Abstract
Background
Muscle splitting augmentation mammoplasty is the creation of a submuscular pocket which is gaining attention and acceptance by plastic surgeons worldwide. First introduced in 2007, muscle splitting augmentation mammoplasty has since been used for primary and secondary augmentation mammoplasty and augmentation mastopexy procedures. A personal experience of revision surgeries following muscle splitting augmentation mammoplasty is presented.
Methods
A retrospective data analysis for revision surgeries, following muscle splitting primary augmentation mammoplasties, performed between October 2005 and October 2018 was carried out.
Results
A total of 1511 primary augmentation mammoplasties were performed. Of these 1511 patients, 93 (6.1%) patients had revisionary or secondary surgery. The mean age of the patients was 33.8 + 9 years (range 20–60). Of the 93 patients, 78 patients had same size implants, mean 337 cc + 53.5 (range 230–495), and 14 had different size implants. Of these 14 patients, mean implant size on right and left was 331 cc + 59.4 (range 225–425) and 351 cc + 61.7 (range 260–450), respectively. Of the recorded texturing in 1495 patients, only 3.1% had smooth implants. Leading causes for revision were implant exchange for various reasons, in 33 (35.4%); 25 (26.8%) wanted larger implants, revisionary surgery for capsular contracture in 18 (19.3%), implant rupture was seen in 9 (9.6%), 4 (4.3%) patients had surgery for recurrent back-to-front flipping, 2 (2.1%) patients wanted a smaller size, 1 (1.07%) patient had fold flaw failure, and in 1 (1.07%) the cause was not recorded. There were no haematoma and breast implant-associated anaplastic large cell lymphoma (BIA ALCL) recorded in the series.
Conclusion
The incidence of revisionary surgery following muscle splitting primary augmentation mammoplasty is acceptable and can be corrected using the described techniques.
Level of Evidence IV
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary augmentation mammoplasty in muscle splitting biplane is a partial submuscular pocket where an implant is placed behind and in front of the pectoralis major at the same time (Figs. 1, 2, 3) [1]. It is an extension of partial submuscular augmentation as the implant is not covered completely by the pectoralis (Figs. 1b, 2d, 3a) [2]. The concept allows better lower pole definition and closer breast cleavage without lateral displacement of the implant as the device gets locked at the lateral end of the intact pectoralis (Figs. 1b, 2c, 3a) [1,2,3]. The procedure has been used for primary [1, 4,5,6,7,8] and secondary procedures [9,10,11,12] including correction of animation or dynamic deformity [1, 3, 13,14,15] commonly seen following partial submuscular or its popular extension dual plane pocket (Fig. 3b) [16, 17]. The procedure is reproducible with longevity of results and acceptably low revision rate [3, 18,19,20] with no anatomical disruption of muscle activity or risk to its nerve supply [1, 3, 18, 21]. However, there is no detailed account, appraisal or nature of revision surgeries arising from the primary augmentation mammoplasties in muscle splitting pocket. As such, this is the first article, in which the number, distribution and nature of the revisionary surgeries are documented since the pocket was first described in 2007 [1].
