Abstract
Purpose
To clinically evaluate an arthroscopic autologous chondrocyte implantation (ACI) technique with an in situ crosslinking matrix for the treatment of full thickness cartilage defects of the knee and to present histological results of a graft cartilage biopsy obtained after 1.5 years.
Methods
Fifteen cases of arthroscopic autologous chondrocyte implantation in the knee performed between November 2011 and October 2012 were included in the study. Medical charts and operational reports were screened and the patients were contacted after 0.8 ± 0.3 years (0.4–1.3) and 4.3 ± 0.3 years (4.0–4.8) to asses subjective IKDC and re-operation. The Tegner activity scale was collected at the second follow-up time point. Subjective IKDC response rates were assessed at both follow-up time points.
Results
The first and second follow-up was completed by all 15 patients (100%). The subjective IKDC scores showed a significant improvement (pre-operative 44.5 ± 15.9, first follow-up 71.1 ± 15.9, p < 0.001, second follow-up 72.6 ± 17.3, p < 0.001). The overall response rate was 66.7% (n = 10) at follow-up one and two. There were no significant differences in pre-injury (4, range 1–9) and follow-up two (4, range 2–7) Tegner activity scales (p = n.s.). Two patients required re-operation in the index knee, not related to the ACI procedure. No complication related to the ACI or the implantation technique occurred. The histological results showed excellent cartilage regeneration.
Conclusion
Arthroscopic ACI using an in situ crosslinking matrix is a safe and reliable treatment option for full-thickness cartilage defects of the knee.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cartilage defects of the knee are frequently diagnosed, and show a limited potential to heal and a predisposition for the development of osteoarthritis [1,2,3,4,5,6]. Repair techniques range from autologous osteochondral transplantation (OAT) to abrasion, microfracturing or Pridie drilling [6, 7]. Abrasion, Pridie drilling and microfracturing mobilise subchondral bone marrow, to form a fibrocartilaginous repair tissue [8, 9]. These bone marrow stimulating techniques can be combined with the implantation of a scaffold to improve cell differentiation (autologous matrix-induced chondrogenesis, AMIC) [10]. In 1994, Brittberg et al. [11] introduced the autologous chondrocyte implantation (ACI) technique for the treatment of full-thickness cartilage lesions. In some studies, compared to bone marrow-stimulating techniques, the long-term results of ACI for the treatment of large cartilage defects are reported to be superior [7, 12,13,14,15,16]. Until today, the surgical techniques for ACI have been modified and further generations of ACI have been developed [17]. Nowadays, the standard technique for chondrocyte implantation is arthrotomy and therefore comes with surgical morbidity and the risk of arthrofibrosis as relevant complications [18, 19]. To overcome this problem, arthroscopic ACI techniques have been developed.
Purpose
To clinically evaluate an arthroscopic ACI technique with an in situ crosslinking matrix for the treatment of full-thickness cartilage defects of the knee. The study presents the first mid-term results and one histological evaluation.
Materials and methods
All cases of arthroscopic ACI (n = 15) performed between November 2011 and October 2012 were included in the study. Inclusion criteria were focal cartilage defects of grade 3 or 4 according to ICRS [20] with a minimum size of 2 cm2.
Exclusion criteria were the need for bone grafting because of osteochondral lesion, malalignment of more than 4 degree, untreated ligamentous instability, osteoarthritis (more than Kellgren–Lawrence grade 2 [21]) and kissing lesions (second cartilage defect located on the opposite surface). More than one cartilage defect, previous or concomitant procedures were not included in exclusion criteria. The medical charts and operational reports were retrospectively screened for the patient’s parameters, previous and concomitant procedures, complications, operation time, and surgical details. For demographical data, see Table 1.
The patients were contacted at two points: after 0.8 ± 0.3 years (0.4–1.3) and after 4.3 ± 0.3 years (4–4.8). At both follow-up points, patients were asked to provide information on any re-operations they might have had and to complete the IKDC subjective questionnaire. At the second follow-up, the Tegner activity scale was also collected.
Subjective IKDC response rates were assessed at both follow-up time points. A response in the subjective IKDC was defined as an absolute increase by more than 20.5 score points from baseline [22]. Patients were divided into subgroups based on the number of cartilage lesions (one or two) and whether they had prior cartilage surgery. Responder rates and IKDC scores were analysed for the different subgroups.
