Abstract
Background
The Fistula Risk Score (FRS), a ten-point scale that relies on weighted influence of four variables, has been shown to effectively predict clinically relevant postoperative pancreatic fistula (CR-POPF) development and its consequences after pancreatoduodenectomy (PD). The proposed FRS demonstrated excellent predictive capacity; however, external validation of this tool would confirm its universal applicability.
Methods
From 2001 to 2012, 594 PDs with pancreatojejunostomy reconstructions were performed at three institutions. POPFs were graded by International Study Group on Pancreatic Fistula standards as grades A, B, or C. The FRS was calculated for each patient, and clinical outcomes were evaluated across four discrete risk zones as described in the original work. Receiver operator curve analysis was performed to judge model validity.
Results
One hundred forty-two patients developed any sort of POPF, of which 68 were CR-POPF (11.4 % overall; 8.9 % grade B, 2.5 % grade C). Increasing FRS scores (0–10) correlated well with CR-POPF development (p < 0.001) with a C-statistic of 0.716. When segregated by discrete FRS-risk groups, CR-POPFs occurred in low-, moderate-, and high-risk patients, 6.6, 12.9, and 28.6 % of the time, respectively (p < 0.001). Clinical outcomes including complications, length of stay, and readmission rates also increased across risk groups.
Conclusion
This multi-institutional experience confirms the Fistula Risk Score as a valid tool for predicting the development of CR-POPF after PD. Patients devoid of any risk factors did not develop a CR-POPF, and the rate of CR-POPF approximately doubles with each subsequent risk zone. The FRS is validated as a strongly predictive tool, with widespread applicability, which can be readily incorporated into common clinical practice and research analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most formidable complications after pancreatoduodenectomy (PD) is postoperative pancreatic fistula (POPF). This problem has been shown to directly lead to other complications, an overall increase in morbidity, longer hospital stays, and greater costs after PD.1–4 The International Study Group on Pancreatic Fistula (ISGPF) defines a threshold for POPF as an anastomotic leak with elevated fluid amylase activity and then further classifies them as grade A, “biochemical fistulas”, or grades B and C, which are considered clinically relevant postoperative pancreatic fistula (CR-POPF). Through consensus, the ISGPF created a globally accepted definition for POPFs, opening the field to further study of the prediction and management of these ominous events.5 Distinct risk factors for ISGPF CR-POPFs have since been identified as small duct size, soft gland texture, high-risk pathology (anything other than pancreatic cancer or pancreatitis), and increased blood loss, although some prediction scales have been based selectively on only certain subsets of these factors.6–11
The Fistula Risk Score (FRS) is a prospectively validated scale that predicts development of CR-POPF after PD based upon the four aforementioned risk factors for CR-POPF, which were originally discerned from a multivariate analysis of 18 preoperative and 24 intraoperative factors.12 The FRS refined these elements to be a small pancreatic duct size of less than 5 mm, soft gland texture, any pathology except chronic pancreatitis or pancreatic adenocarcinoma, and blood loss of greater than 400 mL. Derived at a single institution, the proposed FRS was a ten-point scale based on the proportional weights of these four variables and it demonstrated excellent predictive capacity with a concordance index of 0.942. Historically, preoperative prediction of POPF has hardly ever disqualified patients needing resection, or changed the course of postoperative care. The FRS, however, can be tabulated intraoperatively at the point of the creation of the pancreatic anastomosis and can therefore change the course of intraoperative decision making and even postoperative care.
As a part of the creation of the FRS, it was prospectively validated internally at the institution of origin. Since its introduction, the FRS has also been shown to correlate to complication severity using the Postoperative Morbidity Index (PMI).13,14 This correlation was seen both in terms of the overall PMI (including all postoperative complications) and the PMI specific to complications directly caused by the fistula. Although the FRS has already been internally validated and shown to predict morbidity in a quantified fashion, the current study seeks to externally validate this tool using a multi-institutional approach in order to confirm its universal applicability.
