Abstract
Background
Pancreaticojejunal anastomotic leakage remains a major complication after pancreatoduodenectomy, and various means of preventing pancreatic leakage have been studied over the past few decades. The purpose of this study was to determine whether closed suction drainage provided a better option than gravity drainage in pancreaticojejunostomy.
Methods
Between 2004 and 2006, a total of 110 patients who underwent pancreaticojejunostomy at our institute were enrolled in this prospective randomized pilot study. Fifty-five patients were allocated to the closed suction drainage (CD) group and 55 to the gravity drainage (GD) group. In each patient a polyethylene pediatric feeding tube was inserted into the remnant pancreatic duct across a duct-to-mucosa type pancreaticojejunostomy and totally externalized. The tube was then connected to the aspiration bag of a Jackson–Pratt drain to generate negative pressure or to a bile bag for natural drainage. Pancreatic fistulas were defined and graded as A, B, or C according to the international study group for pancreatic fistulas (ISGPF) criteria.
Results
No differences were found between the GD and CD groups in age, sex distribution, or diagnosis. A pancreatic fistula occurred in 24 patients (43.6%) in the GD group and in 14 (25.5%) in the CD group (P = 0.045). In the GD group, grade B and C fistula occurred in 6 patients (10.9%), whereas in the CD group, this occurred in 5 patients (9.1%).
Conclusion
In this study, temporary external drainage of the pancreatic duct with closed suction drainage significantly reduced the incidence of grade A pancreatic fistula. A follow-up randomized prospective multicenter study has been initiated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreaticojejunal anastomotic leakage is a major complication after pancreatoduodenectomy, and various technical methods have been examined to improve the situation, e.g., pancreatic duct occlusion [1], anastomosis reinforcement with fibrin glue [2], placement of an internal stent [3], and pancreaticogastrostomy [4, 5]. However, none of these methods have been successful at improving results according to the findings of prospective randomized studies.
Some retrospective studies [6, 7] have reported a low pancreatic fistula rate when a catheter is inserted into the pancreatic duct to externally drain the pancreatic juice. Furthermore, a recent prospective randomized trial [8] showed that external drainage of the pancreatic duct decreased the rate of pancreatic fistula formation, indicating that diverting pancreatic juice from an anastomosis can theoretically reduce the incidence of pancreaticojejunostomy anastomotic leakage. We have experienced more than 1000 cases of pancreatoduodenectomy at our institution, and based on the above theory, have performed external pancreatic duct stenting to achieve gravity drainage. It was recently proposed by Dr. Ahn, one of the present authors that active drainage of pancreatic juice using suction drainage might maximize the advantage of a stent. Thus, before conducting a large-scale randomized prospective multicenter study to evaluate the feasibility of this method, we decided to conduct this prospective randomized pilot study.
Methods
During the period 2004–2006, we enrolled 110 patients who underwent duct-to-mucosa pancreaticojejunostomy reconstruction after pancreatoduodenectomy, and we randomly allocated them to two groups before surgery (using blinded envelopes) to rule out any influence on selection based on intraoperative findings. Equal numbers of envelopes with protocols for a either closed suction drainage group or a gravity drainage group were prepared in a blinded fashion. Fifty-five patients were allocated to the closed suction drainage group (CD group) and the other 55 patients to the gravity drainage group (GD group; Fig. 1). This study was approved by the Institutional Review Board of Seoul National University Hospital, and informed consent was obtained from all patients participating in the trial before surgery. Operations were performed using a standardized technique by two surgeons who each had experience of more than 200 cases of pancreatoduodenectomy before this study.
