Abstract
Background
Augmentation mammoplasty is a commonly performed procedure by plastic surgeons with a high satisfaction outcome. Muscle splitting augmentation mammoplasty was first described in 2007 and has been since used for primary and secondary augmentation mammoplasty as well as for primary and secondary augmentation mastopexy procedures.
Methods
A retrospective analysis of data for muscle splitting primary augmentation mammoplasties performed between October 2005 and October 2018 was carried out.
Results
A total of 1511 patients had their primary augmentation mammoplasty consecutively performed in muscle splitting pocket. Mean age of the patient was 29.4 ± 8.56 years (range 18–67). Of the 1502 patients with documented implant sizes, 1272 patients had same-size implants, mean 340 cc ± 58.3 (range 170–700), and 230 patients had two different-size implants for correction of asymmetry. Of these 230 patients, mean implant size on right and left was 341 cc ± 61.5 (range 200–655) and 345 cc ± 67.4 (range 200–605), respectively. Of the 1495 known texturing, only 3.1% patients had smooth implants. Periprosthetic infection was seen in 10 patients, 38 patients had wound-healing issues and 5 patients had late seroma. Capsular contracture (CC) was recorded at three monthly, six monthly, one yearly and two yearly or longer period. Secondary procedures were performed for various reasons in 93 (6.15%) of the patients. Leading causes for revision were implant exchange in 33 (2.2%), to go for bigger size in 25 (1.65%), CC in 18 (1.2%) and implant rupture in 9 (0.6%). There was no ALCL recorded in the series.
Conclusion
Muscle splitting pocket for primary augmentation mammoplasty is a reliable, reproducible procedure with acceptable revision rate.
Level of Evidence IV
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authorswww.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary augmentation mammoplasty, using breast implants, is a commonly performed procedure by plastic surgeons. Since the report of the use of the first breast implant in 1964 in a subglandular pocket [1], the plane for breast implants has been primarily referred to by its position in relation to the pectoralis major (PM) muscle. Regardless of the name given to the technique, a breast implant almost lies in front of or behind the PM with the exception of the muscle splitting biplane pocket where the implant lies in front of and behind the pectoralis at the same time. The concept of muscle splitting technique in combination with subfascial augmentation was first given by Baxter for the correction of dynamic deformity or its selective use in primary subfascial augmentation in 2005 [2]. The muscle was split at varying levels and was used in conjunction with the subfascial technique [2, 3]. The muscle splitting biplane technique was fully described later as a separate technique with well-defined anatomical landmarks and was reported for its use in primary augmentation mammoplasty in 2007 [4]. The technique and the concept have been extended for their use in other implant-related primary procedures such as mastopexy with augmentation [5, 6] and multiplane internal mastopexy with augmentation [7]. The technique also has been reported for secondary corrective procedures following subglandular and submuscular augmentation mammoplasties [8,9,10,11,12]. The technique has been used and reported by various other authors, for its use in primary as well as secondary procedures [13,14,15,16,17]. Follow-up and review articles of muscle splitting biplane technique have been frequently reported and published at regular intervals and have shown acceptable results and outcomes [18,19,20]. The current article is a 13-year data analysis of 1511 consecutive muscle splitting biplane primary augmentation mammoplasties carried out by a single surgeon.
Materials and Methods
A retrospective analysis of data for muscle splitting primary augmentation mammoplasties performed between October 2005 and October 2018 was carried out.
