Abstract
Postoperative pancreatic fistula (POPF) is a major source of morbidity following pancreatic resection. Surgically placed drains under suction or gravity are routinely used to help mitigate the complications associated with POPF. Controversy exists as to whether one of these drain management strategies is superior. The objective was to identify and compare the incidence of POPF, adverse events, and resource utilization associated with passive gravity (PG) versus active suction (AS) drainage following pancreatic resection. MEDLINE, EMBASE, CINAHL, and Cochrane Library databases were searched from inception to May 18, 2020. Outcomes of interest included POPF, post-pancreatectomy hemorrhage (PPH), surgical site infection (SSI), other major morbidity, and resource utilization. Descriptive qualitative and pooled quantitative meta-analyses were performed. One randomized control trial and five cohort studies involving 10 663 patients were included. Meta-analysis found no difference in the odds of developing POPF between AS and PG (p = 0.78). There were no differences in other endpoints including PPH (p = 0.58), SSI (wound p = 0.21, organ space p = 0.05), major morbidity (p = 0.71), or resource utilization (p = 0.72). The risk of POPF or other adverse outcomes is not impacted by drain management following pancreatic resection. Based on current evidence, a suggestion cannot be made to support the use of one drain over another at this time. There is a trend toward increased intra-abdominal wound infections with AS drains (p = 0.05) that merits further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic resection is commonly performed for diseases of the pancreas, duodenum, and distal bile duct [1, 2]. Despite advancements in surgical and perioperative management, morbidity following pancreatic surgery remains high, approaching 50% [2, 3]. The most common cause of major morbidity is the development of a postoperative pancreatic fistula (POPF), with reported incidence between 10 and 35% [3,4,5]. The resulting leakage of pancreatic effluent can lead to a cascade of complications including surgical site infections (SSI), hemorrhage, end organ failure, death and in the case of oncologic indications, a delay to adjuvant therapy [6,7,8,9,10].
The 2016 classification system for POPF by the International Study Group for Pancreatic Fistula (ISGPF) defines biochemical leak (Grade A) and clinically relevant POPF (Grade B,C). [4, 5] Patients developing POPF are more likely to undergo additional procedures, have increased length of stay (LOS) and increased likelihood of hospital re-admissions [1, 5]. Furthermore, the healthcare costs of POPF are an estimated 1.5- 2.0 times that of patients without POPF [11,12,13]. Reducing the development and mitigating the severity of POPF are necessary given the associated clinical and economic burden.
Several strategies have been investigated to decrease POPF risk; however, incidence has remained relatively stable [5,6,7]. The effect of different intra-abdominal drainage systems on the development of POPF has yet to be robustly investigated. Intra-abdominal drains are commonly placed close to the pancreaticojejunal anastomosis following pancreaticoduodenectomy (PD) and the pancreatic transection line following distal pancreatectomy (DP). These drains can be managed with active suction (AS) or passive gravity (PG). The former is attached to a reservoir generating negative pressure while the latter is attached to a reservoir that acts as a vessel for effluent to flow by gravity. It has been postulated that an AS system promotes improved drainage and collapse of the surgical dead space, thereby potentially decreasing the severity of POPF, were it to occur [8, 14]. However, AS systems generate pressure measured at − 150 mm Hg when the bulb is fully decompressed and up to − 200 mm Hg when the drain is stripped [8, 14]. As such, it is also theorized that this pressure gradient could promote the development of a POPF. [8, 14] At this time, the utilization of one system over another largely depends on surgical dogma or institutional practice.
Considering both AS and PG are cost-effective, simple interventions, one would rapidly become standard of care over the other, should a true difference exist. Therefore, the objective of this systematic review and meta-analysis was to synthesize existing evidence comparing PG and AS drainage systems in patients undergoing pancreatic resection.
Materials and methods
This review was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) and Assessing the Methodological Quality of Systematic Reviews (AMSTAR) Guidelines (Appendix A) [15]. It was prospectively registered on Prospero on February 26, 2019, (CRD42019123647) and the protocol was published in BMJ Open [16].
Literature search strategy
A reference librarian (AD) developed database-specific search strategies (Appendix B). These were used to conduct a systematic literature search of the following databases, from inception to May 18, 2020: MEDLINE (PubMed, PubMed in Process and Ovid), EMBASE, CINAHL, and Cochrane Central Registry of Controlled Trials. A manual search of references in primary studies, relevant reviews, and conference proceedings was conducted. Cited references were also searched using Web of Science.
Identified articles were exported to a citation manager (Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia [17]). Title and abstract screening were conducted independently and in duplicate. Three independent reviewers (LP, LB, HS) subsequently undertook a full-text review of eligible articles. Disagreements were resolved by consensus.