Surgical technique
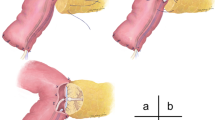
Pancreatoduodenectomy was performed using conventional pancreatoduodenectomy or pylorus-preserving pancreatoduodenectomy (PPPD). Anastomosis to the remnant pancreas was performed between the pancreas and jejunum by two-layer pancreaticojejunostomy, as described in our previous report [9]. The outer layer, consisting of the remnant pancreatic parenchyma and the seromuscular layer of the jejunum, was sutured using a 5-0 polypropylene suture (Prolene; Ethicon, Somerville, NJ, USA) and the inner layer, consisting of the pancreatic duct and mucosa of the jejunum, was sutured using an interrupted 5-0 polydioxanone suture (PDS II; Ethicon). A Fr 5–8 silastic polyethylene pediatric feeding tube (NIPRO disposable feeding tube; Nipro, Tokyo, Japan) with multiple side holes was then inserted 2 cm into the pancreatic duct. The stents used were the largest that could easily pass into the pancreatic duct. Catheter migration was prevented by placing an anchoring suture, using one of the inner posterior layer sutures. The catheter exited via a small enterotomy in the jejunal loop of the distal portion of the hepaticojejunostomy. The enterostomy site used for catheter exit was closed with a purse-string suture and anchored using absorbable suture material. Stents were externalized by making a stab incision in the anterior abdominal wall, and totally externalized pancreatic stents were connected to the aspiration bag of a Jackson–Pratt drain to generate negative pressure, or to a bile bag for gravity drainage. The bags for gravity drainage were kept at floor level. Three drains were routinely placed anterior and posterior to the pancreatico-jejunal anastomoses and exteriorized through the lateral abdominal wall.
Perioperative management
Perioperative management was standardized. All patients received broadspectrum antibiotics for 2 days and an H2 blocker (famotidine) during the no-oral-intake period after surgery. Postoperatively, octreotide was routinely given subcutaneously (100 μg every 8 h for 5 days). The volume of fluid drained from the peripancreatic drains and that drained from the pancreatic duct were measured daily, and serum and drain fluid amylase levels were measured on postoperative days 1, 3, 5, 7, and 10. A computed tomography (CT) scan was performed on postoperative day 7, and if there was no evidence of leakage or fluid collection, the peripancreatic drains were removed on postoperative day 8. Patients were discharged with the pancreatic duct catheter in situ, and this was removed at our outpatient clinic after the fourth postoperative week. If the patients were discharged after more than 28 days postoperative hospitalization, the pancreatic duct catheter was removed during hospitalization.
Data collection
Preoperative demographic and clinical data, and surgical procedure, pathologic diagnosis, postoperative course, and complication details were collected prospectively.
Study endpoints
The primary study endpoint was the pancreatic fistula rate. Pancreatic fistula was defined as any measurable drainage from an operatively placed drain (or a subsequently placed percutaneous drain), on or after postoperative day 3, with an amylase content greater than three times the upper limit of the normal serum amylase level (i.e., >300 IU/L) [10] (International Study Group for Pancreatic Fistulas [ISGPF] definition) or drainage of more than 30 mL of fluid with an amylase level higher than 600 U/dL on or after postoperative week 1 [11] (Seoul National University Hospital [SNUH] definition). In addition, fistula severity was graded as A, B, or C according to the ISGPF clinical criteria [9], as follows; grade A fistula—a transient, asymptomatic fistula with only elevated drain amylase levels; treatments or deviation in clinical management are not required; grade B fistula—a symptomatic, clinically apparent fistula requiring diagnostic evaluation and therapeutic management; and grade C fistula—a severe, clinically significant fistula requiring major deviations in clinical management and unequivocal aggressive therapeutic interventions. Major pancreatic leakage was defined as drainage of more than 200 mL of fluid or the development of an intraabdominal abscess [9].
Delayed gastric emptying (DGE) was defined as nasogastric drainage for more than 10 days, or the requirement for nasogastric tube reinsertion due to vomiting, or failure to tolerate a semisolid diet at 14 days after surgery [12].
Statistical analysis
Continuous data are expressed as means ± SD. The χ2 test was used to compare qualitative parameters, and Student’s t test was used for quantitative parameters. P values of <0.05 were accepted as significant. Statistical analyses were performed using SPSS version 12.0 for windows (SPSS, Chicago, IL, USA).
Results
Demographics
No differences were observed between the gravity drainage (GD) group and the closed suction drainage (CD) group in terms of age, sex distribution, diagnosis, pancreatic texture, pancreatic duct diameter, or stage (Table 1).