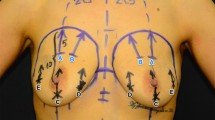
a A diagrammatic and anatomical illustration of pectoralis major origin and extent along with the level of muscle split. The muscle split starts at the level of the middle and lower third of the sternum, outlined by blue dots. b Schematic illustration of an implant in muscle splitting biplane showing upper pectoralis covering the implant while lower pectoralis lies behind the implant
a Intraoperative picture showing right split upper and lower pink and healthy pectoralis major held with silk sutures in a patient for implant replacement, who had her breast augmentation in muscle splitting biplane 12 years ago. b Silk sutures have been pulled apart to show lower subglandular pocket communicating through the split with submuscular space. Fourth rib and third intercostal space are clearly visible under the pectoralis major through an inframammary incision. c A schematic intraoperative drawing of muscle splitting pocket seen through inframammary incision marked in yellow outline as rectangle. For the sake of simplicity and understanding of the dissected pocket anatomy, total extent of pectoralis major split is shown from the junction of the lower and middle third of the sternum up to its joined lateral end in bright red. Lower subglandular pocket is communicating with the upper submuscular pocket through the gap in the split muscle, separated in the drawing by forceps below and breast retractor above, respectively. d Smooth cohesive gel silicone implant in place through the split pectoralis. Lower split pectoralis is visible through the implant with the silk suture in place to upper border of lower split pectoralis. The silk suture in the lower border of the upper split pectoralis delineates upper submuscular pocket from the lower subglandular pocket
a Pectoralis major relationship to implant in muscle split technique as seen in front view. Upper split pectoralis major lies in front and lower split pectoralis major lies behind the implant. b An illustration showing implant in dual plane. Pectoralis major muscle in dual plane lies completely in front of the implant as shown in front view
Materials and Methods
A retrospective data analysis for revision surgeries following muscle splitting primary augmentation mammoplasties performed between October 2005 and October 2018 was carried out.
Methods
All examinations, measurements, planning, surgeries and follow-ups were done by the author. All patients were marked preoperatively in the standing position, according to the procedure planned. Patients were operated under general anaesthesia with full muscle relaxation and arms extended less than 90°. The inframammary incision is generally used for revisionary surgery excluding those requiring mastopexy. All patients had nipple shields to minimize incision site contamination. Double gloves were used routinely of which the outer layer was changed before device handling; perioperative intravenous antibiotics were given and pockets irrigated with diluted povidone iodine before the insertion of devices. All procedures are performed as a day procedure, and drains are not used as a routine unless necessary. Pocket adjustments, using capsulotomies or capsulorrhaphies, are performed where necessary and to accommodate a smaller or larger implant. Also muscle splitting is extended, where necessary, to accommodate a larger implant diameter. Patients are followed for at least 2 years at 2 weekly, 3 weekly, 3 monthly and 6 monthly intervals.
Statistical Analysis
The data were analysed using the Statistical Package for the Social Sciences (SPSS), version 19.0. The results are presented in the text as frequency, percentage for qualitative/categorical variable (difference in implant size) and mean ± S.D for quantitative/continuous variables (age and implant size). The Chi-square test is used to compare the categorical variable, and t test for quantitative/continuous variables. In all statistical analyses, only p values < 0.05 are considered significant.
Results
A total of 1511 primary augmentation mammoplasties were performed by the author during the period specified. Of these 1511 patients, 93 (6.1%) had their revisionary or secondary surgery following primary augmentation mammoplasty in the muscle splitting pocket. The mean age of the patients was 33.8 ± 9 years (range 20–60). Of the 93 patients, 78 patients had the same size implants (mean 337 cc ± 53.5, range 230–495) and 14 had different size implants. Of these 14 patients, mean implant size on the right and left was 331 cc ± 59.4 (range 225–425) and 351 cc ± 61.7 (range 260–450), respectively (Table 1). Of the recorded texturing in 1495 patients, only 3.1% had smooth implants. All revisionary surgeries involved those patients who had textured implants. The leading causes for revision were implant exchange for various reasons and wanting larger implants in 33 (35.4%), 25 (26.8%) wanted to go for bigger implants and had no other associated problems, capsular contracture was seen in 18 (19.3%), implant rupture in 9 (9.6%), 4 (4.3%) patients had surgery for recurrent back-to-front flipping, 2 (2.1%) patients requested to smaller implants, 1 (1.07%) patient had fold flaw failure, and in 1 (1.07%) patient, the cause was not recorded (Table 2). Periprosthetic infection and wound healing issues were seen in 1 (1.1%) patient, and 5 patients presented with late seroma. No haematoma, breast implant-associated anaplastic large cell lymphoma (BIA ALCL) were recorded in the series.