Informed consent was obtained by each patient in the study. The study protocol of this retrospective case series was approved by the institutional research ethics board (F-2018-031) and the study was done in agreement with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration.
Surgical technique
In this study, NOVOCART inject® (TETEC—Tissue Engineering Technologies AG, Reutlingen, Germany) was used, which represented an injection system comprising two components: The first component consists of in vitro expanded human autologous articular chondrocytes suspended in a solution containing modified human albumin (maleimido-albumin, MAHSA), isotonic sodium hyaluronate, human serum, and cell culture media. The second component consists of α ω-bisthio-polyethylene glycol functioning as a crosslinker. By simultaneous injection of the two components via a special application system (dual-chamber syringe with a mixing unit), in situ formation of the hydrogel is achieved by crosslinking of the MAHSA molecules.
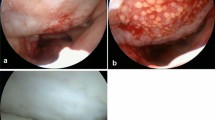
In the first step, arthroscopic assessment of the cartilage lesions (defect size, depth and location, Fig. 1a) and additional injuries is performed. During this first step, procedure additional factors like axis deviations, meniscal injury or ligamentous instability are treated with corrective osteotomy, meniscal surgery or ligament reconstruction, respectively. The chondral defects are prepared and unstable cartilage borders are resected to form a stable bed for implantation (Fig. 1b and c). Then three osteochondral cylinders (diameter of 4 mm, length of 10 mm) are harvested from the anterolateral or anteromedial notch entrance with a special trephine, placed in a nutrient solution and sent in a refrigerated container for processing. The defect preparation is performed during this first step procedure, because the debridement can cause bleeding and the chondral flakes need to be washed out. This is more reasonable and easier to perform during the first step arthroscopy than during the second step dry arthroscopy. Additionally, the harvesting of the osteochondral cylinders can cause some bleeding as well and if needed, a drain can be used (what is not recommended after second step cell implantation). In the second step procedure, the processed autologous chondrocytes are implanted in an arthroscopic fashion usually 21 (17–29) days later. After pre-operative single shot antibiotics (first generation cephalosporin), the patient is placed in supine position, a tourniquet is inflated to 250 mmHg and the leg is placed in a leg holder. Dry arthroscopy with anterolateral and anteromedial standard portals is performed. For implantation into retropatellar defects changing the patient’s position is not necessary, because of the solution’s adhesiveness.
Surgical technique. a Medial femoral condyle (MFC) of a right knee, showing cartilage defect ICRS grade 3b. b MFC of the same knee during defect preparation. c MFC of the same knee after defect preparation (defect size 5 cm2). d Dry arthroscopy 21 days later, showing same defect with regenerative tissue. e Same defect after cleaning and drying. f MFC of the same knee during chondrocyte injection with a syringe
The already prepared defect usually shows some regenerative tissue (Fig. 1d). At first, using small swabs, the defect is cleaned from this tissue and dried (Fig. 1e). A dry defect without bleeding is necessary for chondrocyte implantation, especially for the solution’s adhesiveness. Further defect preparation is usually not necessary. Afterwards, the chondrocytes are implanted using a dual-chamber syringe, the autologous cell suspension is injected together with the crosslinker into the prepared site of the defect until the defect is completely filled up to the surrounding cartilage height (Fig. 1f). The resulting bioresorbable hydrogel keeps the cells in the desired location without the need for additional fixation. The hydrogel takes several minutes (1 to 3 min) until crosslinking is completed; during this time, the leg is held in position. If lesions located on the femoral or tibial condyles and patellofemoral lesions are treated during one procedure, after defect cleaning and drying, at first, the femoral or tibial lesions are treated in flexed knee position, and as second (after first crosslinking), patellofemoral defects are treated in extended knee position. The reason of this sequence is that after knee extension no further movement that can harm or dislocate the graft is necessary. No drains are used. Standard duration of surgery is around 15 min (see Table 1).
Standard in-hospital time is 2 days. After implantation, the knee is fixed in an extension brace for 3–4 days. Afterwards, brace-free full range of motion is allowed. Partial weight bearing (10 kg) is allowed. In cases of isolated patellofemoral implantation, full weight bearing is allowed after 4 weeks. Main reason for limited weight bearing for 4 weeks, even in cases of isolated patellofemoral transplantation, is the brace-free rehabilitation after 3–4 days and with the absence of pain and swelling the risk that patients forget to save the patellofemoral joint from pressure (for example, during walking stairs or raising from a chair). In tibiofemoral locations, full weight bearing is allowed after 8 weeks. Continuous passive motion (CPM) is applied for 8 weeks (20 min, three to four times a day).
