Abstract
Purpose
Third generation autologous chondrocyte implantation (ACI) is an established treatment for full thickness cartilage defects in the knee joint. However, little is known about cases when revision surgery is needed. The aim of the present study is to investigate the complication rates and the main reasons for revision surgery after third generation autologous chondrocyte implantation in the knee joint. It is of particular interest to examine in which cases revision surgery is needed and in which cases a “wait and see” strategy should be used.
Methods
A total of 143 consecutive patients with 171 cartilage defects were included in this study with a minimum follow-up of two years. All defects were treated with third generation ACI (NOVACART®3D). Clinical evaluation was carried out after six months, followed by an annual evaluation using the International Knee Documentation Committee (IKDC) subjective score and the visual analogue scale (VAS) for rest and during activity. Revision surgery was documented.
Results
The revision rate was 23.4 % (n = 36). The following major reasons for revision surgery were found in our study: symptomatic bone marrow edema (8.3 %, n = 3), arthrofibrosis (22.2 %, n = 8) and partial graft cartilage deficiency (47.2 %, n = 17). The following revision surgery was performed: retrograde drilling combined with Iloprost infusion therapy for bone marrow oedema (8.4 %, n = 3), arthroscopic arthrolysis of the suprapatellar recess (22.2 %, n = 8) and microfracturing/antegrade drilling (47.3 %, n = 17). Significant improvements of clinical scores after revision surgery were observed.
Conclusion
Revision surgery after third generation autologous chondrocyte implantation is common and is needed primarily in cases with arthrofibrosis, partial graft cartilage deficiency and symptomatic bone marrow oedema resulting in a significantly better clinical outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cartilage repair is challenging for patients and orthopaedic surgeons. Autologous chondrocyte implantation (ACI) in the knee joint using a periosteal flap [1] has become a promising treatment with encouraging results for femoral and patellar cartilage defects [2–6]. In the last two decades since the first study by Brittberg et al., its further developments including the third generation autologous chondrocyte implantation using collagen I/III scaffolds has become increasingly popular for treatment of large full thickness cartilage defects. The simplification of the operative procedure had made the third generation ACI more common [7–10].
However, the knowledge of revision surgery and the complication rate after third generation autologous chondrocyte implantation is still low. Very few studies addressing the complication rates and revision surgery have been performed. Jungmann et al. analysed the complication rates of patient after autologous chondrocyte implantation with regard to the risk factors for revision surgery [11]. To date, there is no information about the revision rate and their risk factors after third generation autologous chondrocyte implantation.
Given the increasing number of cases including problems and complications related to revision surgery, it is necessary to get more information about common problems and appropriate revision procedures. This information is needed to be able to inform patients and for them to make informed decisions.
The aim of the present study is to investigate the complication rates and common revision operations after third generation autologous chondrocyte implantation. It is of particular interest to determine in which cases and what revision surgery is necessary. We also examined the success rate after revision surgery.
The following hypothesis was stated: Third generation ACI is an appropriate therapy for patients with a full thickness cartilage defect of the knee joint resulting in an acceptable number of complications and revision operations. We also hypothesize that cartilage-inducing operating procedures are appropriate treatments in cases with partial graft cartilage deficiencies.
Materials and methods
A total of 143 consecutive patients with 171 cartilage defects are included in this study with follow up of a minimum of two years. The average ACI follow-up was 5.0 (SD 2.1) years. All cartilage defects were full thickness cartilage defects classified as III and IV using the Classification of the International Cartilage Repair Society (ICRS). The mean patient age was 35.1 years (11–66). The study was comprised of a total of 58.7 % men (n = 84) and 41.3 % (n = 59) women. Defects were located in 44.4 % femoral (n = 76) and in 55.6 % (n = 95) femoropatellar. The femoral defects were mainly on the medial femoral condyle (84.2 %, n = 64). A total of 18.9 % (n = 18) of the femoropatellar defects were located in the trochlea. The average defect size was 4.0 cm². The average body mass index was 26.3. In 80.1 % of the defects, ACI was performed as a firstline therapy (n = 137). In 19.9 % (n = 34) of the treated defects, ACI was conducted as secondline therapy after a previously failed cartilage repair procedure (e.g. microfracturing, drilling, first generation ACI, third generation ACI) (Table 1).
