Abstract
Background
Muscle-splitting breast augmentation, initially described by Baxter and later popularized by Khan, has proved to be an effective technique in terms of implant coverage, bypassing, and even solving of some issues associated with the dual-plane technique. A muscle-splitting breast augmentation technique recently has been used in combination with mastopexy. However, no reports have described muscle-splitting techniques accomplished by the transaxillary route.
Methods
A prospective study was conducted to evaluate the outcomes and complications of a novel approach to a specific breast augmentation technique. A total of 160 patients underwent bilateral transaxillary muscle-splitting breast augmentation between October 2007 and July 2010. All the patients were treated on an outpatient basis and received epidural anesthesia. Soft, round, textured, cohesive gel implants ranging in size from 200 to 350 ml were used.
Results
All the patients recovered quickly. To date, no infection, capsular contracture, rippling, double-bubble deformity, muscle contracture-associated deformities, or implant migration has occurred. Four patients (2.5%) experienced hematomas, all of which resolved before discharge. All the patients were discharged less than 24 h postoperatively and had an aesthetically natural result.
Conclusion
Transaxillary muscle-splitting breast augmentation, a novel approach to a technique that has been described previously, provides consistent, satisfactory results and good reproducibility. This new approach provides an excellent anatomic final appearance with no risk of displacement, rippling, double-bubble deformity, or contracture-associated deformities. Furthermore, this technique avoids any visible scars on the breast and features a low complication rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Augmentation mammoplasty is one of the most common aesthetic surgery procedures worldwide. Breast augmentation was the second most common invasive procedure in the International Society of Aesthetic Plastic Surgery (ISAPS) international survey of aesthetic/cosmetic procedures performed in 2009. Brazil was no exception. Among its total of 172,615 surgeries, breast augmentation was the second most common invasive procedure performed in the country in 2009 [17].
The main indications for augmentation are amastia, hypoplastic breasts, breast asymmetry, and age-related changes [5]. The most common surgical planes for breast implant placement, each with its particular advantages and drawbacks, are the subglandular, submuscular, and subfascial planes.
The position of the implant, which depends on both operator preference and patient anatomy, determines the adequacy of soft tissue coverage over the implant for the patient’s lifetime, the pressure that the implant device will exert on tissues over time, and the position of the breast on the chest wall [16]. In lean patients with little subcutaneous tissue in the upper pole of the breast, the subglandular pocket can result in visible edges, increased implant palpability, and traction rippling [5, 8, 12]. In these patients, full or partial submuscular implant placement may limit implant setting, jeopardizing skin envelope expansion and leading to decreased lower pole fullness and high positioning of the prosthesis [5, 8].
The dual-plane technique, which involves varying degrees of pectoralis release from the sternocostal attachment in a posterior plane and from the soft tissues in an anterior plane, has enabled greater expansion of the lower pole and more natural cosmetic outcomes [14, 15]. However, it is sometimes associated with a complication, the dynamic breast, brought about precisely by the release of the pectoralis major from its inferior attachments [6].
With the muscle-splitting technique, the implant is placed posterior to the muscle in the upper pole and anterior to the muscle in the lower segment. There is no need to detach muscle fibers from their natural sites [1, 5–7]. This technique allows good coverage of the breast’s upper pole while imposing no limitation to expansion of the lower pole or precluding any muscle contraction-associated breast deformity.
Transaxillary breast augmentation was first described by Hoehler [4] in 1973 and then modified in 1979 by Peterson [13], who placed the implant subpectorally. This approach is becoming increasingly popular due to its cosmetic outcomes and because scars are placed outside the breast. Most surgeons prefer implant placement approaches other than the axillary route, however. The complexity of the procedure is dependent on the surgeon’s technical expertise. Therefore, the more surgeons dedicate themselves to the transaxillary approach, the simpler this route becomes. Many operators still use endoscopy as a technical aid during transaxillary surgery [8, 11, 12], which makes the procedure more difficult. Other authors, however, have managed to break free from these unwieldy devices, making the procedure faster with no decline in safety [10, 11]. Surgical planes can be fashioned through the transaxillary route just as they are created using inframammary or periareolar approaches [10, 12].
Muscle-splitting breast augmentation via the transaxillary route has not been reported in the literature to date. This study presents a large case series with long-term follow-up evaluation and provides evidence of the extensive advantages offered by performing muscle-splitting breast augmentation, an established technique, through a transaxillary approach.
Materials and Methods
From January 2007 to July 2010, a total of 160 patients underwent transaxillary muscle-splitting breast augmentation. The criteria for inclusion required that patients be 18 years of age or older and agree to take part in the current study. The procedure was performed with the patient under epidural anesthesia, which shows that complete relaxation of the pectoral muscles is not necessary for proper reproduction of the technique. All the surgeries were performed on an outpatient basis, with patients discharged home on the same day.
Soft cohesive gel microtextured, round, high-profile implants ranging in size from 200 to 350 ml were used. Textured silicone implants were used because of their association with a lower incidence of capsular contracture [3], and high-profile implants were chosen because they provide better projection of the breast’s upper pole, a characteristic desired by most of our patients. Antibiotic prophylaxis with a first-generation cephalosporin (cefazolin, 1–2 g) was provided at the time of anesthesia induction.