Histological evaluation
After informed consent was obtained, in one patient a graft cartilage biopsy was taken during revision surgery because of new traumatic posterior cruciate ligament (PCL) rupture, 1.5 years after ACI. The biopsy was fixed in 4% para-formaldehyde and then decalcified using Osteosoft solution (Merck, Darmstadt, Germany). After decalcification was terminated, the biopsy was infiltrated with Tissue-Tek O.C.T (Sakura Finetek, USA), shock frozen with dinitrogen gas (N2) and then stored at − 20 °C. The frozen tissue sample was sliced by a cryotome to prepare 10 μm sections. The sections were analysed by immunohistology with antibodies against collagen type II (clone II-II6B3; 1 μg/ml) and aggrecan (SM1353, Acris Antibodies GmbH, Herford, Germany). The collagen type II antibody was obtained from the Developmental Studies Hybridoma Bank maintained by the Department of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA, and the Department of Biological Sciences, University of Iowa, Iowa City, IA 52242, USA. Secondary antibodies were Cy3-conjugated IgG+M antibodies (Dianova, Hamburg, Germany). Control sections were prepared without primary antibody. The stained sections were analysed by fluorescence microscopy (Axiophot, Zeiss; Jena, Germany). Photography was performed with an electronic camera (Axiocam, Zeiss, Jena, Germany).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows (version 24, IBM, Corp., Armonk, NY, USA). The Chi-square test and Fisher’s exact test were used in evaluation of nominal data. For statistical evaluation of non-parametric data, the Mann–Whitney U test was used in unrelated samples and the Wilcoxon-signed-rank test was used in related samples. The Student’s t test was used for parametric data. All reported p values are two tailed, with an alpha level < 0.05 considered as significant. Unless otherwise stated, descriptive data are demonstrated as mean ± standard deviation (and range). The data were collected prospectively and analysed retrospectively.
Results
From the total number of 15 patients, 100% completed the first and second follow-up. The majority of patients (11 patients) had one defect and four patients had two defects treated. The site of the defects was the medial femoral condyle (ten defects), the lateral femoral condyle (four defects), the femoral trochlea (four defects) and the lateral tibial plateau (one defect). The average size of the defects was 4.3 ± 1.6 cm2 (range 2.0–7.5 cm2). Four patients (27%) had undergone prior bone marrow stimulation. In three patients, other procedures were combined with ACI treatment (see Table 1).
There was a significant improvement in subjective IKDC scores comparing mean pre-operative scores (subjective IKDC 44.5 ± 15.9) with the first follow-up (at an average of 9.6 months) (subjective IKDC 71.1 ± 15.9, p < 0.001) and the second follow-up (at an average of 4.3 years) (subjective IKDC 72.6 ± 17.3, p < 0.001) (Fig. 2). The overall response rate was 66.7% (n = 10) at the first and second follow-up. There were no significant differences in subjective IKDC scores and responder rates between patients with or without previous cartilage surgeries at both follow-up time points (Tables 2 and 3).
Patients with two cartilage defects showed a tendency towards inferior subjective IKDCs but superior responder rates in the first follow-up and had significant inferior subjective IKDC scores in the second follow-up (Tables 2 and 3), compared to patients with one cartilage defect.
There were no significant differences in Tegner activity scales from pre-injury (4, range 1–9) to the second follow-up (4, range 2–7) (p = n.s.).
Two patients required further surgery on the index knee after 1.5 and 3.5 years, respectively. Both were not related to the ACI. The first patient had a traumatic posterior cruciate ligament (PCL) rupture (during handball match) treated with PCL reconstruction. The second patient was initially treated with ACI because of chondrolysis (of unknown cause) of the lateral femoral condyle with a size of 4 cm2. He developed an analogous chondrolysis 3.5 years after ACI on the medial femoral condyle (8 cm2) of the same knee, which was again treated with arthroscopic ACI.
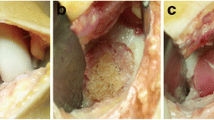
Both patients had a decrease in subjective IKDC score from first to second follow-up (74–54 and 82–69, respectively). In both patients, the assessment of the initially treated cartilage defects during re-operation showed chondral coverage of 100% without signs of delamination or graft hypertrophy (Figs. 3 and 4). During one of the revision procedures (patient with traumatic PCL rupture 1.5 years after ACI), a graft cartilage biopsy was obtained. The histological results showed cartilage tissue with a high proportion of collagen type II and aggrecan (Fig. 5), as well as complete integration at the borders to the native cartilage (Fig. 6).