Eligibility criteria
Published studies comparing the incidence of postoperative adverse events in adult patients who had a drain placed to PG or AS at the time of an elective pancreatic resection were considered for inclusion. Pancreatic resections included patients undergoing PD, DP, central pancreatectomy, and pancreatic enucleation. A PG drainage system was defined as a drain that maintains a pathway for fluid to flow from the surgical site by gravity, connected to a reservoir maintained at atmospheric pressure. An AS surgical drainage system was defined as a drain connected to a collapsible reservoir, which generates a negative pressure relative to atmospheric pressure. Studies were excluded if they included patients who underwent pancreatic necrosectomy or total pancreatectomy. Studies involving external pancreatic stents, drains managed with continuous irrigation or open drains not connected to a reservoir were also excluded.
Outcomes of interest
The primary outcome of interest was development of POPF (Grade B and C), as defined by the 2016 ISGPF criteria [4, 5].
Secondary outcomes included incidence of biochemical pancreatic leak, postoperative adverse events, resource utilization, and quality of life (QOL). Postoperative adverse events of interest included overall and major morbidity (as defined by the Clavien–Dindo Grade 3 and above)[18], SSI (wound or intra-abdominal infections), delayed gastric emptying (DGE), postoperative pancreatitis, post-pancreatectomy hemorrhage (PPH), percutaneous drain insertion, re-operation, and death. Resource utilization was to be assessed by comparing: LOS, re-intervention, and re-admission to hospital. Surrogate QOL measures were to be assessed by the number of days a drain was in situ and the presence of a drain at discharge.
Data extraction
Data extraction for included studies were conducted independently, in triplicate, by three reviewers (LP, LB, HS) using a standardized electronic data extraction form. Disagreements were resolved by the senior author (KB). The following data were extracted from each study: study identifiers, study design characteristics, patient characteristics (inclusion/exclusion criteria, baseline demographics, underlying pathology, fistula risk score (FRS) [19, 20]), intervention details, and primary and secondary outcomes of the present review, as previously described.
Study authors were contacted for missing information. In the event studies referring to the same patient population were identified, only the most comprehensive or recent study was included.
Risk of bias/quality assessment
The Cochrane Collaboration’s tool for assessing risk of bias in randomized controlled trials (RCT) was used to assess randomized interventional trials. The Methodological Index for Non-Randomized Studies (MINORS) tool was used for non-randomized interventional studies. [21, 22]. A MINORS score ≥ 17 was considered high quality [22, 23]. Additionally the Risk of Bias in Non-Randomized Studies of Interventions tool (ROBINS-I) was used to supplement the evaluation of non-randomized studies. [24,25,26,27,28,29]
Data synthesis
Meta-analysis, where appropriate, was conducted using the RevMan 5.3 software (Copenhagen: The Nordic Cochrane Centre, 2014).
Using the Mantel–Haenszel method, odds ratio (OR) values were calculated for dichotomous variables. Inverse-variance weighting was used to calculate difference in means for continuous variables. All values were reported with 95% confidence intervals (CI), where possible, adjusted OR values were used. If the data were reported as a median and range, authors were contacted for mean and standard deviation (SD) values. If unsuccessful, an established method was used to translate the values into their mean and SD estimates [30]. Due to anticipated heterogeneity between studies, a random effects model was employed.
Clinical and methodological heterogeneity between studies was assessed by the I2 statistic. The threshold for interpretation was defined in accordance with the Cochrane Handbook for Systematic Reviews of Interventions [31]. When significant heterogeneity was identified, sensitivity analysis was performed to explore potential sources. Given that the complication profile of PD and DP are unique, all endpoints, besides POPF, were analyzed separately for PD and DP.
Results
Literature search and study characteristics
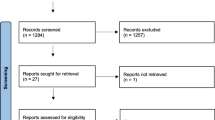
The search strategy identified 2454 references (Fig. 1). After the duplicate references were removed, 2113 studies remained. Thirty articles were advanced to full-text review, of which 6 satisfied the eligibility criteria.
Table 1 outlines the characteristics of the included studies. One RCT [32] and five cohort studies (two prospective [25, 26], three retrospective [27,28,29]) were included in the review. One study utilized the 2016 American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) pancreas-specific procedure target participant use data file (PUF) [29]. Altogether, the selected studies included 10 663 patients. All studies were published in English between 2006 and 2020. Two studies included patients undergoing PD [27, 28], 1 study included patients who underwent laparoscopic DP [25], and the 3 remaining studies [26, 29, 32] included patients who underwent either DP or PD. Of note, the RCT by Cecka et al.[32] included patients undergoing both DP and PD, but the majority of outcomes were reported exclusively for the PD cohort. The authors were contacted for data pertaining to the DP cohort; however, additional data were only obtained for the outcome of POPF. Table 2 outlines variations in postoperative drain management strategies as well as the types of bulbs and drains that were used across institutions.