Surgical Procedure
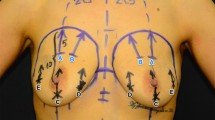
Markings are made with the patient in the orthostatic and supine positions. The nipple–areolar complex is the anatomic landmark for the center of the breast (i.e., the most projected part of the breast). The inframammary crease is lowered 1 to 2 cm when necessary, whereas the lateral dissection cannot cross the anterior axillary line. The patient is placed in a supine position with both arms abducted 90°. A 1:250,000 epinephrine solution is used to infiltrate the breast and the axillary tunnel (100 ml on each side).
The rectilinear incisions are designed to be 4 to 5 cm long at the natural skin folds behind the midaxillary front line to hide the scars. The axillary tunnel is started, with the anterior flap kept quite thin to preserve the lymphatic supply of the area and the intercostobrachial nerve. The tunnel is advanced under direct visualization with the aid of a cold light source.
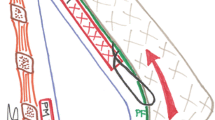
After the axillary tunnel has been created, the lateral border of the pectoralis major muscle is reached and the fibers are split obliquely from the anterior axillary line toward the junction of the middle and lower third of the sternum. The cranial portion is elevated (muscle and gland) to create a pocket to cover 20 to 30% of the implant’s upper pole. The caudal portion of the muscle is not elevated, and the inferior pocket is created in a subfascial plane (Fig. 1). However, the fascia over the inferior half of the pectoral muscles is extremely thin, and the subfascial and subglandular planes are difficult to distinguish. The medial two thirds of the muscle fibers are divided, whereas the lateral third is kept intact (i.e., the muscle is in no way separated from its original attachment). The dissection plane never advances past the anterior axillary line, thus avoiding lateral displacement of the implant and reducing the risk of injury to the main pedicle of the pectoralis. Hemostasis is maintained with the aid of the cold light source and a bipolar cautery.
The implant then is placed using only the cold light retractor to lift the upper segment of the muscle flap and a Montellano dissector to slide in the implant. One important directive is that a simple stitch should be placed at the end of the axillary incision before introduction of the device. This prevents tearing of the skin during placement of the prosthesis. Breast symmetry and projection are assessed with the implants in place and the patient in a semi-sitting position looking over her feet. No drains are used, and the dressing exerts sufficient compression during the early postoperative period.
Results
A total of 160 bilateral transaxillary muscle-splitting breast augmentations were performed between October 2007 and July 2010, with a maximum follow-up period of 30 months. All the patients were women with a mean age of 25 years (range, 18–41 years). The average operating time was 50 min (range, 40–90 min). The implant size ranged from 200 to 350 ml (mean, 250 ml).
Table 1 summarizes the main findings of the study. Intraoperative bleeding was negligible. No drains were placed. Four patients (2.5%) experienced hematomas, which were promptly drained with the patient under sedation and local anesthesia through the same incision. The vessels were coagulated under direct visualization using a light retractor. The patients were discharged at the end of the day and instructed to call the surgeon in case of any adverse events such as pain, wet dressings, or swelling occurred. To date, no infection, capsular contracture, rippling, double-bubble deformity, muscle contracture-associated deformities, or migration of the implants has occurred.
The patients were discharged in less than 24 h postoperatively. All were encouraged to lift their arms above their shoulders from the second postoperative week, although all had regained this range of motion long before. Pain complaints were minor. Most of the patients discontinued analgesia a few days after the procedure. The axillary scars healed satisfactorily for all the patients, with no cases of hypertrophy or keloid formation.
Discussion
The benefits of muscle-splitting breast augmentation were first reported in 2005 by Baxter [1] in a series of four cases in which the author was able to address issues associated with the dual-plane technique (dynamic breasts). Baxter named his procedure “subfascial breast augmentation associated with segmental pectoralis muscle flaps.”
In 2007, Khan [5] published a series of 125 bilateral breast augmentations using a technique similar to that described by Baxter but titled it the “muscle-splitting technique.” The best candidates for this technique are thin patients with little subcutaneous tissue in the upper pole of the breast [1, 2, 5, 6] (Figs. 2, 3). The technique provides excellent muscle coverage of the upper third of the prosthesis while imposing no restrictions to expansion of the lower pole. In fact, for patients with a loose skin envelope or slight ptosis, the implant instantly fills the entire lower pole of the breast. The surgeon can therefore rest assured that little likelihood of double-bubble deformity exists for patients with minor ptosis and hardly any risk of complications such as deformity-associated contraction because the upper fibers of the pectoralis are shorter and have less contraction strength than its lower fibers [1, 5]. Therefore, some technical distinctions from Tebbets’ dual-plane technique [15] are worth stressing, namely, that no muscle fibers are detached from their origins or attachments. That is, the pectoral muscles undergo no anatomic changes, and the implant is placed anteriorly and posteriorly to the muscle, not purely posteriorly, as with the dual-plane technique (Fig. 4).