Surgical Procedure
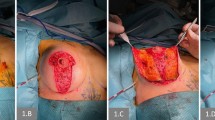
All patients had their procedures done using general anaesthetics with complete muscle relaxation as a day case, and all patients had perioperative intravenous antibiotics. Arms were placed and supported on an arm board at an angle less than 90°. A line is marked from mid-suprasternal notch to xiphoid process. A pocket is approached through an inframammary incision 5 cm wide and starting about 6 cm from midline. With nipple as the central point, a 2.5–3-cm-wide cleavage is marked bilaterally as the medial boundary. Measurements from the nipple to marked medial boundary are taken and transposed laterally as the lateral margin of the pocket. Lateral boundary of the pocket is limited by anterior axillary line, and implant base is always selected corresponding to the footprint of the breast. Vertical boundary is normally about 14.5 to 15 cm measured from inframammary incision upwards. Medial and lateral boundaries are joined together in a circumferential manner with special reference to inframammary crease. All patients have their nipple covered using a transparent sticky dressing. Subglandular pocket is created using cutting diathermy, extending from the junction of the middle and lower third of the sternum to the anterior axillary fold and crossing just below the level of nipple-areolar complex (NAC). Muscle splitting is commenced from the junction of middle and lower third and extends up and laterally to the anterior axillary point and crossing just below the NAC (Fig. 1a, b). Lateral third of the muscle split is done slowly using electro-cautery on coagulation mode as branches of thoracoacromial axis are often encountered. Irrigation of the pocket is performed using saline and diluted povidone-iodine. Implants are placed in the pocket, haemostasis rechecked before closure. Wounds are closed in three layers using 2–0, 3–0 and 4–0 absorbable sutures. Light adhesive dressings are placed, and a support brassiere is put on. Patients are discharged 3–4 h post-operatively with a supply of oral antibiotics (co-amoxiclav 500 mg/125 mg 8 hourly for 5 days or a substitute, if allergic to penicillin) and analgesia (Diclofenac 50 mg 8 hourly for 3 days followed by paracetamol 60 mg 4–6 hourly for 5 days). Wounds are checked in 2 weeks’ time.
a A diagrammatic and anatomical illustration of the pectoralis major origin and extent along with the level of muscle split. The muscle split starts at the level of middle and lower third of the sternum, outlined by blue dots. Reprinted with permission from Khan [20]. b Schematic illustration of a side view of an implant in muscle splitting biplane showing upper pectoralis covering the implant, while lower pectoralis lies behind the implant. Reprinted with permission from Riaz M, Khan UD. 2015. Use of multiplane internal mastopexy for ptosis correction revision-augmentation mammoplasty. Plast Aesthet Res 2:120–6
Statistical Analysis
The data were analysed using the Statistical Package for the Social Sciences (SPSS), version 19.0. The results are presented in the text as frequency, percentage for qualitative/categorical variable (difference in implant size) and mean ± S.D for quantitative/continuous variables (age and implant size). The Chi-square test is used to compare the categorical variable and t test for quantitative/continuous variables. In all statistical analyses, only p values < 0.05 are considered significant.
Results
A total of 1511 patients had their primary augmentation mammoplasties consecutively performed in muscle splitting biplane pocket. The mean age of the patients was 29.4 ± 8.56 years (range 18–67). Of the 1502 patients with documented implant sizes, 1272 patients had same-size implants, mean 340 cc ± 58.3 (range 170–700), and 230 patients had two different-size implants for correction of asymmetry. Of these 230 patients, mean implant size on right and left was 341 cc ± 61.5 (range 200–655) and 345 cc ± 67.4 (range 200–605), respectively (Table 1). Of the 1495 recorded surface texturing, only 3.1% patients had smooth-surface implants (Fig. 2). Periprosthetic infection was seen in 10 (0.6%) patients, 38 (2.51%) had wound-healing issues and 5 had late seroma. A haematoma was seen in 10 (1.5%) out of recorded 1501 patients (Fig. 3). The presence of capsular contracture (CC) was recorded at three monthly, six monthly, one yearly and two yearly or a longer period. Of the recorded follow-up in 1260 patients at three-months, three had grade III/IV CC. At six-month follow-up recorded in 360 patients, three patients showed grade III/IV CC. A one-year follow-up recorded in 216 patients showed Grade III/IV CC in another three patients. Of the 133 patients followed up for two years or longer (range 2–12 years, median 6) another nine patients were found to have Grade III/IV CC (Fig. 4). Secondary procedures were performed for various reasons in 93 (6.15%) of the 1511 patients. The leading reasons for revisions in 93 (6.1%) patients were: 25 (1.65%) for bigger implants, 33 (2.2%) wanted to replace implants for various reasons and wished for bigger implants as well. Of these 93 revision cases, 18 (1.2%) patients were treated for capsular contracture and 9 (0.6%) cases for implant rupture (Table 2 and Fig. 5). Average time between primary and revision surgery was 5.4 ± 3.5 years (range 0.3–11.0) (Table 2).