Cartilage therapy was performed according to the guidelines of the German Working Group Tissue Regeneration [12]. Therefore the exclusion criteria for matrix based ACI was: osteoarthritis of the knee, joint instability, arthritis, corresponding cartilage defects or more than two focal cartilage defects. All defects were treated with NOVACART®3D (TETEC AG, Reutlingen, Germany). The operation technique was performed as described in a previous study [13].
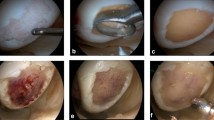
At first, the sampling of two to three osteochondral plugs was obtained from an unloaded area of the intercondylar notch with a diameter of 3 mm and a thickness of 5–10 mm. Afterwards the osteochondral plugs were sent to the manufacturer (TETEC GmbH, Reutlingen, Germany) in sterile nutrient solution. The cultivation time was three to four weeks. After a sufficient proliferation, the chondrocytes were seeded on a collagen I/III biphasic scaffold. In a second operation, a parapatellar arthrotomy was performed and the cartilage defects were carefully debrided with curettes until a stable rim of healthy surrounding cartilage was prepared. The scaffolds were implanted in the prepared cartilage defect. Afterwards, the scaffolds were fixed with absorbable sutures in the defect (Fig. 1). In cases without a stable cartilage shoulder, an additional fixation using PLLA pins was conducted (Aesculap AG, Tuttlingen, Germany).
Autologous chondrocyte implantation of a retropatellar cartilage defect (a). Cartilage defects were carefully debrided with curettes until a stable rim of healthy surrounding cartilage was prepared (b). The scaffolds were implanted in the prepared cartilage defect (c). Afterwards, the scaffolds were fixed with absorbable sutures in the defect (d)
In regards to the postoperative rehabilitation, patients did a standardized protocol as described earlier [13]. After 24 hours of bed rest and drain removal, continuous passive motion device (CPM) was done and weight-bearing was limited to 20 kg for six weeks for femoral cartilage defects. Flexion was increased quickly. In cases with patella defects, flexion was limited to 30° for two to three weeks and gradually increased in the following weeks. Weight bearing was allowed with full extension after wound healing.
In order to assess the clinical evaluation, the following standardised protocol was performed. The first clinical evaluation was done after six months, followed by an annual evaluation. Then the subjective knee evaluation form of the International Knee Documentation Committee (IKDC) was completed. For pain detection, the visual analogue scale (VAS) during activity and at rest was used. Both scores were established and validated [14, 15] and were obtained in previous studies analysing cartilage reconstruction [16–18].
To get information about complications and revision surgeries, the patients were assessed using a standardised form and the data was documented. We also collected the information about the time point of revision operations. The clinical evaluation after revision surgery was continued as described above.
Furthermore, we analysed individual parameters like patients’ age, gender, body weight and body height to determine the body mass index (BMI). In regards to the defect associated parameters we collected the information about defect size, aetiology, and localisation as well as first- and secondline therapy. The detailed information about these parameters is given in Table 1.
Statistical analysis
The statistical analysis was done using the statistics program SPSS (Statistical Package of Social Sciences, Version 22, Chicago, IL, USA). The Wilcoxon test was used to examine differences regarding the clinical outcome in the postoperative course. The level of statistical significance was set at P < 0.05. Significant differences before and after revision surgery were determined using the Wilcoxon test for dependent samples. For statistical analysis of possible risk factors of revision surgery, we used the Mann–Whitney-U test for independent samples in the comparison of two groups. The level of statistical significance was also set at P < 0.05.
Results
The clinical evaluation showed a significant improvement of the analysed clinical scores compared to the pre-operative values over the whole observation period. The subjective IKDC scores increased significantly from 39.4 to 60.0 points after two years. The measured subjective IKDC scores showed a slight decline especially up to five years with an IKDC score of 58.7. Significant differences between the follow-up examinations were found in the first two postoperative years. The VAS score during activity and at rest showed significant improvements compared with the pre-operative results over the whole observation period with significant improvements between the follow-up examinations up to two years. Detailed information of the overall clinical results is given in Table 2.
The revision rate was 22.4 % (n = 32). On average, the revision surgery was performed after 418 days after ACI with a range from 10–1172 days. The following reasons for revision surgery were given: symptomatic bone marrow oedema (8.3 %, n = 3) with an intact cartilage surface, arthrofibrosis (22.2 %, n = 8) and partial graft cartilage deficiency (47.3 %, n = 17) (Table 3).
The following revision surgeries were made: Iloprost therapy for five days combined with retrograde drilling (8.4 %, n = 3), arthroscopic arthrolysis (22.2 %, n = 8) and microfracturing or antegrade drilling (47.3 %, n = 17).