Surgical finding, ACI and re-operation 1.5 years after ACI because of acute posterior cruciate ligament (PCL) rupture. a Medial femoral condyle (MFC) of a right knee, showing cartilage defect after preparation (defect size 4.5 cm2). b MFC of the same knee during dry arthroscopy and injection of the solution for chondrocyte implantation. c Same patient during PCL reconstruction 1.5 years after ACI, showing the MFC with complete cartilage covering
Surgical finding, ACI and re-operation after 3.5 years because of chondrolysis (not related to primary ACI). a Lateral femoral condyle (LFC) of a left knee, showing chondrolysis with cartilage lesion. b Same lesion after preparation (defect size 6 cm2). c Same lesion during dry arthroscopy after implantation of the chondrocytes. d LFC of the same patient, 3.5 years after ACI, showing complete cartilage covering
Histological examination 1.5 years after ACI showing bone and subchondral bone plate (right) and cartilage graft (left) with high proportion of aggrecan and collagen type II. Some wrinkling, that demonstrates a cutting artifact from processing for histological evaluation, can be observed. a Graft biopsy with aggrecan immunohistology (aggrecan = red). b Graft biopsy with collagen type II immunohistology (collagen type II = red). c Graft biopsy without antibodies (negative control)
There were no complications, especially no cases of arthrofibrosis or infection. No re-operations because of failure of the ACI, graft hypertrophy, delamination or symptomatic edema were necessary.
Discussion
The most important finding of the present study is that arthroscopic ACI using an in situ crosslinking matrix is a safe procedure to repair full-thickness cartilage defects with promising results in the medium term follow-up of 4 years and without the occurrence of severe complications.
Arthroscopic techniques for ACI have been reported by several authors in the past [23,24,25], all dealing with the problems of fixation and trying to reach the different regions of the knee joint.
Filardo et al. [26] reported on the treatment of 62 cases with full-thickness cartilage defects in the technique described by Marcacci et al. [25]. The implantation of the cell seeded scaffold was performed arthroscopically and only fixed by its adhesiveness. The clinical results were promising and also stable over time (IKDC: pre-operative 39.6 ± 15; follow-up 7 years 77.3 ± 21.5). In a total of seven cases (11%) treatment failed. The average defect size was 2.5 ± 1 cm2 and the treatment was restricted to defects located on the medial or lateral condyles. One year later, Filardo et al. reported on the treatment of focal degenerative cartilage lesions with the same arthroscopic technique for ACI [27]. They showed a significant improvement of the clinical scores, but a higher failure rate of 18.5%, as well as inferior clinical outcome compared to the prior study population. They also reported on good results in the treatment of osteochondritis dissecans (OCD) with arthroscopic bone grafting and arthroscopic ACI in a second stage [28]. In all three studies, no cases of infection or arthrofibrosis were reported.
Ibarra et al. [29] reported on an arthroscopy-based technique for ACI with the use of a collagen scaffold, implanted into the defect by means of a suture anchor. The results were comparable to ours according to IKDC values and the absence of complications (IKDC: pre-operative 46 ± 18.5, follow-up 3 years 77.2 ± 12.8). However, the cohort consisted of only ten patients with a single full-thickness cartilage defect of 1 cm2, located either on the medial or the lateral femoral condyle.
Siebold et al. [30] reported on 41 patients with full-thickness cartilage lesions in the knee, treated with all-arthroscopic ACI using spheroids and dry arthroscopy. In all patients, a second-look arthroscopy was performed and the cartilage regeneration was rated normal or nearly normal in 91.3% of the defects. The IKDC value after 34 ± 19 months was 63.0 ± 18.8. However, only 31 out of 41 patients completed the follow-up examination, resulting in a follow-up rate of 75.6% and the timing of second-look arthroscopy and clinical follow-up had a wide range (6–72 months). Therefore, different stages of cartilage maturation were assessed. In addition, 65.8% of the ACIs were combined with additional procedures. Therefore, a direct comparison with our results is not appropriate.