Smoking status was recorded in 1491 primary augmentation mammoplasty patients, and of these, 438 (29.4%) were smokers. Smoking status also was recorded in 85 revision surgeries, and of these, 16 (18.8%) were smokers. Drain was used in 79 (5.3%) and 7 (7.5%) of primary and revision groups, respectively.
There was no anaplastic large cell lymphomas (ALCL) or deep venous thrombosis recorded in the series.
Discussion
An ideal pocket for augmentation mammoplasty procedure is challenging. All techniques described so far may have certain advantages over the other procedures, and despite the fact that how meticulously or carefully these techniques are performed, the element of individual variations of breast at the time of presentation of surgery and its natural history later on in each patient make it extremely difficult to declare which can fit and suit all individuals. Anatomically, the breasts lie in front of the PM and for an ideal and most natural three-dimensional result, an augmentation should be performed using an ideal filler material in the subglandular or prepectoral plane and hence the reason for implant placement in this pocket when first described [1]. The subglandular pocket yields a three-dimensional natural result when compared to an implant placed in a two-dimensional total submuscular pocket. The subglandular pocket has various advantages because of its easy and quicker pocket dissection along with its reproducible, predictable and natural results in suitably selected patients. However, the marriage between the consistent filler material of the implant and the inconsistent physical characteristics of the breasts may not remain in harmony for too long, and the initial good results cannot be relied upon in the long term, requiring revision surgeries in many patients [21]. The initial subglandular pocket introduced in 1963 was quickly changed to total submuscular augmentation [22], not for the lack of appearance but due to a high and unacceptable rate of capsular contracture [23]. The submuscular technique has been evolved since then to render the submuscular results into more three-dimensional results comparable to subglandular pocket and to bring harmony in the outcome between its early and long-term results [24]. One such commonly used partial submuscular technique is dual-plane technique [25]. The technique is very commonly used but is not without an avoidable complication generally known as dynamic breast deformity or breast animation deformity (BAD).
Dynamic deformity or animation deformity is generating a lot of interest and concern among surgeons and patients alike and merits special consideration in this follow-up article. The complication is totally due to PM release from its fixed point on the sternum, costal margins, ribs and rectus fascia, allowing freed muscle to retract, reposition and reattach to the breast envelope and later to the implant capsule. The released muscle leads to the creation of a panniculus-carnosus muscle effect, which eventually results in breast skin envelope distortion and deformity when the muscle is activated [9, 10]. This disfiguring deformity is well described [2, 26], its grading system well outlined [26, 27] and its incidence well reported in primary augmentation mammoplasty as well implant-based breast reconstructions as 53% and 75.6%, respectively [27, 28]. However, a well-researched meta-analysis of the topic suggests that the incidence could be higher in primary augmentation mammoplasty as the response rate of the questionnaire was low of a small sample. The research article also reports that breast animation deformity or dynamic deformity is not only a cosmetic problem but also a functional problem which may eventually affect patient’s quality of life [29]. The deformity can be reversed either converting dual plane to the muscle splitting biplane technique [9, 10, 30–32], combining the muscle splitting technique with the subfascial technique [2, 3], or converting retropectoral to prepectoral position of the implant in breast reconstructions cases [30,31,32].