The clinical scores after revision surgery in general showed significant improvements of the subjective IKDC and the VAS during activity and at rest (Fig. 2). The subjective IKDC score increased from 30.0 to 50.1 after the first evaluation after revision surgery. Significantly better results regarding the VAS during activity and at rest were also found (Table 4).
Considering the special revision operations, we found the following: better clinical results after arthroscopical arthrolysis in cases with arthrofibrosis in all clinical scores. In regards to the partial graft cartilage deficiencies we performed microfracturing or antegrade drilling of the non-covered area. They were located at the border zone of the implanted ACI implants with well-developed cartilage regeneration in the central part of the ACI scaffolds. Afterwards we observed significantly better results in the subjective IKDC scores and the VAS during activity (Fig. 3). The overall IKDC result after revision surgery was 50.1 (17.8; 21.8–82.8), which is comparable to the clinical results of the patients without revision surgery.
The analysis of the individual and defect associated parameters to get more information about risk factors which could predict revision surgery did not show a higher complication rate in view of the individual parameters patient’ age, gender, body weight, body height and body mass index. The defect-associated parameters such as defect size, localization and defect aetiology also had no influence on the revision rate. Furthermore, cartilage defects with secondline therapy after a previously performed cartilage repair before ACI, did not show a higher complication or revision rate (Table 1).
In this study, in 14 % (n = 24) of the treated cartilage defects, an additional fixation of the ACI graft with PLLA pins was performed. A significant influence of the different types of fixation and the revision rate could not be found. Interestingly, we found a statistical trend without significant results regarding the partial graft deficiencies and the types of fixation. No partial graft deficiency was found in cases with an additional pin fixation (p-value 0.109).
Discussion
The most important finding of the present study is that revision surgery after third generation autologous chondrocyte implantation is common and is often needed in cases with arthrofibrosis, partial graft cartilage deficiency and symptomatic bone marrow oedema with significant better clinical outcome afterwards.
Autologous chondrocyte implantation is an established procedure for treatment of full cartilage defects in the knee joint with several satisfactory results in the literature [19–23]. Although little is known about cases when joint revision surgery is needed. The aim of the present study is to investigate the complication rate and common revision surgery after third generation autologous chondrocyte implantation. It is of particular interest in which cases a revision surgery is indicated and leads to better clinical results.
In this study, for the first time complication rates after third generation ACI in the knee joint are standardised and analysed. Jungmann et al. described risk factors which are associated with the cause of reintervention after first, second and third generation ACI [11]. Typical complications are analysed. But no special data is given about third generation ACI which is now a common orthopaedic practice. Minas described the complication rate of a large patient cohort over ten years. This complication rate involves only patients after first generation ACI in the knee joint [24]. Vijayan et al. showed the revision cartilage transplantation after primary ACI can yield acceptable functional results [25].
In the present study, a standardised protocol was performed to analyse clinical results after third generation ACI. Clinical evaluation was carried out for the first time after six months, followed by an annual evaluation using IKDC subjective score and the VAS score for rest and during activity. These scores are established in case series after ACI procedure and were used in several previous studies [16–18, 26]. We included 143 consecutive patients with 171 cartilage defects in this study.
The minimum follow-up was two years for detecting typical complications and revision surgeries. With a mean follow-up of 5.0 (SD 2.1) years, this observation period is also comparable to previous studies analysing this topic [7, 27, 28]. All revision surgeries and their underlying complications were documented.
In general, we observed significantly better clinical results in the IKDC subjective score after ACI treatment. However, in a total of 36 cases, a revision surgery had to be performed. The revision rate in this study was 23.4 %. This result is similar to previously performed studies [11].
The following must be taken into consideration in evaluating the relatively high revision rates. One of the major reasons for revision surgery was a postoperative arthrofibrosis. As a result, arthroscopic arthrolysis of the suprapatellar recess were performed in 22.2 % of the cases. This relatively high rate of arthrofibrosis was caused by the restricted flexion after the surgery. Arthrofibrosis was observed in 71.4 % (n = 5) in retropatellar cartilage defects and in 21.6 % (n = 2) in femoral cartilage defects, without significant differences (p = 0.781).
