku T, Tanaka N, Yamamoto M, Mizushima H, Miyakawa T, Tanokura M (2014) A novel Ca2+-activated, thermostabilized polyesterase capable of hydrolyzing polyethylene terephthalate from Saccharomonospora viridis AHK190. Appl Microbiol Biotechnol 98:10053–10064
Kawasaki N, Nakayama A, Yamano N, Takeda S, Kawata Y, Yamamoto N, Aiba S (2005) Synthesis, thermal and mechanical properties and biodegradation of branched polyamide 4. Polymer 46:9987–9993
Kerem Z, Jensen KA, Hammel KE (1999) Biodegradative mechanism of the brown rot basidiomycete Gloeophyllum trabeum: evidence for an extracellular hydroquinone-driven fenton reaction. FEBS Lett 446:49–54
Kermanshahi pour A, Cooper DG, Mamer OA, Maric M, Nicell JA (2009) Mechanisms of biodegradation of dibenzoate plasticizers. Chemosphere 77:258–263
Khalil A, Domeizel M, Prudent P (2008) Monitoring of green waste composting process based on redox potential. Bioresour Technol 99:6037–6045
Kim S, Chmely SC, Nimos MR, Bomble YJ, Foust TD, Paton RS, Beckham GT (2011) Computational study of bond dissociation enthalpies for a large range of native and modified lignins. J Phys Chem Lett 2:2846–2852
Klun U, Friedrich J, Krzan A (2003) Polyamide-6 fibre degradation by a lignolytic fungus. Polym Degrad Stab 79:99–104
Knyazev VD (2007) Effects of chain length on the rates of C–C bond dissociation in linear alkanes and polyethylene. J Phys Chem A 111:3875–3883
Koutny M, Sancelme M, Dabin C, Pichon N, Delort A-M, Lemaire J (2006) Acquired biodegradability of polyethylenes containing pro-oxidant additives. Polym Degrad Stab 91:1495–1503
Kramer C, Kreisel G, Fahr K, Kassbohrer J, Schlosser D (2004) Degradation of 2-fluorophenol by the brown-rot fungus Gloeophyllum striatum: evidence for the involvement of extracellular Fenton chemistry. Appl Microbiol Biotechnol 64:387–395
Krueger MC, Hofmann U, Moeder M, Schlosser D (2015) Potential of wood-rotting fungi to attack polystyrene sulfonate and its depolymerisation by Gloeophyllum trabeum via hydroquinone-driven Fenton chemistry. PLoS One 10:e0131773. doi:10.1371/journal.pone.0131773
Kümmerer K (2011) Emerging contaminants. In: Wilderer P (ed) Treatise on water science. Elsevier, Oxford, pp. 69–87
Lapworth DJ, Baran N, Stuart ME, Ward RS (2012) Emerging organic contaminants in groundwater: a review of sources, fate and occurrence. Environ Pollut 163:287–303
Larking DM, Crawford RJ, Christie GBY, Lonergan GT (1999) Enhanced degradation of polyvinyl alcohol by Pycnoporus cinnabarinus after pretreatment with Fenton’s reagent. Appl Environ Microbiol 65:1798–1800
Law KL, Moret-Ferguson SE, Goodwin DS, Zettler ER, Deforce E, Kukulka T, Proskurowski G (2014) Distribution of surface plastic debris in the eastern pacific ocean from an 11-year data set. Environ Sci Technol 48:4732–4734738
Loredo-Trevino A, Gutierrez-Sanchez G, Rodriguez-Herrera R, Aguilar CN (2012) Microbial enzymes involved in polyurethane biodegradation: a review. J Polym Environ 20:258–265
Lundell TK, Makela MR, Hilden K (2010) Lignin-modifying enzymes in filamentous basidiomycetes—ecological, functional and phylogenetic review. J Basic Microbiol 50:5–20
Luo Y-R (2007) Comprehensive handbook of chemical bond energies. CRC Press, Boca Raton, FL
Luo ZH, Pang KL, Wu YR, Gu JD, Chow RK, Vrijmoed LL (2012) Degradation of phthalate esters by Fusarium sp. DMT-5-3 and Trichosporon sp. DMI-5-1 isolated from mangrove sediments. Prog Mol Subcell Biol 53:299–328
Marco-Urrea E, Pérez-Trujillo M, Caminal G, Vicent T (2009) Dechlorination of 1,2,3- and 1,2,4-trichlorobenzene by the white-rot fungus Trametes versicolor. J Hazard Mater 166:1141–1147