Risk of bias assessment
Table 3 outlines the risk of bias assessment for the included studies. One cohort study was determined to be low quality with a total MINORS score < 17. [27] The remaining observational studies were considered high quality according to the MINORS criteria [22]. The ROBINS-I tool was applied, and all five non-randomized studies were considered to be of moderate risk of bias, which is interpreted to be sound for a non-randomized study, but not comparable to a rigorous randomized trial (Supplementary table 1). [24,25,26] For the single included RCT, high risk of bias was suspected in blinding and baseline imbalance [32]. Other factors, as outlined by the Cochrane risk of bias tool, were found to be at a low risk of bias [21].
Postoperative Pancreatic Fistula (POPF) development
All six studies reported on the incidence of POPF. While five of the studies followed the 2016 ISGPF definition of POPF [5], the study by Schmidt et al. included patient cohorts from 1980 to 2002, during which, different POPF definitions were used [27].
Aumont et al.[28] reported an association between AS and increased incidence of biochemical pancreatic leaks (21.5% vs. 8.3%, p = 0.03) [30], whereas Dokmak et al.[25] demonstrated an association between AS and decreased grade B POPF incidence (3% vs. 37%, p < 0.001). These findings were not replicated by the other studies, which did not find relations between drainage system and POPF.
Pooled analysis (n = 5 studies) demonstrated no difference in POPF between the AS and PG drainage groups (Fig. 2a). A sensitivity analysis was undertaken whereby the removal of the Dokmak et al.[25] study considerably reduced heterogeneity without meaningful impacts on the effect size (OR 1.14 [0.79, 1.64], p = 0.49, i2 = 53%). This is likely explained by their unique population profile of solely laparoscopic DP [25]. Subgroup analysis by type of surgery also did not demonstrate significant differences in neither PD (Fig. 2b) nor DP (Fig. 2c) cohorts. The pooled analyses excluded the study by Schmidt et al.[27] because it did not follow the ISGPF definition of POPF.
a All POPF in both PD and DP b POPF (ISGPF definition) in patients undergoing PD c POPF (ISGPF definition) in patients undergoing DP d Major Complications (Clavien–Dindo > III) e Surgical site infections–wound infections in patients undergoing PD f Surgical site infections–intra-abdominal infections in patients undergoing PD
Pooled analyses of biochemical leak (formerly grade A) and individual grades of POPF severity (B, C) [4] also did not demonstrate differences between the PG and AS groups.
Overall complications
Overall complications were reported by three studies [26, 28, 32] as a proportion of patients that experienced at least one of the following complications: wound infection, intra-abdominal infection, DGE, PPH, pneumonia, abdominal wound dehiscence, cardiac event, and neurologic complication. The RCT [32] reported a unique trend between AS drainage and major morbidity (p = 0.053) after DP. However, pooled analyses for neither overall (OR 1.11, CI [0.77, 1.60], p = 0.56, i2 = 2%) nor major complications [18] (Fig. 2d) in patients undergoing PD demonstrated significant differences between the two drains. There were also no differences in 30-day mortality (OR 0.92, CI [0.31, 2.72], p = 0.88, i2 = 0%, n = 2 studies) [26, 32].
Post-pancreatectomy hemorrhage.
The incidence of PPH was reported by four studies. [25, 26, 28, 32] Individual and pooled analyses did not demonstrate statistically significant differences by drain type in neither PD (OR 1.18 [0.66, 2.09], p = 0.58, i2 = 0%) nor DP (OR 0.68 [0.04, 12.97], p = 0.80, i2 = 84%).
Surgical site infection
Three studies [26, 29, 32] reported on the incidence of wound infections and two [29, 32] on the incidence of intra-abdominal infections. The studies by Cecka et al.[32] and Marchegiani et al.[26] did not demonstrate associations between SSI and either drainage groups. Kone et al.[31] reported an association between increased intra-abdominal SSI with AS drains in PD cohorts (12% vs. 16%, p = 0.004) on univariate and multivariate analysis, which was not maintained in propensity score matching (p = 0.088). There was no difference demonstrated in the patients undergoing DP.
Of note, the study by Marchegiani et al.[26] used open Penrose drains as their PG system, which theoretically holds a higher risk for ascending infection than a conventional closed PG system [26, 33, 34]. Therefore, this study was excluded from the meta-analyses for SSI as to avoid introduction of possible bias.
Thus, pooled analyses of two studies were undertaken for intra-abdominal and wound SSI among the PD cohort. These analyses did not demonstrate significant associations between drain type and wound infections (Fig. 2e) or intra-abdominal infections (Fig. 2f). There was a trend toward increased intra-abdominal infections with AS drains, but this did not reach statistical significance.
Resource utilization
To compare resource utilization, the following outcomes were assessed: LOS, re-admission, and re-intervention (including percutaneous drain insertion and re-operation).