Another meta-analysis on animation deformity concluded that animation deformity was treated by muscle splitting biplane technique with least complications [9, 10] when compared with conversion of dual plane to prepectoral or subfascial plane in terms of skin envelope viability, capsular contracture and excessive rippling.[32] In selected group for subglandular and subfascial conversion, patients often needed advanced skin viability tests, acellular dermal matrix or auto-fat grafting in breast reconstruction group-related animation deformities.[32] However, the critique of the muscle splitting technique in this meta-analysis article erroneously reported the technique as being “excessively destructive” citing a reference in which Scott Spear referred excessive destruction, thinning and depression of the lower pole to the Pelle-Ceravolo technique [27, 32]. In the Pelle-Ceravolo technique, the pectoralis was bisected vertically, transecting the lower half of the pectoralis major as opposed to pectoralis splitting along the muscle fibres with no release in muscle splitting biplane technique [4, 26, 32]. In the current series, revision rate including bottoming down was well within the acceptable range (Fig. 5). Alnaif’s meta-analysis also has referenced the limitation of the size of the implants (greater than 400 cc) used in muscle splitting biplane. There was no such limitation or size restriction in earlier or current series (Table 1).
The muscle splitting biplane technique itself is an evolution of partial submuscular where the technique gives very natural lower pole fullness along with adequate muscle cover superomedially. There is no release of the muscle and hence no animation deformity as reported in the original article with a follow-up of 1.2 years [4]. Subsequent 6- and 9-year follow-up articles did not report this deformity either [8,9,10,11,12,13,14,15,16,17,18,19,20]. Similarly, there was no animation or dynamic deformity reported by other authors to date [13,14,15,16,17, 33]. The muscle splitting technique gives a natural and longer-lasting stable results (Figs. 6, 7 and 8). The muscle splitting biplane procedure is reproducible with a quick learning curve, and the safety of dissection has been established with no risk to the innervation of the pectoralis major muscle [33]. Lack of animation deformity prevents physical or chemical denervation [26, 28, 31, 34]. However, the dynamic movement of the implant resulting from muscle contraction that displaces the implant laterally but without a pull or distortion of breast or breast envelop skin can still happen [35]. This lateral mild displacement is a Grade II of the Spear [27] type and clinically not noticeable in the muscle splitting biplane technique due to the locking of the implant superolaterally at the anterior axillary fold where the muscle split ends (Fig. 1a) [4].
a, b Preoperative pictures of a 24-year-old patient following childbirth. c, d Post-operative pictures taken 4 months following augmentation mammoplasty in muscle splitting pocket using 300 cc round cohesive gel silicone implants. e–g Pictures showing results 13 years following augmentation mammoplasty. Patient had two more children following her mammoplasty
a, b Front and side views of a 20-year-old nulliparous female with breast hypoplasia. c, d Front and side view six weeks following augmentation mammoplasty in muscle splitting biplane pocket, using 300 cc round microtextured cohesive gel silicone implants. e–g Front and side views 13 years following augmentation mammoplasty showing good and stable results
a, b Preoperative pictures of a 25-year-old nulliparous patient presenting with hypoplastic breasts. c, d Post-operative pictures taken two months following augmentation mammoplasty using 300 cc cohesive silicone gel microtextured implants in muscle splitting biplane. e–g Pictures taken 13 years following augmentation mammoplasty showing stable results
Following the report of absence of animation deformity, in the first original article [4], no animation deformity evaluation was carried out in the later part of the series [19, 20, 33]. Similarly, no animation deformities have been reported by other authors as well [13,14,15,16,17, 32]. Another recent development is the concept of the triple-plane technique, a submuscular pocket approached through its lateral edge [36]. After the creation of the submuscular pocket, the pectoralis is incised from its lateral edge to its sternal insertion at the nipple level, with no muscle release and no animation deformity noted in 524 patients [36].
Many authors have reported various revision rates in their follow-ups depending on the timescale the patients were followed up. In early follow-up of 1 to 3 years, revision rates of 0% to 1.97% were reported [4, 37, 38]. Follow-ups ranging between 6 and 7 years showed a revision rate of 3.7% and 4.2%, respectively [39, 40].