Marten E, Muller RJ, Deckwer WD (2003) Studies on the enzymatic hydrolysis of polyesters I. Low molecular mass model esters and aliphatic polyesters. Polym Degrad Stab 80:485–501
Marten E, Muller RJ, Deckwer WD (2005) Studies on the enzymatic hydrolysis of polyesters. II. Aliphatic-aromatic copolyesters. Polym Degrad Stab 88:371–381
Martinez AT, Speranza M, Ruiz-Duenas FJ, Ferreira P, Camarero S, Guillen F, Martinez MJ, Gutierrez A, del Rio JC (2005) Biodegradation of lignocellulosics: microbial chemical, and enzymatic aspects of the fungal attack of lignin. Int Microbiol 8:195–204
McCormick A, Hoellein TJ, Mason SA, Schluep J, Kelly JJ (2014) Microplastic is an abundant and distinct microbial habitat in an urban river. Environ Sci Technol 48:11863–11871
McKeen LW (2013) 2 - introduction to the weathering of plastics. In: McKeen LW (ed) The effect of UV light and weather on plastics and elastomers (3rd edition). William Andrew Publishing, Boston, pp 17–41
Mikulasova M, Kosikova B, Alexy P, Kacik F, Urgelova E (2001) Effect of blending lignin biopolymer on the biodegradability of polyolefin plastics. World J Microbiol Biotechnol 17:601–607
Milstein O, Gersonde R, Huttermann A, Chen MJ, Meister JJ (1992) Fungal biodegradation of lignopolystyrene graft-copolymers. Appl Environ Microbiol 58:3225–3232
Mor R, Sivan A (2008) Biofilm formation and partial biodegradation of polystyrene by the actinomycete Rhodococcus ruber. Biodegradation 19:851–858
Muller RJ, Kleeberg I, Deckwer WD (2001) Biodegradation of polyesters containing aromatic constituents. J Biotechnol 86:87–95
Muller RJ, Schrader H, Profe J, Dresler K, Deckwer WD (2005) Enzymatic degradation of poly(ethylene terephthalate): rapid hydrolyse using a hydrolase from T-fusca. Macromol Rapid Commun 26:1400–1405
Murray KE, Thomas SM, Bodour AA (2010) Prioritizing research for trace pollutants and emerging contaminants in the freshwater environment. Environ Pollut 158:3462–3471
Nakajima-Kambe T, Onuma F, Kimpara N, Nakahara T (1995) Isolation and characterization of a bacterium which utilizes polyester polyurethane as a sole carbon and nitrogen-source. FEMS Microbiol Lett 129:39–42
Nakamiya K, Sakasita G, Ooi T, Kinoshita S (1997) Enzymatic degradation of polystyrene by hydroquinone peroxidase of Azotobacter beijerinckii HM121. J Ferment Bioeng 84:480–482
Negoro S (2000) Biodegradation of nylon oligomers. Appl Microbiol Biotechnol 54:461–466
Negoro S, Shibata N, Tanaka Y, Yasuhira K, Shibata H, Hashimoto H, Lee YH, Oshima S, Santa R, Oshima S, Mochiji K, Goto Y, Ikegami T, Nagai K, Kato D, Takeo M, Higuchi Y (2012) Three-dimensional structure of nylon hydrolase and mechanism of nylon-6 hydrolysis. J Biol Chem 287:5079–5090
Ng KL, Obbard JP (2006) Prevalence of microplastics in Singapore’s coastal marine environment. Mar Pollut Bull 52:761–767
O’Brine T, Thompson RC (2010) Degradation of plastic carrier bags in the marine environment. Mar Pollut Bull 60:2279–2283
Oberbeckmann S, Loeder MGJ, Gerdts G, Osborn AM (2014) Spatial and seasonal variation in diversity and structure of microbial biofilms on marine plastics in Northern European waters. FEMS Microbiol Ecol 90:478–492
OECD (2013) Policies for bioplastics in the context of a bioeconomy. OECD Science, Technology and Industry Policy Papers, no. 10. OECD Publishing, Paris
Ogawa G, Ishida M, Urano N (2009) Isolation and identification of dibutyl phthalate-degrading bacteria from hydrospheres in Tokyo. J Gen Appl Microbiol 55:261–265
Ohki T, Mizuno N, Shibata N, Takeo M, Negoro S, Higuchi Y (2005) Crystallization and X-ray diffraction analysis of 6-aminohexanoate-dimer hydrolase from Arthrobacter sp KI72. Acta Crystallogr Sect F: Struct Biol Cryst Commun 61:928–930
