Abstract
Purpose
Third-generation autologous chondrocyte implantation (ACI) is an established and frequently used method and successful method for the treatment of full-thickness cartilage defects in the knee. There are also an increasing number of patients with autologous chondrocyte implantation as a second-line therapy that is used after failed bone marrow stimulation in the patient’s history. The purpose of this study is to investigate the effect of previous bone marrow stimulation on subsequent autologous chondrocyte implantation therapy. In this study, the clinical results after the matrix-based autologous chondrocyte implantation in the knee in a follow-up over 3 years postoperatively were analysed.
Methods
Forty patients were included in this study. A total of 20 patients with cartilage defects of the knee were treated with third-generation autologous chondrocyte implantation (Novocart® 3D) as first-line therapy. The mean defect size was 5.4 cm2 (SD 2.6). IKDC subjective score and VAS were used for clinical evaluation after 6, 12, 24 and 36 months postoperatively. The results of these patients were compared with 20 matched patients with autologous chondrocyte implantation as second-line therapy. Matched pair analysis was performed by numbers of treated defects, defect location, defect size, gender, age and BMI.
Results
Both the first-line (Group I) and second-line group (Group II) showed significantly better clinical results in IKDC score and VAS score in the follow-up over 3 years compared with the preoperative findings. In addition, Group I showed significantly better results in the IKDC and VAS during the whole postoperative follow-up after 6, 12, 24 and 36 months compared to Group II with second-line autologous chondrocyte implantation (IKDC 6 months p = 0.015, 1 year p = 0.001, 2 years p = 0.001, 3 years p = 0.011). Additionally, we found a lower failure rate in Group I. No revision surgery was performed in Group I. The failure rate in the second-line Group II was 30%.
Conclusion
This study showed that third-generation autologous chondrocyte implantation is a suitable method for the treatment of full-thickness cartilage defects. Both, Group I and Group II showed significant improvement in our follow-up. However, in comparing the results of the two groups, autologous chondrocyte implantation after failed bone marrow stimulation leads to worse clinical results.
Level of evidence
III
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Full-thickness cartilage defects, known as pre-arthritic lesions [7], often cause significant pain and disability for the patient [1]. It is well known that the intrinsic regeneration capacity of the cartilage is very limited and the healing likelihood of once damaged cartilage is very small [16]. Surgical medical options in these cases include bone marrow stimulation (BMS) techniques such as microfracturing, osteochondral cylinder transplantation and autologous chondrocyte implantation (ACI) [23, 37, 45].
Bone marrow stimulation techniques like microfracture expose the subchondral bone marrow and create a blood clot in the chondral defect, ultimately recruiting mesenchymal stem cells that heal the defect with a fibrocartilaginous scar [41]. Due to the technical simplicity, short surgical times, low cost, and lack of need for additional equipment, BMS has become a popular first-line treatment for chondral defects [28, 45]. Microfracture has historically demonstrated good to excellent results in active patients with small defects at short-term follow-ups [13, 14, 26, 40, 45]. However, the quality of the new regenerated tissue after microfracturing seems to be inferior to that after ACI, as shown by an investigating the histomorphometry and the overall histologic evaluation in the randomised control trial of patients [38].
Autologous chondrocyte implantation, which is a more elaborate and expensive procedure has been proven by several studies to be an appropriate method for treatment of larger full-thickness cartilage defects in knee [10, 21, 29,30,31, 35]. Since the first ACI, there have been many improvements in this procedure. The third-generation autologous chondrocyte implantation, where the chondrocytes are seeded on an absorbable matrix, simplified the operative procedure and produced comparable clinical results [12].
There are many studies comparing the ACI with BMS options and they have provided various results [3, 18, 42]. There seems to be a general agreement that BMS is appropriate for small defects, while ACI is more suitable for larger defects > 3.5 cm2 [30]. In selecting the appropriate procedure, a variety of patient-related (sex, age, BMI, activity level) and defect-related (lesion size, location, prior procedures) aspects need to be considered. To date, there has not been much information regarding the results of the subsequent third-generation ACI after the failure of the first-line cartilage therapy.
The aim of this study is the investigation of the clinical results after third-generation autologous chondrocyte implantation as first-line and second-line therapy after failed previous cartilage therapy with bone marrow stimulation technique. This study was focused on investigating the effect of previous BMS on subsequent autologous chondrocyte implantation therapy. The question arises, if autologous chondrocyte implantation is a suitable method for treatment of full-thickness cartilage defects in both situations. The following hypothesis was generated from the questions above: second-line autologous chondrocyte implantation after failed BMS leads to inferior outcomes in comparison to patients with the autologous chondrocyte implantation as first-line therapy.