Discussion
The quest for an ideal breast implant pocket is far from over. Various implant pockets and techniques have been described so far, but essentially, the pectoralis major remains the anatomical divide for all the techniques described so far, that is, prepectoral or subpectoral. An ideal breast pocket to enhance a breast should ideally be prepectoral, where the anatomical position of the breast gland is located. However, changes related to ageing, pregnancy, breast-feeding and weight loss bring a discord between the ever-changing breast gland and the permanent physical nature of the breast implants currently in use. The harmony between the filler and the target gland is likely to dissociate over a period of time and eventually break down in the majority of cases. A submuscular position of the implant, even though aesthetically less rewarding, does provide stability to the results and extends support to the ever-changing physical characteristics of the breast tissue. To improve the aesthetic appearance, to enhance the three-dimensional outcome of the breast and to bring harmony between the permanent nature of the device and often ever-changing breast tissue, various modifications have been introduced. Two such recent modifications are the dual plane pocket and subfascial techniques [16, 22]. Dual plane is a modified partial submuscular and subfascial being an extension of the subglandular technique, where pectoral fascia is incorporated into the breast gland. However, when the dual plane is used, animation or dynamic deformity can be distressing with high incidence [17, 18, 23,24,25] and may require repositioning of the implant in the prepectoral location [26] or conversion of the dual plane or partial submuscular plane to a muscle splitting pocket [9, 13,14,15, 23, 25]. On the other hand, the longevity of the subfascial pocket has not shown much advantage over a simple submammary pocket [27]. The muscle splitting pocket, another modification of partial submuscular, is unique in that the implant lies in front of and behind the pectoralis at the same time and a three-dimensional result is achieved without releasing the muscle; hence, there is no animation or dynamic distortion of the breast (Figs. 1a, 2b, 3a). The implant placement in this pocket gives a natural close cleavage due to the commencement of split above the cleavage hindering costal margin of the pectoralis and the locking effect of the device at the un-split lateral end of the pectoralis (Fig. 4a–f). The locking effect is produced when an implant is placed after splitting the muscle, a part of the muscle is over and a part of it behind the implant; however, the two split ends join laterally where the locking effect is created (Figs. 1a–b, 2c, 3a). The superior pole of the submuscular implant is prevented from being displaced or slipping out laterally, where the two ends of the split pectoralis meet together (Figs. 1b, 2c, 3a). Similarly, the lateral subglandular part of the implant, extending beyond and laterally to the confluence of the split pectoralis, disallows the implant to displace superiorly, and hence, no high riding breasts are seen or special bands or dressings are required to keep the implants down (Figs. 1b, 3a). The revision rate following muscle splitting augmentation mammoplasty was significantly lower when compared with submammary and partial submuscular pocket. In a large series with a follow-up of 6 years in subglandular, partial submuscular and muscle splitting pockets, the revision rate was reduced from 7.3% in submuscular and 6.0% in subglandular to 1.2% in muscle splitting pocket, respectively [3]. The longevity of the results in muscle splitting pocket has been documented [18], making the technique a useful option for augmentation mammoplasty.
a–c Preoperative views of a 32-year-old patient who had her augmentation mammoplasty 4 years ago. She had 340 cc cohesive gel silicone implants in the muscle splitting biplane pocket. She was not happy with her cup size and wanted to go larger. d–f Postoperative views showing results following revision surgery using 560 cc microtextured implants in muscle splitting biplane pocket
The muscle splitting pocket, however, is not devoid or free of complications or revision surgeries. Surprisingly, the largest proportion of the revision surgeries (35.4%) comprised those patients who wanted to replace implants before the expiry of its presumed life expectancy of 10 years. Not surprising, most of these patients wished for larger implants and some required treatment for associated breast changes like ptosis requiring mastopexy [4, 6], sliding ptosis requiring internal lift with device size increment [5, 28], improvement of cleavage using multilayer lateral capsulorrhaphy or higher device repositioning requiring inframammary crease relocation using multilayer capsulorrhaphy [11]. Requests for smaller size implants were not frequently made. Smallest size implants used in revisionary surgery in the series were 230 cc. In such a scenario and to match the breast footprint, exchanging a high profile implant for a lower profile is extremely important. These patients may require lateral capsulorrhaphies and/or inframammary crease relocation to reduce the dimensions of the pocket and minimize skin excess issues. In some cases, mastopexy is strongly advised if the patient decides to have a significant drop in implant size or requests explanation altogether.