Ojeda TFM, Dalmolin E, Forte MMC, Jacques RJS, Bento FM, Camargo FAO (2009) Abiotic and biotic degradation of oxo-biodegradable polyethylenes. Polym Degrad Stab 94:965–970
Otake Y, Kobayashi T, Asabe H, Murakami N, Ono K (1995) Biodegradation of low-density polyethylene, polystyrene, polyvinyl-chloride, and urea-formaldehyde resin buried under soil for over 32 years. J Appl Polym Sci 56:1789–1796
Pastorelli G, Cucci C, Garcia O, Piantanida G, Elnaggar A, Cassar M, Strlič M (2014) Environmentally induced colour change during natural degradation of selected polymers. Polym Degrad Stab 107:198–209
Peng YH, Shih YH, Lai YC, Liu YZ, Liu YT, Lin NC (2014) Degradation of polyurethane by bacterium isolated from soil and assessment of polyurethanolytic activity of a Pseudomonas putida strain. Environ Sci Pollut Res 21:9529–9537
PlasticsEurope (2015a) Business data and charts 2013/2014. PlasticsEurope, Brussels
PlasticsEurope (2015b) Plastics - the facts 2014/2015. PlasticsEurope, Brussels
Pradeep S, Faseela P, Josh MK, Balachandran S, Devi RS, Benjamin S (2013) Fungal biodegradation of phthalate plasticizer in situ. Biodegradation 24:257–267
Pradeep S, Sarath Josh MK, Binod P, Sudha Devi R, Balachandran S, Anderson RC, Benjamin S (2015) Achromobacter denitrificans strain SP1 efficiently remediates di(2-ethylhexyl)phthalate. Ecotoxicol Environ Saf 112:114–121
Prahl FG, Ertel JR, Goni MA, Sparrow MA, Eversmeyer B (1994) Terrestrial organic carbon contributions to sediments on the Washington margin. Geochim Cosmochim Acta 58:3035–3048
Purdy RE, Kolattuk PE (1973) Depolymerization of a hydroxy fatty-acid biopolymer, cutin, by an extracellular enzyme from Fusarium solani f. pisi—isolation and some properties of enzyme. Arch Biochem Biophys 159:61–69
Pushpadass HA, Weber RW, Dumais JJ, Hanna MA (2010) Biodegradation characteristics of starch-polystyrene loose-fill foams in a composting medium. Bioresour Technol 101:7258–7264
Restrepo-Florez JM, Bassi A, Thompson MR (2014) Microbial degradation and deterioration of polyethylene—a review. Int Biodeterior Biodegrad 88:83–90
Rochman CM, Kurobe T, Flores I, Teh SJ (2014) Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci Total Environ 493:656–661
Ronkvist AM, Xie WC, Lu WH, Gross RA (2009) Cutinase-catalyzed hydrolysis of poly(ethylene terephthalate). Macromolecules 42:5128–5138
Roth C, Wei R, Oeser T, Then J, Follner C, Zimmermann W, Strater N (2014) Structural and functional studies on a thermostable polyethylene terephthalate degrading hydrolase from Thermobifida fusca. Appl Microbiol Biotechnol 98:7815–7823
Roy PK, Hakkarainen M, Varrna IK, Albertsson AC (2011) Degradable polyethylene: fantasy or reality. Environ Sci Technol 45:4217–4227
Ruiz-Duenas FJ, Martinez AT (2009) Microbial degradation of lignin: how a bulky recalcitrant polymer is efficiently recycled in nature and how we can take advantage of this. Microb Biotechnol 2:164–177
Russell JR, Huang J, Anand P, Kucera K, Sandoval AG, Dantzler KW, Hickman D, Jee J, Kimovec FM, Koppstein D, Marks DH, Mittermiller PA, Nunez SJ, Santiago M, Townes MA, Vishnevetsky M, Williams NE, Vargas MPN, Boulanger LA, Bascom-Slack C, Strobel SA (2011) Biodegradation of polyester polyurethane by endophytic fungi. Appl Environ Microbiol 77:6076–6084
Ryan PG, Moloney CL (1993) Marine litter keeps increasing. Nature 361:23–23
Santana VT, Goncalves SPC, Agnelli JAM, Martins-Franchetti SM (2012) Biodegradation of a polylactic acid/polyvinyl chloride blend in soil. J Appl Polym Sci 125:536–540
Santo M, Weitsman R, Sivan A (2013) The role of the copper-binding enzyme—laccase—in the biodegradation of polyethylene by the actinomycete Rhodococcus ruber. Int Biodeterior Biodegrad 84:204–210