Four studies [25, 26, 28, 32] reported data pertaining to LOS. Independent and pooled analyses for PD ( − 0.22 days [ − 1.42, 0.98], p = 0.72, i2 = 11%) and DP ( − 4.54 days [ − 13.36, 4.28], p = 0.31, i2 = 94%) did not demonstrate significant differences in LOS. The heterogeneity in the DP cohort is likely contributed by the Dokmak et al.[25] study, which reported range as opposed to SD, requiring estimation of the SD [30]. Of note, Dokmak et al. demonstrated that PG drains were significantly associated with a longer LOS in their cohort of laparoscopic DP (11 days (5–44) vs. 20 days (7–73), p < 0.001) [25].
Incidence of re-operation was only reported by Cecka et al. [32] Greater re-operation rate with AS drains (p = 0.053) and greater re-admission rates with PG (p = 0.053) were reported in patients undergoing DP.
Percutaneous drainage (OR 0.91 [0.27, 3.10], p = 0.88, i2 = 34%) and re-admission to hospital (OR 1.39 [0.57, 3.36], p = 0.47, i2 = 0%) were meta-analyzed, including three [25, 26, 32] and two studies [25, 26], respectively. Pooled and individual analyses of both outcomes did not demonstrate differences between drainage systems.
Quality of life
Effect on QOL was assessed by comparing surrogate outcomes including the length of time drains remained in situ and the presence of a drain at discharge.
Marchegiani et al.[26] demonstrated a statistically significant association between the PG drainage system and an increased incidence of hospital discharge with a drain (12.1% vs. 3.8%, p = 0.009). However, there was no difference between PG and AS in the number of days the drain remained in situ (8.1 ± 11.1 days vs. 6.8 ± 8.1 days, p = 0.20) or LOS (14.3 days vs. 9.5 days, p = 0.20). Conversely, Cecka et al.[32] demonstrated an association between AS and longer time to drain removal (median 6 days vs. 5 days, p = 0.047).
Discussion
The present review identified six studies comparing the incidence of adverse events following pancreatic resection with AS versus PG drainage systems. The type of drainage system was not found to influence the development of POPF. Furthermore, there were no associations between drainage systems and other outcomes of interest including SSI, mortality, morbidity, PPH, resource utilization, or QOL.
Interesting associations were reported independently by select studies. Dokmak et al.[25] reported increased Grade B POPF incidence with PG drain use, an association that was not demonstrated elsewhere. Whereas surgeon preference determined the selection of PG versus AS drains in other observational studies, the Dokmak study initially used PG drains and transitioned to using AS drains at the latter period of the 8-year project [25]. Additionally, the study solely included patients undergoing laparoscopic DP. Thus, the temporal switch in drainage systems in conjunction with a possible learning curve associated with laparoscopic DP may have contributed to reduced POPF with AS drains.
Kone et al. demonstrated increased intra-abdominal SSI with AS drains in univariate and multivariate analyses, which was not maintained in the propensity score matching [29]. This was the largest study included in the review and involved 9 232 patients. Therefore, this study strongly influenced the results when pooled with the study by Cecka et al. [32]. Meta-analysis demonstrated a trend favoring PG, which did not reach statistical significance (p = 0.05). Theoretically, PG drains allow for pooling of fluids, more so than AS drains, and therefore would present higher risk of infections. Studies across various surgical disciplines that compared PG and AS drainage systems reported increased SSI risk with passive, albeit open, drains [35]. Nonetheless, the unique trend seen in a large sample population [32] merits further investigations.
The present study is the first systematic review and meta-analysis comparing the effect of intra-abdominal drainage selection on outcomes following all pancreatic resections. It is distinct from similar reviews primarily by its strict inclusion criteria limited to papers comparing operatively placed drains on suction versus drainage by gravity. Specifically, existing reviews by Zhang et al.[36] and Gachabayov et al.[37] included studies by Jiang et al.[38] and Lee et al.[39]. Although both these studies are RCTs, they were excluded from the present review as the drainage systems failed to meet the inclusion criteria. Jiang et al.[38] utilized an AS system that involved two drains, one that was kept on continuous suction and another that was primarily used for irrigation until POD 3. Lee et al.[39] described the use of an external pancreatic duct stent that was connected to negative suction or gravity drainage. This is entirely distinct from an intra-abdominal drain. Careful methodology screening with strict inclusion criteria for drain systems was done with intention to avoid inclusion of such studies. Furthermore, the present review compared AS versus PG drains in 10 663 DP and PD patients; this is a substantially greater sample population compared to the 1 519 and 160 PD patients included in the Gachabayov et al. and Zhang et al. studies, respectively. [36, 37]
While the present review includes the best available evidence on outcome differences between AS and PG drains following pancreatic surgery, some limitations remain. Although the included non-randomized studies were carefully screened for bias, observational studies cannot replace the level of evidence from a high-quality RCTs. Given that there is only one RCT on this subject, the strength of this review in answering whether drain management can alter outcomes is limited. Across the published literature, there are variations in postoperative management of drains as well as inconsistencies in the type of drain and bulb used across different institutions. Although this allows for the advantage of generalizability, these factors may have contributed heterogeneity to the analyses. Specifically, among AS drains, maximum negative suction occurs with an empty bulb and rapidly decreases as the bulb fills [8, 14, 40]. Thus, differences in how often and at what point the drain is emptied or stripped could impact the amount of time a drain is truly imparting a negative pressure. Currently, available data on the topic of interest are largely observational, with the only included RCT having omitted most outcome data pertaining to the DP group. Two of the six included studies were also considered to be low-quality evidence according to the MINORS and Cochrane Risk of bias criteria. Finally, this review included patients undergoing either PD or DP. Understanding that differences between surgical procedures and complication profiles may introduce heterogeneity, all endpoints were analyzed separately by PD or DP. This was done at the cost of the sample size, thereby decreasing the power to detect differences, should they exist. This further highlights the need for larger trials to definitively answer the question of whether suction or gravity drainage is superior.