Materials and methods
In this prospective study, 40 patients with cartilage defects of knee classified as grades III–IV according to the International Cartilage Repair Society (ICRS) were treated with third-generation ACI (NOVOCART® 3D, TETEC AG, Reutlingen, Germany). All patients were treated according to the guidelines of the working group Tissue Regeneration of the German Society for Orthopaedic and Trauma Surgery [30]. A matched pair analysis was performed with 20 patients with ACI after failed previous cartilage therapy with bone marrow stimulation technique (Group II) and 20 patients with ACI without previous cartilage therapy (Group I) was created. The criteria for pair matching were numbers of treated defects and defect location. If there were multiple options, the criteria gender, aetiology, defect size, BMI and age were used for pair matching (Table 1).
The International Knee Documentation Committee (IKDC) Subjective Knee Form and the visual analogue scale (VAS) at rest and during activity were used after 6, 12, 24, and 36 months postoperatively to evaluate the clinical outcomes.
Surgical technique
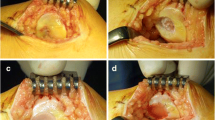
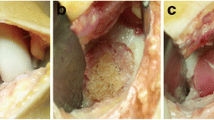
All patients were treated with NOVOCART® 3D (TETEC AG, Reutlingen, Germany), a third-generation ACI. In the initial arthroscopic procedure of the knee joint, which confirmed the full-thickness cartilage defect of ICRS III–IV, two or three osteochondral cylinders were harvested from the non-weight bearing place at the intercondylar notch and sent in a sterile nutrient solution to the manufacturer. Developing and cultivation time was approximately 3–4 weeks. After cultivation, the cells were seeded on a collagen I/III biphasic scaffold, with a dense membrane and a spongy part of pores. The ACI procedures were performed with a parapatellar arthrotomy of the knee joint, the cartilage defect measured and debrided to create the healthy rim. The ACI scaffolds were cut to the needed size and placed with the cell-seeded spongy part into the debrided cartilage defect. Afterwards the grafts were fixed with absorbable sutures. Perioperative antibiotic prophylaxis was done with intravenous Cefuroxim 1.5 g. In cases with existing deformities or pathologies, additional co-operations were performed.
Rehabilitation
Postoperative rehabilitation was carried out with a standardised protocol. In patients with femoral cartilage defects, rehabilitation began with use of a continuous passive motion (CPM) device after 24 h of bed rest and drain removal. During the first 6 weeks post-operation, only a partial load of 20 kg for the femoral cartilage defects was permitted. In patients with patellar defects, a limited knee brace was fitted with flexion to 30° for 2–3 weeks and was gradually increased in the next weeks. Full weight bearing was allowed with full extension after wound healing. All patients were also treated by physical therapists. Moderate physical activities such as cycling, swimming, and Nordic walking were not allowed until 3 months postoperatively. High-impact sports (e.g., soccer, basketball) were not allowed earlier than 12 months after surgery. Institutional review board (IRB) approval was obtained from the ethical committee of the Ludwig Maximilian University (LMU) of Munich (344-12).
Statistical analysis
For the statistical analysis of the clinical data, the statistic program SPSS (Statistical Package for the Social Sciences, Chicago, IL, USA) was used. All patients were detected with a failed previous BMS. Accordingly, a matched paired analysis was performed as described above. To calculate the required sample size, power analysis was performed with G*Power (version 3.1) using a t test (alpha 0.05, power of 0.95, two-tailed). A minimum total sample size of 57 were calculated (medium effect size of 0.5). For the detection of significant differences between the two groups at the same time of investigation, the Wilcoxon or Friedman test was carried out for paired samples. To compare multiple groups of non-related samples at one point, the Mann–Whitney U test was used. A statistically significant result of p < 0.05 was reported.
Results
Patient characteristics are described in Table 1.
Group I with first-line ACI showed an IKDC subjective score of 37.0 (SD 13.7) preoperatively. The maximum IKDC value was reached after 2 years with 77.7 (SD 19.7) points. Compared to the preoperative IKDC values, a significant improvement at all timepoints was detected (preoperative vs. 6 months p = 0.001, vs 12 months p = 0.000, vs 24 months p = 0.002, vs 36 months p = 0.003).