However, compiling long-term results following primary breast augmentation mammoplasty can be daunting and challenging, and for this reason, these reports are not frequently reported and often involve more than one operating surgeon, more than one technique, more than one type of implant used for various types of breast procedures. Even though there is an advantage that a good mix of surgeons, techniques or implants can allow us to expect a range of acceptable results, the lack of uniformity in execution of a surgical technique, variation of surgical techniques employed or diversity in behaviour of physical characteristics of implants may sway the results between broad ranges [20, 41,42,43,44,45]. One such report of a 25-year-old follow-up performed by Dr Handel showed a reoperation rate of 15.5% in primary augmentation mammoplasties [43]. Results following primary augmentation mammoplasty by a single surgeon are not frequently published and may still have different types of implants or different techniques used [45, 46]. In one such study, where a single surgeon operated on all patients, the overall results showed a 9.4% revision rate in all types of implants and 11.9% of reoperation in round implants alone following 6-year follow-up [46].
A longer follow-up almost always shows a rise of revision surgeries, even when all surgeries are performed by the same surgeon, using the same type of implants with the same technique. In a report of first 125 bilateral primary cases in muscle splitting augmentation mammoplasties with a follow-up of more than a year, there was no reported revision [4]. At six years, the revision rate in 914 muscle splitting primary augmentation mammoplasties rose to 1.2% [20]. An 8-year follow-up in 1298 muscle splitting primary mammoplasties showed a revision rate of 1.4% [18]. However, there was a sharp increase in revisionary procedures, from 1.2% at 6 years to 6.15% at 13 years in the current series.
Early post-operative emergency procedures, whether requiring surgical intervention or not, are not of implant origin and were not included as revision surgeries. There were 10 (0.66%) cases of haematomas, 10 (0.66%) periprosthetic infections and 38 (2.5%) had wound-healing issues (Fig. 3).
Breast implant-associated anaplastic large cell lymphoma (BIA ALCL) is a hot topic and is a serious condition; luckily, the incidence of the condition is rare and acceptable risk is 1 in 30,000 patients and may take up to 8 to 10 years to develop. The condition is predominantly related to textured implants, especially the macrotextured variety (Allergan, Inamed, McGhan, etc.) [47]. Of the 1495 patients in the current series, 700 patients had smooth or microtextured/nanotextured implants, 215 had macrotextured implants and the rest had varying degrees of texturing (Fig. 2). Late seroma was seen in five patients, and all patients had macrotextured implants [48]. These seromas routinely have an ultrasound examination, ultrasound-guided aspiration and immunohistochemistry for CD30 to exclude BIA ALCL. There was no BIA ALCL diagnosed so far in the series probably due to the low incidence of the condition and low number of macrotextured implants used in the series. The author also uses diluted povidone-iodine for pocket irrigation and implants immersion before insertion. Povidone-iodine is quite effective against gram-negative bacteria, which may possibly be involved in the development of BIA ALCL, as proposed in the gram-negative bacteria biofilm pathway [49]. There is a possibility that some patients in the current series may have developed the condition and were treated elsewhere, but no request for release of clinical notes and pictures has been requested by any other physician or an attorney.