Conclusion
Pooled analyses of the best existing evidence on PG versus AS drains following pancreatic surgeries demonstrate that there are no differences between the two with regards to POPF development, mortality, PPH, wound infections, intra-abdominal infections, severe morbidity, resource utilization, and QOL. Based on the existing literature, there lacks evidence to suggest the use of one drain over another at this time. The trend between AS and increased intra-abdominal infections as well as PG and increased Grade B POPF in laparoscopic DP merits further investigation. Greater numbers of well-designed, adequately powered RCTs may prove beneficial to draw robust conclusions as to whether a superior type of drainage system exists in the context of pancreatic surgery.
References
Cameron JL, Riall TS, Coleman J, Belcher KA (2006) One thousand consecutive pancreaticoduodenectomies. Ann Surg 244:10–15. https://doi.org/10.1097/01.sla.0000217673.04165.ea
Mahvi DA, Pak LM, Urman RD, Gold JS, Whang EE (2018) Discharge destination following pancreaticoduodenectomy: a NSQIP analysis of predictive factors and post-discharge outcomes. Am J Surg. https://doi.org/10.1016/j.amjsurg.2018.11.043
Nahm CB, Connor SJ, Samra JS, Mittal A (2018) Postoperative pancreatic fistula: a review of traditional and emerging concepts. Clin Exp Gastroenterol 11:105–118. https://doi.org/10.2147/CEG.S120217
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M (2005) Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138:8–13. https://doi.org/10.1016/j.surg.2005.05.001
Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M (2016) The update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 161(2017):584–591. https://doi.org/10.1016/j.surg.2016.11.014
Bassi C, Buchler MW, Fingerhut A, Sarr M (2015) Predictive Factors for Postoperative Pancreatic Fistula. Ann Surg 261:e99. https://doi.org/10.1097/SLA.0000000000000577
Bassi C, Butturini G, Molinari E, Mascetta G, Salvia R, Falconi M, Gumbs A, Pederzoli P (2004) Pancreatic fistula rate after pancreatic resection: the importance of definitions. Dig Surg 21:54–59
Grobmyer SR, Graham D, Brennan MF, Coit D (2002) High-pressure gradients generated by closed-suction surgical drainage systems. Surg Infect 3:245–249. https://doi.org/10.1089/109629602761624207
Wu W, He J, Cameron JL, Makary M, Soares K, Ahuja N, Rezaee N, Herman J, Zheng L, Laheru D, Choti MA, Hruban RH, Pawlik TM, Wolfgang CL, Weiss MJ (2014) The impact of postoperative complications on the administration of adjuvant therapy following pancreaticoduodenectomy for adenocarcinoma. Ann Surg Oncol 21:2873–2881. https://doi.org/10.1245/s10434-014-3722-6
Turner MC, Masoud SJ, Cerullo M, Adam MA, Shah KN, Blazer DG, Abbruzzese JL, Zani S (2020) Improved overall survival is still observed in patients receiving delayed adjuvant chemotherapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. HPB. https://doi.org/10.1016/j.hpb.2020.03.006
Vallance AE, Young AL, Macutkiewicz C, Roberts KJ, Smith AM (2015) Calculating the risk of a pancreatic fistula after a pancreaticoduodenectomy: a systematic review. HPB 17:1040–1048. https://doi.org/10.1111/hpb.12503
Pratt WB, Callery MP, Vollmer CM (2008) Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg 32:419–428. https://doi.org/10.1007/s00268-007-9388-5
Pratt WB, Maithel SK, Vanounou T, Huang ZS, Callery MP, Vollmer CM (2007) Clinical and economic validation of the International Study Group of Pancreatic Fistula (ISGPF) classification scheme. Ann Surg 245:443–451. https://doi.org/10.1097/01.sla.0000251708.70219.d2
Whitson BA, Richardson E, Iaizzo PA, Hess DJ (2009) Not every bulb is a rose: a functional comparison of bulb suction devices. J Surg Res 156:270–273. https://doi.org/10.1016/j.jss.2009.03.096