Overall morbidity, including pancreatic fistula and mortality
Table 2 shows postoperative outcomes. The overall morbidity rate was 52.7% in the CD group and 63.6% in the GD group (P = 0.246). No hospital mortality occurred in either group.
According to the ISGPF definition, a pancreatic fistula occurred in 24 patients (43.6%) in the GD group and in 14 (25.5%) in the CD group (P = 0.045) and according to our institution’s definition, a pancreatic fistula occurred in 16 patients (29.1%) in the GD group and in 7 (12.7%) in the CD group (P = 0.035). In the GD group, a grade A fistula occurred in 18 patients (32.7%), grade B in 4 (7.3%), and grade C in 2. In the CD group, a grade A fistula occurred in 9 patients (16.4%), grade B in 3 (5.5%), and grade C in 2 (P = 0.614). All patients who developed a grade B fistula received antibiotics and all patients with a grade C fistula received antibiotics and radiologic interventional treatment. However, no sepsis, readmission, reoperation, or hospital mortality occurred in either group. Pseudoaneurysmal rupture occurred in 2 patients in the CD group and in 4 patients in the GD group (P = 0.397) and all 6 patients were successfully managed by radiologic intervention.
Amount of pancreatic juice drainage
Pancreatic duct drainage amounts were significantly higher in the CD group on the first postoperative day (97.5 ± 62.9 vs. 18.6 ± 20.5, P < 0.01) and drainage amounts plateaued on the fourth postoperative day in both groups (Fig. 2).
Discussion
Pancreatic fistula is a leading cause of morbidity and mortality after pancreatoduodenectomy. Moreover, because pancreaticojejunostomy is the most widely used method of reconstruction for the pancreatic stump after pancreatoduodenectomy [13], it is important that technical improvements be found to reduce the pancreaticojejunostomy leakage rates.
At our institution, we perform two-layer end-to-side and duct-to-mucosa pancreaticojejunostomy with temporary external pancreatic drainage, as previously reported [8]. External drainage of pancreatic juice using a stent has some potential advantages [14–16]. First, complete drainage may prevent the activation of pancreatic enzymes by bile soon after surgery. Second, guidance of the tube through the jejunal loop may decompress the denervated jejunal segment, and this may prevent pancreatitis caused by edema of the jejunum with subsequent occlusion of the pancreaticoenterostomy. Third, stenting of the pancreatic duct may allow more precise suture placement, and thus protect the pancreatic duct from suture injury and reduce the risk of iatrogenic pancreatic duct occlusion. Fourth, the stent may provide improved local control of secretions in the presence of a pancreatic fistula and better enable less invasive management. Fifth, the stent may maintain long-term patency of the pancreaticojejunostomy and conserve pancreatic function [17, 18]. Clinical studies [19, 20] and experimental data [21] show that the patency of pancreaticoenteric anastomoses is improved by stent placement. Although no study has compared an external stent with a short internal stent, we note that, compared with a short internal stent, an external stent has the theoretical advantages of more effectively diverting pancreatic secretions away from a pancreaticojejunostomy anastomosis, and thus, it prevents the activation of pancreatic enzymes by bile. Moreover, an external stent is associated with a much lower risk of stent migration from an anastomosis during the first few postoperative days when protection of the anastomosis is most required. Furthermore, a prospective nonrandomized trial [22] and a prospective randomized trial [8] recently showed that external drainage of the pancreatic duct with a stent reduced pancreaticojejunostomy leakage rates.
In the present prospective randomized pilot trial, we examined the hypothesis that external drainage of pancreatic juice after pancreatoduodenectomy, using a closed suction drain, would reduce pancreatic fistula incidence, because pancreatic juice would be extracted more actively and effectively from the remnant pancreas, and because this process increases the security and safety of the anastomosis. Previously, we used a gravity drainage system, whereby pancreatic juice outflow due to intraductal pressure gradients and undrained pancreatic juice remained at the pancreaticojejunal anastomotic site or flowed into the jejunum and were activated by bile juice. On the other hand, the closed suction drainage system works by applying negative pressure and the system drains all pancreatic juice from the remnant pancreas, thus completely diverting it away from the pancreaticojejunal anastomotic site. This may encourage anastomotic healing by preventing the accumulation of pancreatic juice at the anastomotic site and by preventing the activation of pancreatic juice by bile juice; thus improving long-term pancreatic duct patency.