The most postoperative complication was a partial graft cartilage deficiency, which was found in 47.2 % (n = 17). These partial defects were treated with trimming of the rim and microfracturing of the uncovered defect. The remaining part of the ACI implant was firmly attached into the defect. These partial graft cartilage deficiencies were located at the border zone of the implanted ACI implants. The central part of the ACI scaffolds was a well-developed cartilage regenerate.
These partial graft cartilage deficiencies can be interpreted as a problem of bonding to the surrounding healthy cartilage. Hypothetically it can be assumed, that the reduced bonding capacity is caused by a limited regenerative potential of the ACI implants in this area. One reason for that reduced regenerative potential might be due to a localized cellular problem of the implanted autologous chondrocytes. This complication could be solved with an operative revision surgery of this small area with microfracturing or drilling. Accordingly, the patients showed better clinical results and less pain, therefore this procedure appears to be a successful approach.
Symptomatic defect-associated bone marrow edema were found in 8.4 % (n = 3) of the cases. In a previous study, bone marrow edema after autologous chondrocyte implantation was analysed [13]. In this study, it was shown that bone marrow oedema appears in over 78 % of the cases. In cases with postoperative defect-associated bone marrow oedema without cartilage defects, we performed a retrograde drilling combined with intravenous application a prostacyclin analogue Iloprost (Bayer Schering Pharma AG, Berlin, Germany) for a total of five days.
If a symptomatic BME could be observed, an appropriate procedure is recommended. The procedure should be adapted to the graft maturation process, which needs at least 12 months. In cases with symptomatic BME in the early pre-maturation course up to 12 months postoperatively, no operative revision surgery is recommended. But a MR investigation should be performed for control. In the late postoperative course after 12–18 months after completely finished graft cartilage maturation, it must be assumed, that the BME is caused by a partial deficiency of the cartilage. In these cases, we recommended a revision surgery with diagnostic knee arthroscopy and cartilage repair, e.g. microfracture, if necessary. With intact cartilage without bonding problems of the graft cartilage, an operative therapy of the BME could be done with retrograde drilling and, if applicable, followed by Iloprost intravenously, which is an alternative treatment option for patients with symptomatic BME [29].
Analysing the individual und defect associated parameters we could not find higher complication rate in view of the individual and defect-associated parameters. Therefore it is astonishing that no risk factors could be identified for revision surgery. This finding could be confirmed by the results of Jungmann et al. [11]. We expected that patients with large cartilage defects would have a larger rate of revision surgery, but no significant differences could be found.
In regards to the different types of fixation, we found a statistical trend without significant results regarding the partial graft deficiencies. No partial graft deficiency was found in cases with an additional pin fixation. In cases without a stable cartilage shoulder, an additional pin fixation seemed to be a suitable method for stabilization of the graft cartilage, but no significant difference could be found because of the low numbers of cases with pin fixation.
A limitation of the study is the relatively short follow-up. Therefore it would be interesting to analyse the development of osteoarthritis and especially the rate of knee arthroplasty after ACI procedure in the postoperative course. For evaluating this question a longer follow-up with a high number of patients is needed. Furthermore, we included only cases after third generation ACI treatment. A control group with different cartilage repair procedures would be required to detect relevant information about the complication rates of different cartilage repair techniques.
Finally, the results of the present study show that revision surgery after third generation ACI is common. Especially in cases with partial graft cartilage deficiencies after ACI, an operative revision with microfracturing or drilling is a promising method for successful cartilage treatment. Restricted limitation after ACI is associated with a high rate of suprapatellar arthrofibrosis. Therefore, increasing flexion quickly in cases with femoral defects appears advisable.
Conclusion
This study is the first standardized analysis of complication and revision rates after third-generation ACI in the knee joint. The most important finding of the present study is that revision surgery after third generation autologous chondrocyte implantation is common and is needed particularly in cases with arthrofibrosis, partial graft cartilage deficiency and symptomatic bone marrow oedema. We observed significantly better results after revision surgery with reduced pain compared with the pre-revision constitution. In regards to the treatment of partial graft cartilage deficiency, microfracturing seems to be an advisable therapy with promising clinical results afterwards. We could not find any individual or defect-associated parameters which could be seen as predictive of revision surgery.