Request for change of implants for a bigger size only was the most common reason for the revision (Fig. 5). The author’s earlier report for preoperative breast implant size selection, on a 3-year reoperation rate in 507 patients, was noted at 0.2%. However, the current larger data and longer follow-up show that 25 (26.95%) patients of the 93 revision mammoplasties requested bigger implants, forming a total of 1.65% of 1511 patients. The mean duration between surgery and request for reoperation was shortest in this group (Table 2). Of these 25 patients who requested larger implants, 10 patients wanted 800 cc implants with a round fake look and the muscle splitting biplane pocket was changed to a subglandular pocket. Even though the author has used up to 700 cc extra high-profile implants in primary augmentation mammoplasty, larger than 700 cc implants with wide bases are not recommended for primary or secondary mammoplasty as these may interfere with pectoralis innervation [33]. Capsular contracture was the second most common reason for revision seen in 93 revisionary surgeries. As one would expect, there was a sharp rise in capsular contracture when follow-up was carried out for two years or longer. It must be noted that this 6.8% CC was seen in only 133 patients, forming 8% of the 1511 patients (Table 2, Fig. 9). Exchange for implant was performed in patients presenting with various problems and most of them requested larger implants at the same time. In this group, the mean time between surgery and reoperation was longest (7.9 years, range 4–11), reflecting the stability of results (Table 2 and Fig. 5).
Of the 93 revision surgeries performed by the author in this series, nine implants were found ruptured and constituted 9.6% of the 93 revisions or an overall incidence of 0.6% (Fig. 2). True implant rupture, just like capsular contracture, is difficult to estimate in any long-term follow-up as the patient may not be aware of it or may have moved out of the area for various reasons or may have gone to another surgeon. Other 6-year-old and 25-year-old studies showed a similar rupture rate of 1.5 [43] and 1.6%, respectively [42].
The make of the implants also was looked into for their distribution and involvement in revisionary surgeries relative to the number of the implants used (Fig. 2).
Strength and weaknesses of the study
The current study is based upon a large number of augmentation mammoplasties consulted, examined, performed and followed up by a single surgeon. All patients had round silicone gel implants with 97% having varying degrees of textured surfaces and using the single muscle splitting biplane technique. As the data presented in the article suggest, the follow-up number gradually decreased over a period of time, from 83% at three months, 23% at six months, 14% at one year to 8.8% for two years or more. Even though 133 patients were seen for two years or more, with a mean of 6 years and a range of 2–12 years, still 91.2% of patient data were not available for a more reliable or accurate conclusion, especially when the possibilities of complications like capsular contracture and implant rupture are known to occur most. These difficulties are commonly experienced and reported in other long-term follow-ups and largely due to the patients moving to other areas, not coming for follow-up if all is well or may have gone to other surgeons for complications and further treatment [43].
Conclusion
The muscle splitting pocket for primary augmentation mammoplasty is a reliable and reproducible procedure with an acceptable low revision rate.
References
Cronin TD, Gerow RM. (1964) Augmentation mammoplasty: new “natural feel” prosthesis. In: Translation of the third international congress of the plastic surgery. Excerpta medica international congress series, no. 66 Excerpta Medica, Amsterdem, pp. 41–49
Baxter RA (2005) Subfascial breast augmentation: theme and variation. Aesthetic Surg J 25:447–453
Graf RM, Bernardes A, Rippel R et al (2003) Subfascial breast implant: a new procedure. Plast Reconstr Surg 111:904–908