In the present study an external pancreatic duct stent fitted with a closed suction drainage device significantly reduced the pancreatic fistula rate (25.5 vs. 43.6% in the group with gravity drainage, P = 0.045). Although there was no significant difference in the Grade B and C fistula rate between the two groups, this may have been due to the small sample size. Furthermore, the total average amount of pancreatic juice that drained from the external pancreatic duct during postoperative day 1 in the closed suction drainage group was significantly greater than that in the gravity drainage group (97.5 ± 62.9 ml vs. 18.6 ± 20.5 ml, P < 0.01). Although the pancreatic fistula rate recorded during the present study was higher than that in other reports [3, 8], two-thirds of the fistulas in our study were grade A fistulas that showed only elevated amylase level of drained fluid and required no additional management. In the present study, two patients (3.6%) with grade C fistula in each group were maintained with nil by mouth and received total parenteral nutrition and intravenous antibiotics, and underwent radiologic interventional treatment and responded to treatment without developing sepsis or requiring reoperation. Moreover, in the present study, because the diversion of pancreatic juice by external stents was maintained after pancreatic fistula development, even though the total amount of fluid drained was halved, no patient demonstrated worrisome peripancreatic fluid accumulation.
Potential complications associated with stent removal are of concern when an external pancreatic duct stent is used. Although local peritonitis after stent removal has been reported [23], this is preventable by careful suturing of the serosa of the jejunal loop around the exit site of the tube to the peritoneum of the anterior abdominal wall. In the present study, no complications related to either the insertion or the removal of the external stent were encountered, though such events have been reported in association with external pancreatic duct drainage stents [8, 22, 24].
Overall complication rates in the present study (52.7% in the CD group and 63.6% in the GD group) were high compared to those in our previous studies [9, 11]; we attribute this to improved data collection, because of the introduction of electronic data recording and the resultant inclusion of minor complications, such as minor wound problems or minor tractitis.
In the present randomized pilot study, temporary external drainage of the pancreatic duct using a closed suction drainage device was found to significantly reduce the incidence of grade A pancreatic fistula. Currently, a randomized prospective multicenter study is underway to confirm that the closed suction drainage device reduces pancreatic fistula development after pancreaticojejunostomy.
References
Tran K, Van Eijck C, Di Carlo V, Hop WC, Zerbi A, Balzano G, et al. Occlusion of the pancreatic duct versus pancreaticojejunostomy: a prospective randomized trial. Ann Surg. 2002;236:422–8.
Lillemoe KD, Cameron JL, Kim MP, Campbell KA, Sauter PK, Coleman JA, et al. Does fibrin glue sealant decrease the rate of pancreatic fistula after pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg. 2004;8:766–72.
Winter JM, Cameron JL, Campbell KA, Chang DC, Riall TS, Schulick RD, et al. Does pancreatic duct stenting decrease the rate of pancreatic fistula following pancreaticoduodenectomy? Results of a prospective randomized trial. J Gastrointest Surg. 2006;10:1280–90.
Yeo CJ, Cameron JL, Maher MM, Sauter PK, Zahurak ML, Talamini MA, et al. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann Surg. 1995;222:580–8.
Butturini G, Daskalaki D, Molinari E, Scopelliti F, Casarotto A, Bassi C. Pancreatic fistula: definition and current problems. J Hepatobiliary Pancreat Surg. 2008;15:247–51.
Hamanaka Y, Suzuki T. Total pancreatic duct drainage for leakproof pancreatojejunostomy. Surgery. 1994;115:22–6.
Mok KT, Wang BW, Liu SI. Management of pancreatic remnant with strategies according to the size of pancreatic duct after pancreaticoduodenectomy. Br J Surg. 1999;86:1018–9.
Poon RT, Fan ST, Lo CM, Ng KK, Yuen WK, Yeung C, et al. External drainage of pancreatic duct with a stent to reduce leakage rate of pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg. 2007;246:425–33.