The second most common reason for surgery was a request for larger implants with no other associated problems (Fig. 4a–f). A good process of consultation, adequate information and simulation of breast cup size by preoperative trial of implants in a bra may reduce an early 3-year reoperation rate, but the long-term results in this study have shown that reoperation for bigger size, standing at 26.8%, constituted a large proportion of revision surgeries [18, 29, 30]. Two of the patients who requested a larger size and chose 800 cc implants had their implant pockets changed from muscle splitting to subglandular pocket in order not to disturb the medial and lateral pectoral nerves supplying the pectoralis major [21].
The incidence of capsular contracture and its treatment, with an incidence of 19.3% in this series, was the largest clinical reason for reoperation. Capsular contractures are treated according to the degree and symptoms at the time of presentation (Fig. 5a–f). In grade four capsular contractures, total or near total capsulectomies are performed, whereas in grade two and three capsular contractures, capsulotomies are performed. Capsulotomies are also performed in patients requiring larger implants, to accommodate the larger size implant [31].
a–c Preoperative pictures of a 40-year-old patient, who had her augmentation mammoplasty 13 years ago with 350 cc microtextured cohesive gel silicone implants in the muscle splitting biplane pocket. She had a little asymmetry, grade III capsular contracture with left implant sitting a little higher and wanted to go bigger. d–f Postoperative views 1 month following implant exchanged with 500 cc smooth cohesive gel silicone implants placed in the muscle splitting biplane pocket
Implant rupture (9.6%) was the fourth largest group and formed a significant portion of revision surgeries in the current series.
The overall revisionary rate documented in this 13-year follow-up of 1511 mammoplasties stands at an acceptable 6.1%. In another recently published article, timings for these revisions were reported [18]. The mean times for surgeries for larger implants, for capsular contracture, implant rupture and to exchange implants were 2.7 years, 4.5 years, 6.2 years and 7.9 years, respectively. The average timing for revisionary surgeries suggests that the request time for larger implants (26.8%) was shortest, at 2.7 years, within the patients who had revisionary surgery. Surgeries performed for implant rupture constituted 9.6% of the revisionary surgery with an average time of 6.2 years following their first surgery. Most of the implant ruptures were silent, but some were symptomatic with loss of breast shape (Fig. 6a–f). Implant exchange (35.4%) for all reasons took an average time of 7.9 years (Fig. 7a–f). The mean time for capsular contracture surgery (19.3%) was 4.5 years (Table 2). Back-to-front flipping of implants resulting in flattening of the breast is not a well-recognized or reported complication. The condition was seen when microtextured, high profile, low gel fill ratio implants flipped 180° with the flat base lying on the front and round front facing backwards (Table 2) [32]. The flipping can be corrected using outpatient-based external manipulation; however, recurrence of the condition may require surgical intervention and was carried out in 4 patients in the series [32].