A recently published study by Siebold et al. [31] showed good clinical results and magnetic resonance imaging (MRI) scores after 36.0 ± 10.2 months in 30 patients treated for cartilage lesions in the knee, using the same all-arthroscopic technique (spheroids). The defect size ranged from 1.5 to 16 cm2 and defect location, age, duration of symptoms and defect size had no influence on the clinical outcome. The IKDC values at follow-up were comparable to our reported results (IKDC: pre-operative 46.0 ± 4.7, follow-up 84.2 ± 5.6).
There are several studies, dealing with complications after ACI. DiBartola et al. [32] reported on clinical relevant graft hypertrophy to be the most common complication after ACI. However, they mainly included studies with ACI using periosteal or collagen cover and only two studies using matrix-induced ACI.
Niethammer et al. [33] reported on graft hypertrophy after matrix-induced ACI in 25% of their included cases. Remarkably, no patient showed symptomatic hypertrophy with the need for re-operation. But as they used an arthrotomy-based technique, 11.9% of the patients had re-operation because of arthrofibrosis.
In summary and similar to our findings, no complication related to the ACI treatment (especially no case of arthrofibrosis) occurred following arthroscopic ACI in the knee in the above mentioned studies [26,27,28,29,30,31].
Harris et al. [18] reviewed studies using different generations of ACI to compare reoperation, failure and complication rates. Overall re-operation rate was 33% at a mean follow-up of 3.27 years (2–3.77). The rate of patients with unplanned re-operations was higher with periosteal ACI (27%) compared to ACI using a collagen-derived cover or cell-seeded, three-dimensional, bioabsorbable scaffolds (5% each). The lowest rate of unplanned re-operations was reported for all-arthroscopic ACI techniques (1.4%). The most common complication was graft hypertrophy, followed by delamination, arthrofibrosis and infection. Overall, arthroscopic techniques had a significant lower complication rate than arthrotomy-based techniques, especially no cases of arthrofibrosis or infection were reported. This is in line with our results.
In summary, arthroscopic ACI using a crosslinking matrix is a safe and reliable technique with good clinical results, good histological results in one biopsy and without the occurrence of severe complications. An advantage is the avoidance of an arthrotomy and the easy arthroscopic implantation of the autologous cartilage cells with short operation time. In complex injuries with concomitant full -thickness cartilage defects, arthroscopic ACI can easily be combined with other procedures. A further advantage is the possibility to treat multiple defects and especially the possibility to easily treat defects in all knee compartments by a standard arthroscopic approach.
Limitations
There are several limitations in our study. One major limitation is the retrospective setting without strict inclusion criteria, resulting in a heterogenous cohort with different defect sizes, numbers and locations, as well as diverse additional and prior surgeries. On the other hand, this heterogeneity represents daily practice and is underreported in the current literature, coming with strict inclusion and exclusion criteria [34]. Further, there is no control group, treated with conservative treatment, microfracturing or conventional ACI using an arthrotomy-based technique.
Furthermore, the number of included patients is small and, therefore, the influence of factors like defect number and previous cartilage surgery is underpowered. There is no follow-up like second-look arthroscopy or MRI assessment, thus non-symptomatic complications like incomplete defect filling, edema or low-grade graft hypertrophy might have been missed.
Although the minimum follow-up of 4 years is one of the longest reported for arthroscopic ACI, it still represents mid-term results. However, the focus of this study was the evaluation of the technique of completely arthroscopic ACI. Further studies with larger patient number and longer follow-up times are already planned and ongoing.
Conclusion
Arthroscopic ACI using an in situ crosslinking matrix is a safe and reliable treatment option for full-thickness cartilage defects of the knee. Mid-term results are promising without the occurrence of severe complications.