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA (2015) PRISMA-P group, preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Control Found Appl 4:1. https://doi.org/10.1186/2046-4053-4-1
Park L, Baker L, Smith H, Davies A, Abou Khalil J, Martel G, Balaa F, Bertens KA (2019) Passive versus active intra-abdominal drainage following pancreatic resection: does a superior drainage system exist? A protocol for systematic review. BMJ Open 9:e031319. https://doi.org/10.1136/bmjopen-2019-031319
Veritas Health Innovation, Covidence systematic review software, Melbourne, Australia, n.d. www.covidence.org
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications. Ann Surg 240:205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM (2013) A Prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg 216:1–14. https://doi.org/10.1016/j.jamcollsurg.2012.09.002
Miller BC, Christein JD, Behrman SW, Drebin JA, Pratt WB, Callery MP, Vollmer CM (2014) A multi-institutional external validation of the fistula risk score for pancreatoduodenectomy. J Gastrointest Surg 18:172–180. https://doi.org/10.1007/s11605-013-2337-8
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC (2011) The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J (2003) Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg 73:712–716. https://doi.org/10.1046/j.1445-2197.2003.02748.x
Abraham NS, Byrne CJ, Young JM, Solomon MJ (2010) Meta-analysis of well-designed nonrandomized comparative studies of surgical procedures is as good as randomized controlled trials. J Clin Epidemiol 63:238–245. https://doi.org/10.1016/j.jclinepi.2009.04.005
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan A-W, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. https://doi.org/10.1136/bmj.i4919
Dokmak S, Ftériche FS, Meniconi RL, Aussilhou B, Duquesne I, Perrone G, Romdhani C, Belghiti J, Lévy P, Soubrane O, Sauvanet A (2019) Pancreatic fistula following laparoscopic distal pancreatectomy is probably unrelated to the stapler size but to the drainage modality and significantly decreased with a small suction drain. Langenbecks Arch Surg 404:203–212. https://doi.org/10.1007/s00423-019-01756-3
Marchegiani G, Perri G, Pulvirenti A, Sereni E, Azzini AM, Malleo G, Salvia R, Bassi C (2018) Non-inferiority of open passive drains compared with closed suction drains in pancreatic surgery outcomes: A prospective observational study. Surgery 164:443–449. https://doi.org/10.1016/j.surg.2018.04.025
Schmidt CM, Choi J, Powell ES, Yiannoutsos CT, Zyromski NJ, Nakeeb A, Pitt HA, Wiebke EA, Madura JA, Lillemoe KD (2009) Pancreatic fistula following pancreaticoduodenectomy: clinical predictors and patient outcomes. HPB Surg. https://doi.org/10.1155/2009/404520
Aumont O, Dupré A, Abjean A, Pereira B, Veziant J, Le Roy B, Pezet D, Buc E, Gagnière J (2017) Does intraoperative closed-suction drainage influence the rate of pancreatic fistula after pancreaticoduodenectomy? BMC Surg. https://doi.org/10.1186/s12893-017-0257-3
Kone LB, Maker VK, Banulescu M, Maker AV (2020) Should drains Suck? a propensity score analysis of closed-suction versus closed-gravity drainage after pancreatectomy. J Gastrointest Surg. https://doi.org/10.1007/s11605-020-04613-7
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13. https://doi.org/10.1186/1471-2288-5-13
J. Higgins, S. Green, Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011], The Cochrane Collaboration. (2011). www.handbook.cochrane.org (accessed February 19, 2019)
Čečka F, Jon B, Skalický P, Čermáková E, Neoral Č, Loveček M (2018) Results of a randomized controlled trial comparing closed-suction drains versus passive gravity drains after pancreatic resection. Surgery 164:1057–1063. https://doi.org/10.1016/j.surg.2018.05.030
Wang Q, Jiang Y-J, Li J, Yang F, Di Y, Yao L, Jin C, Fu D-L (2014) Is routine drainage necessary after pancreaticoduodenectomy? World J Gastroenterol 20:8110–8118. https://doi.org/10.3748/wjg.v20.i25.8110