a–c Preoperative views of a 35-year-old patient, who had her augmentation mammoplasty 8 years ago. She had 340 cc smooth cohesive gel silicone implants placed in the muscle splitting biplane pocket. She noticed a change of shape of her left breast and had an ultrasound imaging. Her left implant was found ruptured. d–f Postoperative views 6 weeks following revision augmentation mammoplasty in the muscle splitting biplane pocket, using 375 cc round textured cohesive gel silicone implants
a–c Preoperative views of a 37-year-old patient who had her augmentation mammoplasty 10 years ago using 260 cc and 300 cc round microtextured cohesive gel silicone implants in the muscle splitting biplane pocket on her left and right side, respectively. The patient had a little discomfort on her left side, wanted to replace old implants and go larger as well. d–f Postoperative views taken 3 months following revision surgery. She had 340 cc on her right and 375 cc on her left, textured cohesive gel silicone implants, in the muscle splitting biplane pocket
The patients, who developed periprosthetic infections, had their implant removed and replaced after 6 months as a routine [33]. The surgery was not included as revisionary surgery as it was not related to the technique. The patients with wound breakdown were treated conservatively, and all experienced full recovery. Textured implants were involved in all five patients who developed late seroma, all of whom had appropriate investigations and none of whom showed CD 30 on ultrasound-controlled aspiration and immunohistochemistry [18]. No BIA ALCL was reported in the series.
Strength and Weaknesses of the Study
The current study is based upon a large number of augmentation mammoplasties consulted, examined, performed and followed up by a single surgeon. Even though a large number of the patients were followed up for a good period of time, the data on the majority of patients were not available for a more reliable or accurate conclusion, especially when the possibility of complications like capsular contracture and implant rupture is known to occur most. These difficulties are commonly experienced in other long-term follow-ups and are largely due to the patients moving to other areas, not coming for follow-up if all is well or possibly going to other surgeons for complications and further treatment.
Conclusion
The incidence for revisionary surgery following muscle splitting primary augmentation mammoplasty is acceptable and can be corrected using the described techniques.
References
Khan UD (2007) Muscle splitting breast augmentation: a new pocket in a different plane. Aesthet Plast Surg 31:553–558
Regnault P (1977) Partially submuscular breast augmentation. Plast Reconstr Surg 59:72
Khan UD (2013) Muscle splitting, subglandular and partial submuscular augmentation mammoplasties: a 12 year retrospective analysis of 2026 primary cases. Aesthet Plast Surg 37(2):290–302
Khan UD (2010) Augmentation mastopexy in muscle-splitting biplane: an outcome of first 44 consecutive cases of mastopexies in a new pocket. Aesthet Plast Surg 34:313–321
Khan UD (2011) Multiplane technique for simultaneous submuscular breast augmentation and internal glandulopexy using inframammary crease in selected patients with early ptosis. Eur J Plast Surg 34:337–343
Khan UD (2018) One-stage mastopexy and augmentation mammoplasty in layers: outcome analysis of first 50 consecutive cases. Plast Aesthet Res 5:45
Stodell M, McArthur G, James M (2016) Bi-plane breast augmentation: a case series supporting its use and benefits. Plast Aesthet Res 3:17–20
Stumpfle RL, Pereira-Lima LF, Valiati AA, Da Mazzini GS (2012) Transaxillary muscle splitting breast augmentation: experience with 160 cases. Aesthet Plast Surg 36:343–348
Baxter RA (2005) Subfascial breast augmentation: theme and variation. Aesthet Surg J 25:447–453
Khan UD (2009) Acquired synmastia following subglandular mammoplasty and the use of submuscular splitting biplane for its correction. Aesthet Plast Surg 33:605–610
Khan UD (2010) Combining muscle splitting biplane with multilayer capsuloraphy for the correction of bottoming down following subglandular augmentation. Eur J Plast Surg 33:259–269