Khan UD (2007) Muscle splitting breast augmentation: a new pocket in a different plane. Aesthet Plast Surg 31:553–558
Khan UD (2010) Augmentation mastopexy in muscle-splitting biplane: an outcome of first 44 consecutive cases of mastopexies in a new pocket. Aesthet Plast Surg 34:313–321
Khan UD (2018) One-stage mastopexy and augmentation mammoplasty in layers: outcome analysis of first 50 consecutive cases. Plast Aesthet Res 5:45
Khan UD (2011) Multiplane technique for simultaneous submuscular breast augmentation and internal glandulopexy using inframammary crease in selected patients with early ptosis. Eur J Plast Surg 34:337–343
Khan UD (2015) Subglandular to muscle splitting biplane conversion for revision augmentation mammoplasty. In: Mugea TT, Schifmann MA (eds) Aesthetic surgery of the breast. 1st edn. Springer, Berlin, pp. 535–41
Khan UD (2009) Dynamic breasts: a common complication following partial submuscular augmentation and its correction using muscle splitting biplane technique. Aesthet Plast Surg 33:353–360
Khan UD (2012) High transverse capsuloplasty for the correction of malpositioned implants following augmentation mammoplasty in partial submuscular plane. Aesthet Plast Surg 36:590–599
Khan UD (2009) Acquired synmastia following subglandular mammoplasty and the use of submuscular splitting biplane for its correction. Aesthet Plast Surg 33:605–610
Khan UD (2010) Combining muscle splitting biplane with multilayer capsuloraphy for the correction of bottoming down following subglandular augmentation. Eur J Plast Surg 33:259–269
Baxter RA (2011) Update on the split-muscle technique for breast augmentation: prevention and correction of animation distortion and double bubble deformity. Aesthet Plast Surg 33:353–360
Berlanda M (2010) Muscle-splitting augmentation: personal experience with the new technique. X Mied- zynoraodowy Kongress MedycynyEstetycznej I Anti-Aging, 24–26 September 2010, Warsaw
Stodell M, McArthur G, James M (2016) Bi-plane breast augmentation: a case series supporting its use and benefits. Plast Aesthet Res 3:17–20
Astrauskas T, Viksraitis S, Maslauskas K, KaitarisV (2009) Comparison of two methods of breast augmentations: muscle-splitting versus traditional subpectoral method. In: Presented at the 11th Congress of ESPRAS, 26–27 September 2009, Rhodes
Stumpfle RL, Pereira-Lima LF, Valiati AA, Da Mazzini GS (2012) Transaxillary muscle splitting breast augmentation: experience with 160 cases. Aesthet Plast Surg 36:343–348
Khan UD (2016) Augmentation mastopexy and augmentation mammoplasty: an analysis of 1,406 consecutive cases. Plast Aesthet Research 3:26–30
Khan UD (2016) A long term review of augmentation mastopexy in muscle splitting biplane. Plast Aesthet Res 3:21–25
Khan UD. (2013) Muscle splitting, subglandular and partial submuscular augmentation mammoplasties. A twelve year retrospective analysis of 2026 primary cases. Aesthet Plast Surg 37(2):290–302
Khan UD (2009) Selection of breast pocket using pinch test in augmentation mammoplasty: Can it be relied in long term? Aesthet Plast Surg 33:780–781
Dempsey WC, Latham WD (1968) Subpectoral implants in augmentation mammoplasty: a preliminary report. Plast Reconstr Surg 42:515
Biggs TM, Yarish RS (1990) Augmentation mammoplasty: a comparative analysis. Plast Recosnstr Surg 85:368
Regnault P (1977) Partially submuscular breast augmentation. Plast Reconstr Surg 59:72
Tebbet JB (2001) Dual-plane breast augmentation: optimizing implant-soft tissue relationship in a wide range of breast types. Plast Reconstr Surg 107:1255
Pello-Ceravolo M, Del Vescovo A, Bertozzi E et al (2004) A technique to decrease breast shape deformity during muscle contraction in submuscular mammoplasty. Aesthet Plast Surg 28:288–294
Spear SL, Scwartz J, Dayan JH et al (2009) Outcome assessment of breast distortion following submuscular breast augmentation. Aesthet Plast Surg 33:44–48
Nigro LC, Blanchet NP (2017) Animation deformity in postmastectomy implant based reconstruction. Plast Reconstr Surg Glob Open 5:e1407
Dyrberg DL, Camilla B, Gunnarsson GL et al (2019) Breast animation deformity. Arch Plast Surg 46:7–15