Schlosser D, Fahr K, Karl W, Wetzstein HG (2000) Hydroxylated metabolites of 2,4-dichlorophenol imply a Fenton-type reaction in Gloeophyllum striatum. Appl Environ Microbiol 66:2479–2483
Setala O, Fleming-Lehtinen V, Lehtiniemi M (2014) Ingestion and transfer of microplastics in the planktonic food web. Environ Pollut 185:77–83
Shah AA, Hasan F, Hameed A, Ahmed S (2008) Biological degradation of plastics: a comprehensive review. Biotechnol Adv 26:246–265
Sielicki M, Focht DD, Martin JP (1978) Microbial degradation of [C-14]polystyrene and 1,3-diphenylbutane. Can J Microbiol 24:798–803
Silva CP, Otero M, Esteves V (2012) Processes for the elimination of estrogenic steroid hormones from water: a review. Environ Pollut 165:38–58
Sivan A (2011) New perspectives in plastic biodegradation. Curr Opin Biotechnol 22:422–426
Solé M, Schlosser D (2014) Xenobiotics from human impacts. In: Krauss G-J, Niess DH (eds) Ecological biochemistry: environmental and interspecies interactions. Wiley-VCH, Weinheim, pp. 259–275
Song Y, Andrady AL (1991) Fouling of floating plastic debris under Biscayne Bay exposure conditions. Mar Pollut Bull 22:608––6613
Stahl JD, Cameron MD, Haselbach J, Aust SD (2000) Biodegradation of superabsorbent polymers in soil. Environ Sci Pollut Res 7:83––888
Stloukal P, Verney V, Commereuc S, Rychly J, Matisova-Rychlá L, Pis V, Koutny M (2012) Assessment of the interrelation between photooxidation and biodegradation of selected polyesters after artificial weathering. Chemosphere 88:1214–1219
Storz H, Vorlop KD (2013) Bio-based plastics: status, challenges and trends. Landbauforschung-Ger 63:321–332
Suzuki MR, Hunt CG, Houtman CJ, Dalebroux ZD, Hammel KE (2006) Fungal hydroquinones contribute to brown rot of wood. Environ Microbiol 8:2214–2223
Tabone MD, Cregg JJ, Beckman EJ, Landis AE (2010) Sustainability metrics: life cycle assessment and green design in polymers. Environ Sci Technol 44:8264–8269
Tachibana K, Urano Y, Numata K (2013) Biodegradability of nylon 4 film in a marine environment. Polym Degrad Stab 98:1847–1851
Thellen C, Coyne M, Froio D, Auerbach M, Wirsen C, Ratto JA (2008) A processing, characterization and marine biodegradation study of melt-extruded polyhydroxyalkanoate (PHA) films. J Polym Environ 16:1–11
Thompson R, Moore C, Andrady A, Gregory M, Takada H, Weisberg S (2005) New directions in plastic debris. Science 310:1117–1117
Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AWG, McGonigle D, Russell AE (2004) Lost at sea: where is all the plastic? Science 304:838–838
Tokiwa Y, Calabia BP, Ugwu CU, Aiba S (2009) Biodegradability of plastics. Int J Mol Sci 10:3722–3742
Tosin M, Weber M, Siotto M, Lott C, Degli-Innocenti F (2012) Laboratory test methods to determine the degradation of plastics in marine environmental conditions. Front Microbiol 3:225. doi:10.3389/fmicb.2012.00225
Tribedi P, Sil AK (2013) Low-density polyethylene degradation by Pseudomonas sp AKS2 biofilm. Environ Sci Pollut Res 20:4146–4153
Tsuji H, Suzuyoshi K (2002) Environmental degradation of biodegradable polyesters 1. Poly(epsilon-caprolactone), poly[(R)-3-hydroxybutyrate], and poly(l-lactide) films in controlled static seawater. Polym Degrad Stab 75:347–355
Valko L, Tvarovska I, Kellö V (1972) Irregular structures in poly(vinyl chloride). I. Saturated structures. Chem Pap 26:3–17
Van Cauwenberghe L, Vanreusel A, Mees J, Janssen CR (2013) Microplastic pollution in deep-sea sediments. Environ Pollut 182:495–499
Vert M, Doi Y, Hellwich KH, Hess M, Hodge P, Kubisa P, Rinaudo M, Schue F (2012) Terminology for biorelated polymers and applications (IUPAC recommendations 2012). Pure Appl Chem 84:377–408