Methods
This study was conducted under Institutional Review Board (IRB) consent at the Hospital of the University of Pennsylvania (HUP). Four IRB‐approved prospectively accrued and retrospectively reviewed databases at HUP, the University of Alabama at Birmingham (UAB), and Baptist Memorial Hospital, and The University of Tennessee Health Science Center, Memphis (UTHSC) were used to identify patients for the study cohort. Between 2001 and 2012, four pancreatic surgical specialists (CMV, JAD, JDC, and SWB) performed PDs for a full spectrum of indications—all with a duct-to-mucosa pancreatojejunostomy reconstruction. No alternative methods of anastomosis were included in this study. One surgeon (CMV) participated in the original development of the FRS at another institution. Only operations performed in his current practice at HUP (2011–present) were included in this unique dataset.
Intraoperatively placed drains were situated in the proximity of the pancreatic anastomosis in most patients, and the drain amylase activity was tested at various points after postoperative day 3, both according to the individual surgeon's preferences. The highest value encountered served as the basis for defining the presence of a POPF (greater than 3x the normal serum amylase level) or not. All variables, including index length of stay as well as readmissions, overall complications, fistula-related complications, and mortality (all measured up to 90 days), were identified from these practice-centered databases. Although it was analyzed in the original paper, the current study was unable to assess the hospital costs at these particular institutions. Similarly, the individual surgeon's presumed preventative measures against fistulas (e.g., the use of anastomotic stents, prophylactic octreotide, and tissue sealants) were not specifically evaluated on a patient-by-patient basis in this analysis; however, general trends in use were discerned and are summarized in Table 1.
The four risk factors for CR-POPF development necessary to calculate the FRS (soft pancreatic parenchyma, increased blood loss, small duct size, and high-risk pathology) were identified from the operative notes of the attending surgeons at each institution. Each of the risk factors was assigned a discrete numerical value based on the odds ratios set forth in the original paper and summarized in Table 2.12 Two variables (gland texture and pathology) are dichotomous, while the weight of estimated blood loss escalates from 400 mL upward. Conversely, the risk impact of duct size is inversely proportional as the pancreatic duct diameter decreases from 5 mm. The duct size was measured intraoperatively by placing a flexible ruler against the face of the pancreatic transection plane. The gland texture was determined by the operating surgeon as either soft or firm and was not correlated to any histologic analysis. The individual FRS scores were then determined by summing the values for each risk factor for any patient. These values could escalate in risk from zero to ten. For more practical use, each individual FRS score fell into one of four risk zones as defined in the original paper; negligible risk (0 points), low risk (1–2 points), moderate risk (3–6 points), and high risk (7–10 points).12
ISGPF POPFs were identified by the required drain fluid amylase content greater than three times the normal limit (generally 300 IU/L) and calculated according to the standards set out by the Pancreas Club calculator for POPF.15,16 These fistulas were categorized into either “biochemical only” (grade A) or “clinically relevant” (grades B and C) groups. The patients who did not have a raised pancreatic drain amylase level were slated into the “no fistula” group. The severity of the fistula was based on the medical or surgical interventions used to treat the fistula until 90 days postoperatively.
Statistical analyses were performed using IBM Statistical Package for the Social Sciences, version 20 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were accrued and analyzed to determine appropriate rates of occurrence. All continuous variables were scrutinized using analysis of variance, Student's paired t tests, and linear regression. All tests were considered significant at p < 0.05.
Results
Over 11 years, 594 patients underwent PD with pancreatojejunostomy reconstruction at three institutions (N 1 = 306, N 2 = 152, and N 3 = 136). The mean age was 62.2 years and 49.2 % were male. One hundred forty-two patients developed any sort of POPF (23.9 %), of which, 68 were clinically relevant (11.4 % overall; 8.9 % grade B, 2.5 % grade C). Grade A fistulas, which have not previously been shown to be predicted by the FRS, occurred in 74 patients (12.4 % overall). A third of the patients had no complications (N = 194), and 258 suffered a complication not associated with a pancreatic fistula. When a CR-POPF occurred, antibiotics were used in 8.9 % of patients while supplemental nutrition and interventionally placed percutaneous catheters were each required 6.2 % of the time. A total of six out of 21 overall deaths (3.5 % overall 90-day mortality) were directly attributable to pancreatic fistula.