Abbreviations
- ACI:
-
Autologous chondrocyte implantation
- OAT:
-
Autologous osteochondral transplantation
- ICRS:
-
International Cartilage Repair Society
- IKDC:
-
International Knee Documentation Committee
- N2 :
-
Dinitrogen gas
- TETEC:
-
Tissue Engineering Technologies AG
- MAHSA:
-
Monoclonal anti-human serum albumin
- CPM:
-
Continuous passive motion
- PCL:
-
Posterior cruciate ligament
- OCD:
-
Osteochondritis dissecans
- MRI:
-
Magnetic resonance imaging
References
Buckwalter JA (1998) Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther 28(4):192–202. https://doi.org/10.2519/jospt.1998.28.4.192
Buckwalter JA, Mankin HJ (1998) Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect 47:487–504
Buckwalter JA (1995) Osteoarthritis and articular cartilage use, disuse, and abuse: experimental studies. J Rheumatol Suppl 43:13–15
Widuchowski W, Widuchowski J, Trzaska T (2007) Articular cartilage defects: study of 25,124 knee arthroscopies. Knee 14(3):177–182. https://doi.org/10.1016/j.knee.2007.02.001
Hjelle K, Solheim E, Strand T, Muri R, Brittberg M (2002) Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy 18 (7):730–734 (S0749806302000257 [pii])
Niemeyer P, Albrecht D, Andereya S, Angele P, Ateschrang A, Aurich M, Baumann M, Bosch U, Erggelet C, Fickert S, Gebhard H, Gelse K, Gunther D, Hoburg A, Kasten P, Kolombe T, Madry H, Marlovits S, Meenen NM, Muller PE, Noth U, Petersen JP, Pietschmann M, Richter W, Rolauffs B, Rhunau K, Schewe B, Steinert A, Steinwachs MR, Welsch GH, Zinser W, Fritz J (2016) Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: a guideline by the working group "Clinical Tissue Regeneration" of the German Society of Orthopaedics and Trauma (DGOU). Knee 23(3):426–435. https://doi.org/10.1016/j.knee.2016.02.001
Devitt BM, Bell SW, Webster KE, Feller JA, Whitehead TS (2017) Surgical treatments of cartilage defects of the knee: systematic review of randomised controlled trials. Knee 24(3):508–517. https://doi.org/10.1016/j.knee.2016.12.002
Ewing JW, Voto SJ (1988) Arthroscopic surgical management of osteochondritis dissecans of the knee. Arthroscopy 4 (1):37–40 (S0749–8063(88)80010–0 [pii]).
Steadman JR, Rodkey WG, Rodrigo JJ (2001) Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res 391(Suppl):S362–S369
Gao L, Orth P, Cucchiarini M, Madry H (2019) Autologous matrix-induced chondrogenesis: a systematic review of the clinical evidence. Am J Sports Med 47(1):222–231. https://doi.org/10.1177/0363546517740575
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331(14):889–895. https://doi.org/10.1056/nejm199410063311401
Fu FH, Zurakowski D, Browne JE, Mandelbaum B, Erggelet C, Moseley JB Jr, Anderson AF, Micheli LJ (2005) Autologous chondrocyte implantation versus debridement for treatment of full-thickness chondral defects of the knee: an observational cohort study with 3-year follow-up. Am J Sports Med 33(11):1658–1666. https://doi.org/10.1177/0363546505275148
Saris DB, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, Luyten FP (2009) Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med 37(Suppl 1):10S–19S. https://doi.org/10.1177/0363546509350694
Vanlauwe J, Saris DB, Victor J, Almqvist KF, Bellemans J, Luyten FP (2011) Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med 39(12):2566–2574. https://doi.org/10.1177/0363546511422220
Minas T, Von Keudell A, Bryant T, Gomoll AH (2014) The John Insall Award: a minimum 10-year outcome study of autologous chondrocyte implantation. Clin Orthop Relat Res 472(1):41–51. https://doi.org/10.1007/s11999-013-3146-9
Brittberg M, Recker D, Ilgenfritz J, Saris DBF (2018) Matrix-applied characterized autologous cultured chondrocytes versus microfracture: 5-year follow-up of a prospective randomized trial. Am J Sports Med 46(6):1343–1351. https://doi.org/10.1177/0363546518756976
Welch T, Mandelbaum B, Tom M (2016) Autologous chondrocyte implantation: past, present, and future. Sports Med Arthrosc Rev 24(2):85–91. https://doi.org/10.1097/JSA.0000000000000115
Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC (2011) Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthr Cartil 19(7):779–791. https://doi.org/10.1016/j.joca.2011.02.010
Niethammer TR, Valentin S, Ficklscherer A, Gulecyuz MF, Pietschmann MF, Muller PE (2015) Revision surgery after third generation autologous chondrocyte implantation in the knee. Int Orthop 39(8):1615–1622. https://doi.org/10.1007/s00264-015-2792-9
Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, Kandel R, Nehrer S, Pritzker K, Roberts S, Stauffer E (2003) Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J Bone Joint Surg Am 85A(Suppl 2):45–57
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16(4):494–502
Roos EM, Engelhart L, Ranstam J, Anderson AF, Irrgang JJ, Marx RG, Tegner Y, Davis AM (2011) ICRS recommendation document: patient-reported outcome instruments for use in patients with articular cartilage defects. Cartilage 2(2):122–136. https://doi.org/10.1177/1947603510391084
Petersen W, Zelle S, Zantop T (2008) Arthroscopic implantation of a three dimensional scaffold for autologous chondrocyte transplantation. Arch Orthop Trauma Surg 128(5):505–508. https://doi.org/10.1007/s00402-007-0348-1
Erggelet C, Sittinger M, Lahm A (2003) The arthroscopic implantation of autologous chondrocytes for the treatment of full-thickness cartilage defects of the knee joint. Arthroscopy 19(1):108–110. https://doi.org/10.1053/jars.2003.50025
Marcacci M, Zaffagnini S, Kon E, Visani A, Iacono F, Loreti I (2002) Arthroscopic autologous chondrocyte transplantation: technical note. Knee Surg Sports Traumatol Arthrosc 10(3):154–159. https://doi.org/10.1007/s00167-001-0275-6
Filardo G, Kon E, Di Martino A, Iacono F, Marcacci M (2011) Arthroscopic second-generation autologous chondrocyte implantation: a prospective 7-year follow-up study. Am J Sports Med 39(10):2153–2160. https://doi.org/10.1177/0363546511415658
Filardo G, Kon E, Di Martino A, Patella S, Altadonna G, Balboni F, Bragonzoni L, Visani A, Marcacci M (2012) Second-generation arthroscopic autologous chondrocyte implantation for the treatment of degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc 20(9):1704–1713. https://doi.org/10.1007/s00167-011-1732-5
Filardo G, Kon E, Berruto M, Di Martino A, Patella S, Marcheggiani Muccioli GM, Zaffagnini S, Marcacci M (2012) Arthroscopic second generation autologous chondrocytes implantation associated with bone grafting for the treatment of knee osteochondritis dissecans: results at 6 years. Knee 19(5):658–663. https://doi.org/10.1016/j.knee.2011.08.007
Ibarra C, Izaguirre A, Villalobos E, Masri M, Lombardero G, Martinez V, Velasquillo C, Meza AO, Guevara V, Ibarra LG (2014) Follow-up of a new arthroscopic technique for implantation of matrix-encapsulated autologous chondrocytes in the knee. Arthroscopy 30(6):715–723. https://doi.org/10.1016/j.arthro.2014.02.032
Siebold R, Karidakis G, Feil S, Fernandez F (2016) Second-look assessment after all-arthroscopic autologous chondrocyte implantation with spheroides at the knee joint. Knee Surg Sports Traumatol Arthrosc 24(5):1678–1685. https://doi.org/10.1007/s00167-015-3822-2
Siebold R, Suezer F, Schmitt B, Trattnig S, Essig M (2018) Good clinical and MRI outcome after arthroscopic autologous chondrocyte implantation for cartilage repair in the knee. Knee Surg Sports Traumatol Arthrosc 26(3):831–839. https://doi.org/10.1007/s00167-017-4491-0
DiBartola AC, Wright BM, Magnussen RA, Flanigan DC (2016) Clinical outcomes after autologous chondrocyte implantation in adolescents' knees: a systematic review. Arthroscopy 32(9):1905–1916. https://doi.org/10.1016/j.arthro.2016.03.007
Niethammer TR, Pietschmann MF, Horng A, Rossbach BP, Ficklscherer A, Jansson V, Muller PE (2014) Graft hypertrophy of matrix-based autologous chondrocyte implantation: a 2-year follow-up study of NOVOCART 3D implantation in the knee. Knee Surg Sports Traumatol Arthrosc 22(6):1329–1336. https://doi.org/10.1007/s00167-013-2454-7
Engen CN, Engebretsen L, Aroen A (2010) Knee cartilage defect patients enrolled in randomized controlled trials are not representative of patients in orthopedic practice. Cartilage 1(4):312–319. https://doi.org/10.1177/1947603510373917
Funding
There is no funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schlumberger, M., Schuster, P., Bülow, HJ. et al. Arthroscopic autologous chondrocyte implantation in the knee with an in situ crosslinking matrix: minimum 4-year clinical results of 15 cases and 1 histological evaluation. Arch Orthop Trauma Surg 139, 1607–1615 (2019). https://doi.org/10.1007/s00402-019-03243-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-019-03243-2