Fracol M, Feld LN, Chiu WK, Kim JYS (2019) An overview of animation deformity in prosthetic breast reconstruction. Gland Surg 8(1):95–101
Gabriel A, Sigalove S, Sigalove NM et al (2018) Prepectoral revision breast reconstruction for treatment of implant-associated animation deformity: a review of 102 reconstructions. Aesthet Surg J 38(5):519–526
Alnaif N, Safran T, Alex Viezel-Mathieu, Alhalabi B, Dionisopoulos T (2019) Treatment of breast animation deformity: a systematic review. J Plast Reconstr Aesthet Surg 72:781–788
Saleh DB, Callear J, Riaz M (2016) An anatomic appraisal of biplane muscle-splitting breast augmentation. Aesthet Surg J 36(9):1019–1025
Figus A, Mazocchi M, Dessy LA et al (2009) Treatment of muscular contraction deformities with botulinum toxin type A after latismus dorsi flap and subpectoral implant breast reconstruction. J Plast Reconstr Aesthet Surg 62:869–875
Moliver CL (2016) Commentary on: an anatomic appraisal of biplane muscle-splitting breast augmentation. Aesthet Surg J 36(9):1026–1028
Bracaglia R, Tambasco D, Gentileschi S, D'Ettorre M (2013) Triple-plane technique for breast augmentation: solving animation deformities. Aesthet Plastic Surgery 37(4):715–718
Khan UD (2013) The impact of preoperative breast implant selection on the 3-year reoperation rate. Eur J Plast Surg 36:503–510
Tebbets JB (2006) Achieving a zero percent reoperation rate at 3 years in a 50-consecutive case augmentation mammoplasty premarket study. Plast Reconstr Surg 118:1453–1457
Adams WP Jr (2008) The process of breast augmentation: four sequential steps for optimizing outcomes for patients. Plast Reconstr Surg 122:1892–1900
Jewell ML, Jewell JL (2010) A comparison of outcomes involving highly cohesive, form-stable breast implants from two manufacturers in patients undergoing primary breast augmentation. Aesthet Surg J 30:51–65
Stevens WG, Harrington J, Alizadeh K, Broadway D, Zeidler K, Godinez TB. (2015) Eight-year follow-up data from the U.S. clinical trial for sientra’s FDA-approved round and shaped implants with high-strength cohesive silicone gel. Aesthet Surg J 35(S1);S3-S10
Codner MA, Mejia JD, Locke MB, Mahoney A, Thiels C, Nahai FR, Hester TR, Nahai F (2011) A 15-year experience with primary breast augmentation. Plast Reconstr Surg 127:1300–1314
Handel N, Cordray T, Gutierrez J, Jensen JA (2006) A long-term study of outcomes, complications, and patient satisfaction with breast implants. Plast Reconstr Surg 1177:757–767
Maxwell GP, Van Natta BW, Bengston BP, Murphy DK (2013) Ten-year results from Natrelle 410 anatomical form-stable silicone breast implant core study. Aesthet Plast Surg 35:145–155
Khan UD (2017) Low risk primary augmentation mammoplasty and capsular contracture using textured round cohesive silicone gel implants revisited. A long term follow up in a single surgeon’s practice. Pak J Plast Surg 5:6–19
Montemurro P, Cheema M, Heden P et al (2018) Do not fear an implant’s shape: a single surgeon’s experience of over 1200 round and shaped textured implants in primary breast augmentation. Aesthet Surg J 38:254–261
Calobrace MB, Schwartz MR, Zeidler KR, Pitmann TA, Cohen R, Stevens WG (2018) Long term safety of textured and smooth breast implants. Aesthet Surg J 38:38–48
Khan UD (2016) Pathogenesis of late breast autoinflation following augmentation mammoplasty: case series report of three late autoinflation of breast due to seroma and literature search. Plast Aesthet Res 3:31–35
Deva AK, Adams WP Jr, Vickery K (2013) The role of bacterial biofilms in device-associated infection. Plast Reconstr Surg 132:1319–1328
Funding
Author has not received research funding for this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interests.
Ethical Approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, U.D. Muscle Splitting Augmentation Mammoplasty: A 13-Year Outcome Analysis of 1511 Primary Augmentation Mammoplasties. Aesth Plast Surg 43, 1469–1477 (2019). https://doi.org/10.1007/s00266-019-01468-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-019-01468-5