At least one risk factor for CR-POPF was present in 528 patients (88.9 %), while 67.3 % had multiple factors present. Malignancy or chronic pancreatitis was the indication for surgery in 52.7 % of the patients, leaving the additional 47.3 % as “high-risk” pathology (cystic neoplasms, ampullary cancer, neuroendocrine tumors, etc.). Roughly half the patients (N = 304) had a pancreas with soft parenchyma; conversely, fewer than 5 % suffered excessive blood loss (>1,000 mL). Additionally, the rate of soft gland texture increases as the pancreatic duct size diminishes (R 2 = 0.0851; Fig. 1). Table 3 compares features between this series and the original FRS developmental dataset and shows generally similar patient demographics, individual risk factor prevalence, and POPF occurrence between the two study cohorts.
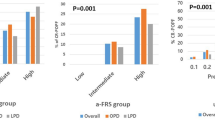
Figure 2 demonstrates the distribution of Fistula Risk Scores across this multi-institutional cohort. The most commonly encountered Fistula Risk Score was 2 (14.1 %), while the least frequent score was 10 (only a single patient). Most patients in the series segregated into the moderate-risk group N = 302 (51 %). The mean, median, and mode of the FRS were 3.54, 3, and 2, respectively, for this series, indicating that most patients harbor a relatively modest level of risk for CR-POPF development. Importantly though, increasing FRS scores (0–10) correlated well with CR-POPF development (p < 0.001) with a concordance index of 0.716. When segregated by defined FRS-risk groups, CR-POPFs occurred in 11 low-risk patients (6.6 %), 29 moderate-risk patients (12.9 %), and 18 high-risk patients (28.6 %) (R 2 = 0.944; Fig. 3). There were 63 patients (10.6 %) with an FRS of 0 (negligible risk); none of whom developed a CR-POPF. As suggested in the original paper, this series could not show that the FRS can distinguish grade B and grade C CR-POPFs. The proportion of grade C fistulas to all CR-POPFs remained fairly consistent, spanning from 18.1 to 25.6 to 16.7 % across the low-, moderate-, and high-risk FRS zones, respectively. In addition to CR-POPF increasing across risk zones, grade A fistulas showed the same trend (Table 4; p < 0.001 for both).
Table 4 shows that overall complication rates increased significantly through the risk zones. The median duration of hospital stay for the entire series was 9 days and increased steadily across risk zones (p < 0.001) to the point where high-risk patients stayed over a third longer (three extra days) than those with negligible risk. There were 106 readmissions in the entire series (17.8 %), and 33 readmissions in the cohort who developed any POPF (23.2 %), a majority of which (24 patients, 35.3 %) were in the group who suffered a CR-POPF, suggesting that pancreatic fistula is a significant driver of readmission following PD. Additionally, for the entire cohort, overall readmissions, as well as readmissions for the patients with POPFs, increased with escalating FRS-risk zones (Table 4, p < 0.001).
The overall C-statistic for the series was 0.716. Variability between the C-statistics of individual surgeons was seen in this series (surgeon 1, N = 306, C-statistic = 0.538; surgeon 2, N = 136, C-statistic = 0.836; surgeon 3, N = 125, C-statistic = 0.753; surgeon 4, N = 27, C-statistic not calculated due to a very low observed CR-POPF rate). Additionally, Table 5 shows that the average FRS vacillated between institutions (p < 0.001) as did the CR-POPF rate (p < 0.001). Similarly, differences in the median and mode FRS were also seen across institutions. These disparities are explained by the variability between the overall breakdown of the four component CR-POPF risk factors seen between institutions (gland texture, p = 0.008; pathology, p < 0.001; duct size, p < 0.001; estimated blood loss, p < 0.001; Table 5).
Discussion
Pancreatic fistula remains the most troublesome complication after pancreatectomy. These problems lead to increased clinical and financial burden on the patient and the medical systems overseeing their convalescence by adding to postoperative complication severity, overall duration of stay, readmissions, reoperations, and even demise.1–3,6–8 Despite the best efforts of surgeons and even with the improved consensus definition of POPF, clinically relevant fistula rates have thus far remained constant at around 15 % after PD.5 Callery et al. have devised an intraoperative prediction tool, the Fistula Risk Score, in order to assess the risk of developing this morbid problem after pancreatoduodenectomy.12 The FRS allows for preemptive intraoperative measures to potentially minimize development of pancreatic fistula and represents a shift away from reactive treatment after fistula occurrence and towards proactive prophylaxis. Although the FRS has already been internally validated in a prospective fashion, this current multi-institutional experience confirms that the Fistula Risk Score is a valid tool for predicting the development of CR-POPF in patients undergoing PD. It also reinforces that the FRS correlates strongly with adverse outcomes and resource utilization.