However, the feasibility of muscle-splitting mammoplasty via the transaxillary route had not been tested to date. Technically simpler than the dual-plane technique, this route is even easier to accomplish without the aid of video endoscopy because the inferior fibers of the pectoral muscles are not detached, which requires greater precision and consequently a clearer view of the field. Division of the pectoralis major fibers from the anterior axillary line laterally to their origin at the junction between the middle and lower third of the sternum is quite easy. Creating this cleavage plane poses no major challenges because the muscle fibers are simply divulsed without compromise to their integrity. Bleeding is minimal.
The widespread fear of hematoma as the main complication of blind dissection is unjustified. The hematoma rates are very low, similar to those reported in the international literature [8, 10–12]. Even during revision surgery for hematoma drainage, endoscopic visualization is not needed because the surgical plane is clearly defined and not associated with any distortion in normal pectoralis anatomy, enabling clear, direct visualization. For safety reasons alone, we do keep a full complement of equipment at the ready in theater should the need arise.
Transaxillary breast augmentation is associated with higher satisfaction rates than transmammary approaches, although the difference is not significant [9]. This provides even greater encouragement for continued improvement of transaxillary techniques. Axillary incisions also are more easily accepted by patients because the scar is kept off the breast, hidden in the armpit [8]. Achieving improved outcomes with lower complication rates is our number one goal.
Conclusion
Muscle-splitting breast augmentation is an established pocket option that can be used in almost all augmentation mammoplasties. This technique has proved to be less painful than the dual-plane approach because the pectoralis major muscle is not detached but rather divided in a specific area along its fibers, preserving all natural insertions. To date, no prospective study had showed the feasibility of performing this technique by a transaxillary approach. Our results are similar to those achieved by Khan, with the added advantage that the surgical scar is placed off the breast.
References
Baxter RA (2005) Subfascial breast augmentation: theme and variations. Aesthet Surg J 25:447–453
Baxter RA (2010) Update on the split-muscle technique for breast augmentation: prevention and correction of animation distortion and double-bubble deformity. Aesthet Plast Surg 35(3):426–429
Berry MG, Davies DM (2010) Breast augmentation: part I: a review of the silicone prosthesis. J Plast Reconstr Aesthet Surg 63:1761–1768
Hoehler H (1973) Breast augmentation: the axillary approach. Br J Plast Surg 26:373–376
Khan UD (2007) Muscle-splitting breast augmentation: a new pocket in a different plane. Aesthet Plast Surg 31:553–558
Khan UD (2009) Dynamic breasts: a common complication following partial submuscular augmentation and its correction using the muscle-splitting biplane technique. Aesthet Plast Surg 33:353–360
Khan UD (2010) Augmentation mastopexy in muscle-splitting biplane: outcome of first 44 consecutive cases of mastopexies in a new pocket. Aesthet Plast Surg 34:313–321
Luan J, Mu D, Mu L (2009) Transaxillary dual-plane augmentation mammaplasty: experience with 98 breasts. J Plast Reconstr Aesthet Surg 62:1459–1463
Momeni A, Padron NT, Fohn M, Bannasch H, Borges J, Ryu SM, Stark GB (2005) Safety, complications, and satisfaction of patients undergoing submuscular breast augmentation via the inframammary and endoscopic transaxillary approach. Aesthetic Plast Surg 29:558–564
Niechajev I (2010) Improvements in transaxillary breast augmentation. Aesthetic Plast Surg 34:322–329
Pacella SJ, Codner MA (2009) The transaxillary approach to breast augmentation. Clin Plast Surg 36:49–61 vi
Pereira LH, Sterodimas A (2009) Transaxillary breast augmentation: a prospective comparison of subglandular, subfascial, and submuscular implant insertion. Aesthet Plast Surg 33:752–759
Peterson R (1979) Transaxillary subpectoral augmentation mammaplasty. Paper presented at American Society of Plastic Surgeons Annual Meeting. Toronto, ON, Canada
Ramirez OM, Heller MDL, Tebbetts JB (2002) Dual-plane breast augmentation: avoiding pectoralis major displacement. Plast Reconstr Surg 110:1198 Author reply 1198–1199
Tebbetts JB (2001) Dual-plane breast augmentation: optimizing implant–soft tissue relationships in a wide range of breast types. Plast Reconstr Surg 107:1255–1272
Tebbetts JB, Adams WP (2005) Five critical decisions in breast augmentation using five measurements in 5 minutes: the high-five decision support process. Plast Reconstr Surg 116:2005–2016
The International Society of Aesthetic Plastic Surgery (ISAPS): ISAPS international survey on aesthetic/cosmetic procedures performed in 2009. St. Gallen, ISAPS, 2010. http://www.isaps.org/uploads/news_pdf/Analysis_iSAPS_Survey2009.pdf. Accessed 28 Jan 2011
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lang Stümpfle, R., Figueras Pereira-Lima, L., Alves Valiati, A. et al. Transaxillary Muscle-Splitting Breast Augmentation: Experience with 160 Cases. Aesth Plast Surg 36, 343–348 (2012). https://doi.org/10.1007/s00266-011-9830-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-011-9830-9