References
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331(14):889–895. doi:10.1056/NEJM199410063311401
Vasiliadis HS, Lindahl A, Georgoulis AD, Peterson L (2011) Malalignment and cartilage lesions in the patellofemoral joint treated with autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc 19(3):452–457. doi:10.1007/s00167-010-1267-1
Kreuz PC, Muller S, von Keudell A, Tischer T, Kaps C, Niemeyer P, Erggelet C (2013) Influence of sex on the outcome of autologous chondrocyte implantation in chondral defects of the knee. Am J Sports Med 41(7):1541–1548. doi:10.1177/0363546513489262
Gomoll AH, Gillogly SD, Cole BJ, Farr J, Arnold R, Hussey K, Minas T (2014) Autologous chondrocyte implantation in the patella: a multicenter experience. Am J Sports Med. doi:10.1177/0363546514523927
Peterson L, Vasiliadis HS, Brittberg M, Lindahl A (2010) Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med 38(6):1117–1124. doi:10.1177/0363546509357915
Niemeyer P, Salzmann G, Feucht M, Pestka J, Porichis S, Ogon P, Sudkamp N, Schmal H (2014) First-generation versus second-generation autologous chondrocyte implantation for treatment of cartilage defects of the knee: a matched-pair analysis on long-term clinical outcome. Int Orthop 38(10):2065–2070. doi:10.1007/s00264-014-2368-0
Nawaz SZ, Bentley G, Briggs TW, Carrington RW, Skinner JA, Gallagher KR, Dhinsa BS (2014) Autologous chondrocyte implantation in the knee: mid-term to long-term results. J Bone Joint Surg Am 96(10):824–830. doi:10.2106/JBJS.L.01695
Goyal D, Goyal A, Keyhani S, Lee EH, Hui JH (2013) Evidence-based status of second- and third-generation autologous chondrocyte implantation over first generation: a systematic review of level I and II studies. Arthroscopy 29(11):1872–1878. doi:10.1016/j.arthro.2013.07.271
Vasiliadis HS, Wasiak J (2010) Autologous chondrocyte implantation for full thickness articular cartilage defects of the knee. Cochrane Database Syst Rev 10:CD003323. doi:10.1002/14651858.CD003323.pub3
Naal FD, Impellizzeri FM, Leunig M (2009) Which is the best activity rating scale for patients undergoing total joint arthroplasty? Clin Orthop Relat Res 467(4):958–965. doi:10.1007/s11999-008-0358-5
Jungmann PM, Salzmann GM, Schmal H, Pestka JM, Sudkamp NP, Niemeyer P (2012) Autologous chondrocyte implantation for treatment of cartilage defects of the knee: what predicts the need for reintervention? Am J Sports Med 40(1):58–67. doi:10.1177/0363546511423522
Niemeyer P, Andereya S, Angele P, Ateschrang A, Aurich M, Baumann M, Behrens P, Bosch U, Erggelet C, Fickert S, Fritz J, Gebhard H, Gelse K, Gunther D, Hoburg A, Kasten P, Kolombe T, Madry H, Marlovits S, Meenen NM, Muller PE, Noth U, Petersen JP, Pietschmann M, Richter W, Rolauffs B, Rhunau K, Schewe B, Steinert A, Steinwachs MR, Welsch GH, Zinser W, Albrecht D (2013) Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: a guideline by the working group "Tissue Regeneration" of the German Society of Orthopaedic Surgery and Traumatology (DGOU). Z Orthop Unfall 151(1):38–47. doi:10.1055/s-0032-1328207
Niethammer TR, Valentin S, Gulecyuz MF, Rossbach BP, Ficklscherer A, Pietschmann MF, Muller PE (2015) Bone marrow edema in the knee and its influence on clinical outcome after matrix-based autologous chondrocyte implantation: results after 3-year follow-up. Am J Sports Med. doi:10.1177/0363546515573935
Higgins LD, Taylor MK, Park D, Ghodadra N, Marchant M, Pietrobon R, Cook C, International Knee Documentation C (2007) Reliability and validity of the International Knee Documentation Committee (IKDC) Subjective Knee Form. Joint Bone Spine 74(6):594–599. doi:10.1016/j.jbspin.2007.01.036
Risberg MA, Holm I, Steen H, Beynnon BD (1999) Sensitivity to changes over time for the IKDC form, the Lysholm score, and the Cincinnati knee score. A prospective study of 120 ACL reconstructed patients with a 2-year follow-up. Knee Surg Sports Traumatol Arthrosc 7(3):152–159
Niethammer TR, Safi E, Ficklscherer A, Horng A, Feist M, Feist-Pagenstert I, Jansson V, Pietschmann MF, Muller PE (2014) Graft maturation of autologous chondrocyte implantation: magnetic resonance investigation with T2 mapping. Am J Sports Med. doi:10.1177/0363546514538756