An important similarity to note between this and the original study is that patients devoid of any risk factors never developed a CR-POPF. This means that no patient in the negligible risk zone, in either study, suffered a clinically relevant fistula and suggests that this may be a targeted population who may not benefit from the placement of intraoperative drains near the pancreaticojejunal anastomosis. It should, however, be emphasized that the four risk factors portrayed in the FRS are not the sole elements of risk any patient may be faced with. This is why the lowest risk category has been termed “negligible” risk and not “no risk”. Obviously, conditions like steroid dependence, poor nutrition status, and impaired wound healing may contribute to anastomotic breakdown. It also cannot be stressed enough that impeccable surgical technique and attention to detail is prerequisite for a successful pancreaticoenteric reconstruction. Such factors were not adequately measurable to be included in the original risk assessment model used to derive the FRS. Additionally, although there is a strong correlation between small duct size and soft pancreas texture, it is not absolute. Figure 1 shows that while this correlation increases steadily from a duct size of at least 5 mm down to 2 mm, it actually decreases from 2 to 1 mm duct size. This is likely why these two variables were independently significant in the original odds ratios developed by Callery et al.12
Also similar to the original study, in the current analysis, the FRS was still unable to differentiate between the two types of CR-POPF (ISGPF grades B and C). However, the most important finding is that as the FRS risk zone increased, the rate of CR-POPF approximately doubled with each subsequent increment. This means a patient in the high-risk group was over four times as likely to develop a CR-POPF as a patient in the low-risk group. Using the information provided by the FRS, a surgeon can perhaps alter intraoperative techniques, such as type of anastomotic reconstruction, application of octreotide, drain usage, or other prophylactic techniques. This knowledge may also assist the surgeon's decision making in the postoperative recovery period. For instance, the timing of drain removal may be influenced; higher FRS scores could perhaps warrant a more cautious management approach, realizing they are more likely to incur a CR-POPF.
One discrepancy of note between the current and original studies is the lower concordance index that was found in this multicenter assessment (0.716 vs. 0.942). This means that for any given patient, the FRS had a 71.6 % chance of correctly predicting the occurrence of a CR-POPF. Although this is lower than the original study, it still indicates strong predictive accuracy. This becomes especially apparent when weighed against other acclaimed surgical prediction tools such as the MSKCC Pancreatic Adenocarcinoma Nomogram (C-statistic = 0.62) and the Gail Model for breast cancer (C-statistic = 0.58).17–19 The lower value of the C-statistic in this analysis is likely attributable to the considerably decreased rate of CR-POPF observed in the high-risk group compared to the original cohort (29 vs. 89 %). This difference might be due to characteristics of the patients, particular CR-POPF risk factor profiles, variations in management styles between surgeons, and a larger overall sample size in the current study. The individual concordance indexes of the institutions along with their sample size could lead to discrepancy based on the surgical techniques and practices of the institution. Although two of the four surgeons fit the model very well, the third and largest single contributor to the study did not show much concordance while a fourth surgeon did not have enough patients with CR-POPFs (only a single incidence of CR-POPF) to be statistically analyzed. Additionally, the difference in risk factors for CR-POPF correlated with the CR-POPF rate at each institution, reinforcing that these risk factors play a role in fistula development. The surgeon with the lowest overall risk profile was the one who did not fit the model as well, suggesting that because of the low risk, fistulas were not often developed.
Although grade A (biochemical) fistulas have historically been considered innocuous, Miller et al. have shown that they are not as harmless as originally believed.10 These regular events (12.4 % in this series) have the capacity to carry some burden. The current analysis shows that the four risk factors for CR-POPF also forecast biochemical grade A fistulas, an outcome not seen in the original cohort. Additionally, the prediction of non-fistulous complications escalates with the risk zones of the FRS in this analysis. This shows that the risk factors that comprise the FRS also provide risk for other potentially harmful complications contributing to the overall morbidity after PD.