Volke-Sepulveda T, Saucedo-Castaneda G, Gutierrez-Rojas M, Manzur A, Favela-Torres E (2002) Thermally treated low density polyethylene biodegradation by Penicillium pinophilum and Aspergillus niger. J Appl Polym Sci 83:305–314
Volova TG, Boyandin AN, Vasil’ev AD, Karpov VA, Kozhevnikov IV, Prudnikova SV, Rudnev VP, Xuan BB, Dung VVT, Gitel’zon II (2011) Biodegradation of polyhydroxyalkanoates (PHAs) in the South China Sea and identification of PHA-degrading bacteria. Microbiology 80:252–260
Wang J, Zhu L, Liu A, Ma T, Wang Q, Xie H, Wang J, Jiang T, Zhao R (2011) Isolation and characterization of an Arthrobacter sp. strain HB-5 that transforms atrazine. Environ Geochem Health 33:259–266
Wang Y, Fan Y, Gu J-D (2004) Dimethyl phthalate ester degradation by two planktonic and immobilized bacterial consortia. Int Biodeterior Biodegrad 53:93–101
Webb JS, Nixon M, Eastwood IM, Greenhalgh M, Robson GD, Handley PS (2000) Fungal colonization and biodeterioration of plasticized polyvinyl chloride. Appl Environ Microbiol 66:3194–3200
Woodall LC, Sanchez-Vidal A, Canals M, Paterson GLJ, Coppock R, Sleight V, Calafat A, Rogers AD, Narayanaswamy BE, Thompson RC (2014) The deep sea is a major sink for microplastic debris. R Soc Open Sci 1:140317. doi:10.1098/rsos.140317
Yamano N, Nakayama A, Kawasaki N, Yamamoto N, Aiba S (2008) Mechanism and characterization of polyamide 4 degradation by Pseudomonas sp. J Polym Environ 16:141–146
Yang J, Yang Y, Wu WM, Zhao J, Jiang L (2014) Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ Sci Technol 48:13776–13784
Yasuhira K, Uedo Y, Takeo M, Kato D, Negoro S (2007) Genetic organization of nylon-oligomer-degrading enzymes from alkalophilic bacterium, Agromyces sp KY5R. J Biosci Bioeng 104:521–524
Yates MR, Barlow CY (2013) Life cycle assessments of biodegradable, commercial biopolymers—a critical review. Resour Conserv Recycl 78:54–66
Yelle DJ, Ralph J, Lu FC, Hammel KE (2008) Evidence for cleavage of lignin by a brown rot basidiomycete. Environ Microbiol 10:1844–1849
Yelle DJ, Wei DS, Ralph J, Hammel KE (2011) Multidimensional NMR analysis reveals truncated lignin structures in wood decayed by the brown rot basidiomycete Postia placenta. Environ Microbiol 13:1091–1100
Yoon GY, Jeon HJ, Kom MN (2012) Biodegradation of polyethylene by a soil bacterium and alkB cloned recombinant cell. J Biorem Biodegrad 3:145. doi:10.4172/2155-6199.1000145
Younker JM, Beste A, Buchanan AC (2012) Computational study of bond dissociation enthalpies for lignin model compounds: beta-5 arylcoumaran. Chem Phys Lett 545:100–106
Zettler ER, Mincer TJ, Amaral-Zettler LA (2013) Life in the “plastisphere”: microbial communities on plastic marine debris. Environ Sci Technol 47:7137–7146
Zolfaghari M, Drogui P, Seyhi B, Brar SK, Buelna G, Dubé R (2014) Occurrence, fate and effects of Di (2-ethylhexyl) phthalate in wastewater treatment plants: a review. Environ Pollut 194:281–293
Acknowledgements
We thank PlasticsEurope for kindly providing the ‘Business Data and Charts 2013/2014’. Funding from the EU project BIOCLEAN, EC Grant Agreement no. 312100, is gratefully acknowledged. This work was further supported by the Helmholtz Association and contributes to the integrated project ‘Controlling Chemicals’ Fate’ in the Chemicals in the Environment (CITE) research programme conducted at the Helmholtz Centre for Environmental Research-UFZ.
Conflict of interest
The authors declare that they have no competing interests.
Compliance with ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krueger, M.C., Harms, H. & Schlosser, D. Prospects for microbiological solutions to environmental pollution with plastics. Appl Microbiol Biotechnol 99, 8857–8874 (2015). https://doi.org/10.1007/s00253-015-6879-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6879-4