Siebold R, Karidakis G, Fernandez F (2014) Clinical outcome after medial patellofemoral ligament reconstruction and autologous chondrocyte implantation following recurrent patella dislocation. Knee Surg Sports Traumatol Arthrosc 22(10):2477–2483. doi:10.1007/s00167-014-3196-x
Muller S, Hirschmuller A, Erggelet C, Beckmann NA, Kreuz PC (2014) Significantly worse isokinetic hamstring-quadriceps ratio in patellofemoral compared to condylar defects 4 years after autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-014-2964-y
Niethammer TR, Muller PE, Safi E, Ficklscherer A, Rossbach BP, Jansson V, Pietschmann MF (2013) Early resumption of physical activities leads to inferior clinical outcomes after matrix-based autologous chondrocyte implantation in the knee. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-013-2583-z
Zak L, Albrecht C, Wondrasch B, Widhalm H, Vekszler G, Trattnig S, Marlovits S, Aldrian S (2014) Results 2 years after matrix-associated autologous chondrocyte transplantation using the Novocart 3D scaffold: an analysis of clinical and radiological data. Am J Sports Med 42(7):1618–1627. doi:10.1177/0363546514532337
Meyerkort D, Ebert JR, Ackland TR, Robertson WB, Fallon M, Zheng MH, Wood DJ (2014) Matrix-induced autologous chondrocyte implantation (MACI) for chondral defects in the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc 22(10):2522–2530. doi:10.1007/s00167-014-3046-x
Brix MO, Stelzeneder D, Trattnig S, Windhager R, Domayer SE (2013) Cartilage repair of the knee with Hyalograft C:(R) magnetic resonance imaging assessment of the glycosaminoglycan content at midterm. Int Orthop 37(1):39–43. doi:10.1007/s00264-012-1700-9
Bode G, Ogon P, Pestka J, Zwingmann J, Feucht M, Sudkamp N, Niemeyer P (2014) Clinical outcome and return to work following single-stage combined autologous chondrocyte implantation and high tibial osteotomy. Int Orthop 39(4):689–696. doi:10.1007/s00264-014-2547-z
Minas T, Von Keudell A, Bryant T, Gomoll AH (2014) The John Insall Award: a minimum 10-year outcome study of autologous chondrocyte implantation. Clin Orthop Relat Res 472(1):41–51. doi:10.1007/s11999-013-3146-9
Vijayan S, Bentley G, Rahman J, Briggs TW, Skinner JA, Carrington RW (2014) Revision cartilage cell transplantation for failed autologous chondrocyte transplantation in chronic osteochondral defects of the knee. Bone Joint J 96-B(1):54–58. doi:10.1302/0301-620X.96B1.31979
Filardo G, Kon E, Perdisa F, Balboni F, Marcacci M (2014) Autologous osteochondral transplantation for the treatment of knee lesions: results and limitations at two years’ follow-up. Int Orthop 38(9):1905–1912. doi:10.1007/s00264-014-2322-1
Niemeyer P, Porichis S, Steinwachs M, Erggelet C, Kreuz PC, Schmal H, Uhl M, Ghanem N, Sudkamp NP, Salzmann G (2014) Long-term outcomes after first-generation autologous chondrocyte implantation for cartilage defects of the knee. Am J Sports Med 42(1):150–157. doi:10.1177/0363546513506593
Pestka JM, Bode G, Salzmann G, Steinwachs M, Schmal H, Sudkamp NP, Niemeyer P (2014) Clinical outcomes after cell-seeded autologous chondrocyte implantation of the knee: when can success or failure be predicted? Am J Sports Med 42(1):208–215. doi:10.1177/0363546513507768
Jager M, Tillmann FP, Thornhill TS, Mahmoudi M, Blondin D, Hetzel GR, Zilkens C, Krauspe R (2008) Rationale for prostaglandin I2 in bone marrow oedema–from theory to application. Arthritis Res Ther 10(5):R120. doi:10.1186/ar2526
Acknowledgments
No financial support of this project has occurred. The authors have received nothing of value. This manuscript does not contain information about medical devices.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niethammer, T., Valentin, S., Ficklscherer, A. et al. Revision surgery after third generation autologous chondrocyte implantation in the knee. International Orthopaedics (SICOT) 39, 1615–1622 (2015). https://doi.org/10.1007/s00264-015-2792-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-015-2792-9