This study has several limitations. First, the data was accrued from three separate individual practice databases by various research associates. Although all variables were verified by the attending surgeon, some inconsistencies between accrual processes may have occurred. The surgeons included in this analysis are all surgical specialists who practice pancreas surgery almost exclusively at high-volume specialty centers, so the predictive capacity of the FRS has not been confirmed in the setting of lower-volume pancreatic surgeons or institutions. Additionally, as discussed above, the patient populations and fistula mitigation approaches varied between surgeons (Table 1), although it is thought that these differences are representative of expected differences between practices and show a realistic idea of the variation that may affect applicability of the FRS. A proper mathematical analysis of variance of fistula risk and actual fistula occurrence is not possible with this particular limited scope of surgeons and institutions. However, we have recently initiated such an investigation to better understand the potential variability of fistula risk as well as actual POPF occurrence. Finally, the three institutions were only asked to fill in a template of a limited scope of predetermined variables so not all outcomes (including the economic evaluation performed in the original study) were available for analysis. However, the variables that were accrued are thought to be adequate and representative of the major outcomes that determine the morbidity of the postoperative course following PD.
Conclusion
The FRS is further validated as a predictive tool with widespread applicability that can be readily adopted into the practice of pancreatic surgical specialists. Since it is predicated on variables best discovered at the time of operation, the FRS can be used to tailor operative technique and postoperative management of patients, depending on risk factors encountered. Depending on the risk strata the patient falls into, a surgeon may choose to alter the anastomotic technique, forgo the anastomosis completely, apply anastomotic stents, use prophylactic octreotide perioperatively, or employ any other approach that may be of value based on either best-available evidence or the individual surgeon's historical experience. These management tools can also help surgeons either minimize unnecessary and invasive precautions in patients with low risk or help minimize the menace of fistula in high-risk patients. Finally, the FRS may have a role by offering a means of risk stratification for future clinical research analyses and trials.20
References
van Berge Henegouwen MI, De Wit LT, Van Gulik TM, Obertop H, Gouma DJ. Incidence, risk factors, and treatment of pancreatic leakage after pancreaticoduodenectomy: drainage versus resection of the pancreatic remnant. J Am Coll Surg. 1997; 185:18–24.
Gouma DJ, van Geenen RC, van Gulik TM, de Haan RJ, de Wit LT, Busch OR, Obertop H. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000; 232:786–795.
Bassi C, Butturini G, Molinari E, Mascetta G, Salvia R, Falconi M, Gumbs A, Pederzoli P. Pancreatic fistula rate after pancreatic resection: the importance of definitions. Dig Surg. 2004; 21:54–59.
Hackert T, Werner J, Buchler M. Postoperative pancreatic fistula. Surgeon. 2011; 9(4):211–17
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005; 138:8–13.
Pratt WB, Callery MP, Vollmer CM. Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg 2008; 32:419–428.
Callery MP, Pratt WB, Vollmer CM. Prevention and management of pancreatic fistula. J Gastrointest Surg 2009; 13:163–173.
Ansorge C, Strommer L, Andren-Sandberg A, Lundell L, Herrington M K, Segersvard R. Structured intraoperative assessment of pancreatic gland characteristics in predicting complications after pancreaticoduodenectomy. British Journal of Surgery. 2012; 99:1076–1082.
Wellner U, Kayser G, Lapshyn H, Sick O, Makewiec F, Hoppner J, Hopt U, Keck T. A simple scoring system based on clinical factors related to pancreatic texture predicts postoperative pancreatic fistula preoperatively. HPB. 2010; 12(10):696–702
Muscari F, Suc B, Kirzin S, Hay J, Fourtanier G, Fingerhut A, Sastre B, Chipponi J, Fagniez P, Radovanovic A, French associations for surgical research. Risk factors for mortality and intraabdominal complications after pancreatoduodenectomy: multivariate analysis in 300 patients. Surgery. 2006; 139(5):591–9
Hashimoto Y, Sclabas G, Takahashi N, Kirihara Y, Smyrk T, Huebner M, Farnell M. Dual-phase computed tomography for assessment of pancreatic fibrosis and anastomotic failure risk following pancreatoduodenectomy. JOGS. 2011; 15(12):2193–204.
Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM. A prospectively validated risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013; 216:1–14.
Strasberg SM, Hall BL. Postoperative morbidity index: a quantitative measure of severity of postoperative complications. J Am Coll Surg. 2011; 213:616–626.
Miller B, Christein D, Behrman S, Callery M, Drebin J, Kent T, Pratt W, Lewis R, Vollmer C. Assessing the impact of fistula after pancreaticoduodenectomy using the postoperative morbidity index. HPB. 2013. doi:10.1111/hpb.12131
The Pancreas Club. ISGPS calculator—The pancreas club. 2012 Available from: http://pancreasclub.com/calculators/isgps-calculator/
Hashimoto Y, Traverso L. Incidence of pancreatic anastomotic failure and delayed gastric emptying after pancreatoduodenectomy in 507 consecutive patients: use of a web-based calculator to improve homogeneity of definition. Surgery. 2010; 147(4):503–15
Ferrone C, Kattan M, Tomlinson J, Thayer S, Brennan M, Warshaw A. Validation of a postresection pancreatic adenocarcinoma nomogram for disease-specific survival. J Clin Oncol. 2005; 23:7529–7535
Brennan M, Kattan M, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004; 240(2):293–8
Rockhill B, Spiegelman D, Byrne C, Hunter D, Colditz G. Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J Natl Cancer Inst. 2001; 93:358–366
Sachs T, Pratt W, Kent T, Callery M, Vollmer C. The pancreaticojejunal stent: Friend or foe. Surgery. 2013; 153(5):651–62
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussant
Dr. David Mahvi (Chicago, IL): First, I want to congratulate Dr. Miller on a great presentation and a very nice study. As Dr. Mathews noted Sunday in his presidential address, many seemingly important studies are never validated. This study validates the previous work from the pancreatic group at BI Deaconess.
To put this in context: the standardization of nomenclature relative to this complication by Mike Saar and Bill Traverso has allowed everyone to speak the same language. Leaks rarely lead to mortality but clearly impact quality of life in the perioperative period. The impact of leak has been extensively studied and presented at DDW by the group at BI Deaconness.
I have two questions:
1. So what? Why is this information helpful? Are you prepared to NOT resect patients with a high risk of a leak. Assuming you are going to proceed with resection: How do you use this data in preoperative decision making?
2. Was there variability between surgeons?
3. It seemed like the site with the softest glands had the lowest fistula rate. How do you explain this discrepancy?
I very much enjoyed your presentation.
Closing Discussant
Dr. Benjamin Miller:
1. The FRS is not meant as a means of disqualifying a patient from resection. Although some of these variables (i.e., duct size and pathology) can be identified in a preoperative setting, they are almost accurately determined intraoperatively. As such, the FRS is really meant as a tool that surgeons can use intra- and postoperatively to mitigate fistula risk in whatever fashion they deem appropriate. This approach may include modifications of drain use and duration, anastomosis type chosen, stent employment, or the use of octreotide to name a few. Such decisions may be based on the surgeon's interpretation of evidence from the literature or rather their own experience with risky scenarios.
2. We did see some variability between surgeons in this study, but three surgeons (the sample size from one was too small to be analyzed) are not large enough sample to make conclusions about the meaning of this variability. For this reason, a multi-institutional study dedicated to variability of the FRS and actual fistula occurrence has been initiated on a much larger scale.
3. This discrepancy could be due to a number of different factors. Potentially, the surgeon could already be performing optimal management techniques to diminish fistula risk, or the resection could be performed with a technique that compensates for this soft gland. Additionally, although the gland was soft, this result further shows that the other factors that contribute to the FRS add significantly to later fistula development. That is to say, although the gland risk was high, other factors may have been lower to balance the risk. The importance of this work is to demonstrate that there is more to the risk equation than the presence of a single risk factor in isolation.
The original article was submitted after oral presentation at the SSAT 5/20/2013 and The Pancreas Club 5/18/13
Rights and permissions
About this article
Cite this article
Miller, B.C., Christein, J.D., Behrman, S.W. et al. A Multi-Institutional External Validation of the Fistula Risk Score for Pancreatoduodenectomy. J Gastrointest Surg 18, 172–180 (2014). https://doi.org/10.1007/s11605-013-2337-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-013-2337-8