Yoshikawa K, Konishi M, Takahashi S, Gotohda N, Kato Y, Kinoshita T (2011) Surgical management for the reduction of postoperative hospital stay following distal pancreatectomy. Hepatogastroenterology 58:1389–1393. https://doi.org/10.5754/hge10811
Reiffel AJ, Barie PS, Spector JA (2013) A multi-disciplinary review of the potential association between closed-suction drains and surgical site infection. Surg Infect (Larchmt) 14:244–269. https://doi.org/10.1089/sur.2011.126
Zhang W, He S, Cheng Y, Xia J, Lai M, Cheng N, Liu Z (2018) Prophylactic abdominal drainage for pancreatic surgery. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD010583.pub4
Gachabayov M, Gogna S, Latifi R, Dong XD (2019) Passive drainage to gravity and closed-suction drainage following pancreatoduodenectomy lead to similar grade B and C postoperative pancreatic fistula rates a meta-analysis. Int J Surg 67:24–31. https://doi.org/10.1016/j.ijsu.2019.05.001
Jiang H, Liu N, Zhang M, Lu L, Dou R, Qu L (2016) A randomized trial on the efficacy of prophylactic active drainage in prevention of complications after pancreaticoduodenectomy. Scand J Surg 105:215–222. https://doi.org/10.1177/1457496916665543
Lee SE, Ahn Y-J, Jang J-Y, Kim S-W (2009) Prospective randomized pilot trial comparing closed suction drainage and gravity drainage of the pancreatic duct in pancreaticojejunostomy. J Hepatobiliary Pancreat Surg 16:837–843. https://doi.org/10.1007/s00534-009-0171-x
Carruthers KH, Eisemann BS, Lamp S, Kocak E (2013) Optimizing the Closed Suction Surgical Drainage System. Plast Surg Nurs 33:38–42. https://doi.org/10.1097/PSN.0b013e31828425db
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This study was presented at the Canadian Surgery Forum, 7 September 2019, Montreal, QC, Canada
Supplementary Information
Appendices
Appendix A: PRISMA Checklist

Appendix B: Search Strategy
Database(s): Ovid MEDLINE(R) ALL 1946 to May 18, 2020, Search Strategy:
# | Searches | Results |
|---|---|---|
1 | Pancreatic diseases/su [Surgery] | 2313 |
2 | exp Pancreatic neoplasms/su [Surgery] | 16,711 |
3 | (pancrea* adj2 surg*).tw | 5135 |
4 | (pancrea* adj2 resection*).tw | 5694 |
5 | (pancrea* surg* or pancrea* resection*).kw | 939 |
6 | PANCREATECTOMY/ or PANCREATICOJEJUNOSTOMY/ or Pancreaticoduodenectomy/ | 20,311 |
7 | (pancreatectom* or pancreaticojejunostom* or pancreaticoduodenectom*).tw,kw | 16,222 |
8 | whipple procedure*.tw,kw | 840 |
9 | or/1–8 | 38,595 |
10 | drainage/ or drain*.tw,kw | 145,869 |
11 | 9 and 10 | 3648 |
12 | (Jackson pratt or jp drain*).tw,kw,kf | 137 |
13 | (closed or active or negative).tw,kw,kf | 2,066,083 |
14 | 12 or 13 | 2,066,180 |
15 | (open or passive or gravity).tw,kf | 622,346 |
16 | 11 and 14 and 15 | 50 |
17 | ((active or closed or negative or jackson pratt or jp) adj4 drain*).tw | 2498 |
18 | ((active or closed or negative or jackson pratt or jp) and drain*).kf | 113 |
19 | 17 or 18 | 2546 |
20 | ((passive or open or gravity) adj4 drain*).tw | 1914 |
21 | ((passive or open or gravity) and drain*).kf | 63 |
22 | 20 or 21 | 1950 |
23 | 9 and (19 or 22) | 130 |
24 | 16 or 23 | 142 |
25 | *DRAINAGE/ or (drains or drainage).ti | 28,790 |
26 | DRAINAGE/is, mt [Instrumentation, Methods] | 15,412 |
27 | 25 or 26 | 33,709 |
28 | 9 and 27 | 1120 |
29 | 24 or 28 | 1205 |
30 | (child/ or infant/) not adult/ | 1,352,637 |
31 | 29 not 30 | 1180 |
32 | animals/ not humans/ | 4,665,913 |
33 | 31 not 32 | 1153 |
Database(s): Embase Classic + Embase 1947 to May 18, 2020, Search Strategy:
# | Searches | Results |
|---|---|---|
1 | pancreas surgery/ or pancreas resection/ | 25,635 |
2 | pancreaticoduodenectomy/ | 21,721 |
3 | pancreatectomy/ | 3260 |
4 | pancreaticojejunostomy/ | 3133 |
5 | (pancrea* adj2 surg*).tw | 9313 |
6 | (pancrea* adj2 resection*).tw | 9524 |