Khan UD (2015) Subglandular to muscle splitting biplane conversion for revision augmentation mammoplasty. In: Mugea TT, Shiffman MA (eds) Aesthetic surgery of breast, 1st edn. Springer, Berlin, pp 535–541
Baxter RA (2011) Update on the split-muscle technique for breast augmentation: prevention and correction of animation distortion and double bubble deformity. Aesthet Plast Surg 33:353–360
Khan UD (2009) Dynamic breasts: a common complication following partial submuscular augmentation and its correction using muscle splitting biplane technique. Aesthet Plast Surg 33:353–360
Khan UD (2012) High transverse capsuloplasty for the correction of malpositioned implants following augmentation mammoplasty in partial submuscular plane. Aesthet Plast Surg 36:590–599
Tebbet JB (2001) Dual-plane breast augmentation: optimizing implant-soft tissue relationship in a wide range of breast types. Plast Reconstr Surg 107:1255
Spear SL, Scwartz J, Dayan JH et al (2009) Outcome assessment of breast distortion following submuscular breast augmentation. Aesthet Plast Surg 33:44–48
Khan UD (2019) Muscle splitting augmentation mammoplasty: a 13-year outcome analysis of 1511 primary augmentation mammoplasties. Aesthet Plast Surg. https://doi.org/10.1007/s00266-019-01468-5
Khan UD (2016) A long term review of augmentation mastopexy in muscle splitting biplane. Plast Aesthet Res 3:21–25
Khan UD (2016) Augmentation mastopexy and augmentation mammoplasty: an analysis of 1,406 consecutive cases. Plast Aesthet Res 3:26–30
Saleh DB, Callear J, Riaz M (2016) An anatomic appraisal of biplane muscle-splitting breast augmentation. Aesthet Surg J 36(9):1019–1025
Graf RM, Bernardes A, Rippel R et al (2003) Subfascial breast implant: a new procedure. Plast Reconstr Surg 111:904–908
Nigro LC, Blanchet NP (2017) Animation deformity in postmastectomy implant based reconstruction. Plast Reconstr Surg Glob Open 5:e1407
Dyrberg DL, Camilla B, Gunnarsson GL et al (2019) Breast animation deformity. Arch Plast Surg 46:7–15
Alnaif N, Safran T, Alex Viezel-Mathieu, Alhalabi B, Dionisopoulos T (2019) Treatment of breast animation deformity: a systematic review. J Plast Reconstr Aesthet Surg 72:781–788
Gabriel A, Sigalove S, Sigalove NM et al (2018) Prepectoral revision breast reconstruction for treatment of implant-associated animation deformity: a review of 102 reconstructions. Aesthet Surg J 38(5):519–526
Brown T (2012) Subfascial breast augmentation: is there any advantage over the submammary plane? Aesthetic Plast Surg 36(3):566–569
Khan UD, Riaz M (2015) Use of multiplane internal mastopexy for ptosis correction revision augmentation mammoplasty. Plast Aesthet Res 2:120–126
Khan UD (2013) The impact of preoperative breast implant selection on the 3-year reoperation rate. Eur J Plast Surg 36:503–510
Tebbets JB (2006) Achieving a zero percent reoperation rate at 3 years in a 50-consecutive case augmentation mammoplasty premarket study. Plast Reconstr Surg 118:1453–1457
Khan UD (2017) Low risk primary augmentation mammoplasty and capsular contracture using textured round cohesive silicone gel implants revisited. A long term follow up in a single surgeon’s practice. Pak J Plast Surg 5:6–19
Khan UD (2011) Back to front flipping of implants following augmentation mammoplasty and the role of physical characteristics in a round cohesive gel silicone breast implant. retrospective analysis of 3458 breast implants by a single surgeon. Aesthet Plast Surg 35:125–128
Khan UD (2010) Breast augmentation, antibiotic prophylaxis and infection: comparative analysis of 1628 primary augmentation mammoplasties to assess the role and efficacy of length of antibiotic prophylaxis. Aesthet Plast Surg 34:42–47
Funding
Author has not received research funding for this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interests.
Ethical Approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individuals participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, U.D. Revisionary Surgery Following Primary Augmentation Mammoplasty in Muscle Splitting Biplane Pocket: An Appraisal of 93 Revisionary Surgeries. Aesth Plast Surg 45, 462–471 (2021). https://doi.org/10.1007/s00266-019-01580-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-019-01580-6