Lee SE, Yang SH, Jang JY, Kim SW. Pancreatic fistula after pancreaticoduodenectomy: A comparison between the two pancreaticojejunostomy methods for approximating the pancreatic parenchyma to the jejunal seromuscular layer: Interrupted vs continuous stitches. World J Gastroenterol. 2007;13:5351–6.
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13.
Kim SW, Youk EG, Park YH. Comparison of pancreatogastrostomy and pancreatojejunostomy after pancreatoduodenectomy performed by one surgeon. World J Surg. 1997;21:640–3.
Park YC, Kim SW, Jang JY, Ahn YJ, Park YH. Factors influencing delayed gastric emptying after pylorus-preserving pancreatoduodenectomy. J Am Coll Surg. 2003;196:859–65.
Watanabe M, Usui S, Kajiwara H, Nakamura M, Sumiyama Y, Takada T, et al. Current pancreatogastrointestinal anastomotic methods: results of a Japanese survey of 3109 patients. J Hepatobiliary Pancreat Surg. 2004;11:25–33.
Smith CD, Sarr MG, van Heerden JA. Completion pancreatectomy following pancreaticoduodenectomy: clinical experience. World J Surg. 1992;16:521–4.
Hamanaka Y, Nishihara K, Hamasaki T, Kawabata A, Yamamoto S, Tsurumi M, et al. Pancreatic juice output after pancreatoduodenectomy in relation to pancreatic consistency, duct size, and leakage. Surgery. 1996;119:281–7.
Okamoto A, Tsuruta K. Fistulation method: simple and safe pancreaticojejunostomy after pancreatoduodenectomy. Surgery. 2000;127:433–8.
Okabayashi T, Kobayashi M, Nishimori I, Sugimoto T, Onishi S, Hanazaki K. Risk factors, predictors and prevention of pancreatic fistula formation after pancreatoduodenectomy. J Hepatobiliary Pancreat Surg. 2007;14:557–63.
Popiela T, Kedra B, Sierzega M, Gurda A. Risk factors of pancreatic fistula following pancreaticoduodenectomy for periampullary cancer. Hepatogastroenterology. 2004;51:1484–8.
Fernández-del Castillo C, Rattner DW, Warshaw AL. Standards for pancreatic resection in the 1990s. Arch Surg. 1995;130:295–9.
Forrest JF, Longmire WP Jr. Carcinoma of the pancreas and periampullary region. Ann Surg. 1979;189:129–38.
Biehl T, Traverso LW. Is stenting necessary for a successful pancreatic anastomosis? Am J Surg. 1992;163:530–2.
Roder JD, Stein HJ, Bottcher KA, Busch R, Heidecke CD, Siewert JR. Stented versus nonstented pancreaticojejunostomy after pancreatoduodenectomy: a prospective study. Ann Surg. 1999;229:41–8.
Ohwada S, Tanahashi Y, Ogawa T, Kawate S, Hamada K, Tago KI, et al. In situ vs ex situ pancreatic duct stents of duct-to-mucosa pancreaticojejunostomy after pancreaticoduodenectomy with Billroth I-type reconstruction. Arch Surg. 2002;137:1289–93.
Langrehr JM, Bahra M, Jacob D, Glanemann M, Neuhaus P. Prospective randomized comparison between a new mattress technique and Cattell (duct-to-mucosa) pancreaticojejunostomy for pancreatic resection. World J Surg. 2005;29:1111–9.
Acknowledgments
This study was supported by the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea. (# 0520320) and Seoul National University Hospital research program (04-2006-0380).
Author information
Authors and Affiliations
Corresponding author
Additional information
S. E. Lee and Y.-J. Ahn contributed equally in this work.
This study was registered at http://www.clinicaltrials.gov/ (Clinical Trials) and the registered number was Seoul National University Hospital protocol record H-0612-018-191.
About this article
Cite this article
Lee, S.E., Ahn, YJ., Jang, JY. et al. Prospective randomized pilot trial comparing closed suction drainage and gravity drainage of the pancreatic duct in pancreaticojejunostomy. J Hepatobiliary Pancreat Surg 16, 837–843 (2009). https://doi.org/10.1007/s00534-009-0171-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00534-009-0171-x