7 | (pancreatectom* or pancreaticojejunostom* or pancreaticoduodenectom*).tw | 26,569 |
8 | whipple procedure.tw | 1350 |
9 | or/1–8 | 55,941 |
10 | abdominal drainage/ or abdominal drain/ | 3498 |
11 | drain*.tw | 197,062 |
12 | suction drainage/ or surgical drainage/ or suction drain/ | 19,950 |
13 | or/10–12 | 205,973 |
14 | 9 and 13 | 5695 |
15 | (Jackson pratt or jp drain*).tw | 372 |
16 | (closed or active or negative).tw | 2,835,747 |
17 | 15 or 16 | 2,835,998 |
18 | (open or passive or gravity).tw | 820,661 |
19 | 14 and 17 and 18 | 88 |
20 | ((active or closed or negative or jackson pratt or jp) adj4 drain*).tw | 3915 |
21 | ((passive or open or gravity) adj4 drain*).tw | 2868 |
22 | 9 and (20 or 21) | 212 |
23 | 19 or 22 | 256 |
24 | 9 and 10 | 381 |
25 | (drainage or drains).ti | 28,765 |
26 | *abdominal drainage/ or *abdominal drain/ | 717 |
27 | *suction drainage/ or *surgical drainage/ | 2649 |
28 | or/25–27 | 29,619 |
29 | 9 and 28 | 988 |
30 | 24 or 29 | 1251 |
31 | case report/ | 2,577,349 |
32 | 30 not 31 | 1024 |
33 | (child/ or infant/) not adult/ | 1,669,345 |
34 | 32 not 33 | 1016 |
Database(s): EBM Reviews—Cochrane Central Register of Controlled Trials April 2020 Search Strategy:
# | Searches | Results |
|---|---|---|
1 | Pancreatic diseases/su [Surgery] | 10 |
2 | exp Pancreatic neoplasms/su [Surgery] | 23 |
3 | (pancrea* adj2 surg*).tw | 820 |
4 | (pancrea* adj2 resection*).tw | 543 |
5 | (pancrea* surg* or pancrea* resection*).kw | 165 |
6 | PANCREATECTOMY/ or PANCREATICOJEJUNOSTOMY/ or Pancreaticoduodenectomy/ | 404 |
7 | (pancreatectom* or pancreaticojejunostom* or pancreaticoduodenectom*).tw,kw | 1371 |
8 | whipple procedure*.tw,kw | 59 |
9 | or/1–8 | 2257 |
10 | drainage/ or (drainage or drain*).tw | 10,978 |
11 | 9 and 10 | 335 |
Cinahl.
# | Query | Results |
|---|---|---|
S28 | S19 OR S23 OR S27 | 328 |
S27 | S10 AND S26 | 315 |
S26 | S24 OR S25 | 6,726 |
S25 | TI (drains or drainage) | 3,814 |
S24 | (MM "Drainage + ") OR (MM "Closed Drainage") | 4,830 |
S23 | S10 AND S22 | 27 |
S22 | S20 OR S21 | 762 |
S21 | TI ( ((passive or open or gravity) N4 drain*)) OR AB ( ((passive or open or gravity) N4 drain*)) | 312 |
S20 | TI ( ((active or closed or negative or jackson pratt or jp) N4 drain*)) OR AB ( ((active or closed or negative or jackson pratt or jp) N4 drain*)) | 481 |
S19 | S14 AND S17 AND S18 | 8 |
S18 | TI ( (open or passive or gravity)) OR AB ( (open or passive or gravity)) | 122,862 |
S17 | S15 OR S16 | 332,486 |
S16 | TI ( (closed or active or negative)) OR AB ( (closed or active or negative)) | 332,457 |
S15 | TI ( (Jackson pratt or jp drain*)) OR AB ( (Jackson pratt or jp drain*)) | 40 |
S14 | S10 AND S13 | 738 |
S13 | S11 OR S12 | 23,692 |
S12 | TI drain* OR AB drain* | 18,008 |
S11 | (MH "Drainage + ") OR (MH "Closed Drainage") | 10,128 |
S10 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 | 6,631 |
S9 | TI whipple procedure OR AB whipple procedure | 169 |
S8 | TI ( (pancreatectom* or pancreaticojejunostom* or pancreaticoduodenectom*)) OR AB ( (pancreatectom* or pancreaticojejunostom* or pancreaticoduodenectom*)) | 2,298 |
S7 | (MH "Pancreaticoduodenectomy") | 1,076 |
S6 | (MH "Pancreatectomy") OR (MH "Pancreaticojejunostomy") | 1,602 |
S5 | TI (pancrea* N2 resection*) OR AB (pancrea* N2 resection*) | 853 |
S4 | TI (pancrea* N2 surg*) OR AB (pancrea* N2 surg*) | 1,162 |
S3 | (MH "Pancreatic Diseases + /SU") | 3,703 |
S2 | (MH "Pancreas + /SU") | 711 |
S1 | (MH "Pancreatic Neoplasms + /SU") | 2,277 |
Rights and permissions
About this article
Cite this article
Park, L.J., Baker, L., Smith, H. et al. Passive Versus Active Intra-Abdominal Drainage Following Pancreatic Resection: Does A Superior Drainage System Exist? A Systematic Review and Meta-Analysis. World J Surg 45, 2895–2910 (2021). https://doi.org/10.1007/s00268-021-06158-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-021-06158-5