In Group II with second-line ACI patients, a significant increase of the subjective IKDC score was found. The IKDC subjective score was 29.9 (SD 17.0) preoperatively and 44.3 (SD 19.5) after 6 months. Afterwards we observed an increasing IKDC subjective score to a maximum value of 50.1 (SD 20.4) after 1 year. After 3 years, the average IKDC subjective score was 49.1 (SD 21.2) (Table 2). A significant improvement of the IKDC subjective score was seen at all times compared to preoperative findings (preoperative vs. 6 months p = 0.05, vs 12 months p = 0.002, vs 24 months p = 0.009, vs 36 months p = 0.011).
Comparing the IKDC results of the two groups over a period of 3 years, a significant difference between the groups was found. In all follow-ups, a significant difference between the IKDC results of both groups was seen. At all time points, the IKDC value of Group I was better than in Group II. After 6 months, the difference was significant: p = 0.015. The significant differences of IKDC subjective scores continued after 1 year p = 0.001, 2 years p = 0.001 and after 3 years p = 0.011 (Table 2) (Fig. 1).
In Group I and Group II, significantly increased IKDC values compared with the preoperative findings were shown. The outcome of ACI as second-line therapy after previous failed cartilage therapy with bone marrow stimulation is worse than ACI as first-line therapy. At all time points, the IKDC value of Group I is better than the Group II (6 months p = 0.015, 1 year p = 0.001, 2 years p = 0.001 and after 3 years p = 0.011) (*p < 0.05)
The VAS for pain in Group I was preoperatively at 6.4, and at rest 1.9. A significant improvement in motion and at rest was shown at all time points postoperatively with significant differences of p = 0.002 (VAS in motion 6 months), p = 0.001 (VAS in motion after 1 year), p = 0.002(VAS in motion after 2 years), p = 0.002 (VAS in motion after 3 years) and p = 0.049 (VAS at rest 6 months), p = 0.007 (VAS at rest after 1 year), p = 0.008 (VAS at rest after 2 years), p = 0.029 (VAS at rest after 3 years) compared to the preoperative values.
In the Group II, a significant improvement in visual analogue scale (VAS) for pain in motion and at rest was observed. The initial VAS pain was 6.8 in motion and 4.4 at rest. Afterwards, a significant improvement in VAS in motion was found only after 6 months and 1 year. In VAS for pain at rest was it after 6 months, 1 year and 3 years compared to the preoperative results. The best value of VAS was achieved after 1 year (3.8 in motion and 1.0 at rest).
The VAS score in motion showed a significant difference between these groups also in all follow-ups. Group I with first-line ACI patients without previous cartilage therapy had less pain in motion and at rest (Fig. 2). The VAS score at rest showed a significant difference between the two groups in all follow-ups except for after 6 months.
Failure was determined by the need of another revision surgery. The failure rate in Group II was 30% (6 of 20). In three cases, microfracturing was performed, because of partial graft insufficiency, which were treated with microfracturing. In two cases, revision surgery was performed with high tibial osteotomy (n = 1) and knee arthroplasty (n = 1), because of osteoarthritis. In one case, a symptomatic bone marrow edema occurred, what was treated with retrograde drilling. In Group I, no revision surgery was performed.
Discussion
The major finding of this study is that third-generation of autologous chondrocyte implantation represents a suitable method and has satisfactory results as first-line and as second-line therapy in treating full-thickness cartilage defects. However, the outcome of ACI as second-line therapy after previous failed cartilage therapy with bone marrow stimulation is worse than ACI as first-line therapy. Our data demonstrate that bone marrow stimulation (BMS) (e.g. microfracturing) has a negative effect on subsequent cartilage repair with ACI.
BMS is one of the most commonly used surgical techniques for the treatment of cartilage defects in the knee. Many studies have reported statistically significant improvements in clinical outcomes after microfracture [26, 40, 45]. Due to its low costs and, compared with other cartilage therapies, less demanding surgical procedure, bone marrow stimulation was performed in cases with small and large cartilage defects.
The evidence regarding ACI procedure has significantly increased over the past years [43, 44]. The efficacy of this procedure has been demonstrated in multiple studies showing a positive effect, with increased functionality and pain reduction [5, 10, 31, 34, 35]. As the use of ACI has been increasing over time, there are also more patients with prior cartilage procedures with bone marrow stimulation as the microfracturing in their patient history. Since the introduction of ACI, several studies have described factors that influence its clinical outcome. Studies have shown the disadvantageous effects of defect chronicity and patient age on the outcome of ACI [19, 42]. To date, there have been only a few studies investigating the outcome of ACI as the second-line therapy after previous cartilage treatment.
As a result, a matched pair analysis of first-line vs. second-line ACI in a follow-up over 3 years was performed. Matched pair analysis is a well-established method allowing the scientific statements, often used in similar types of studies [32]. International Knee Documentation Committee (IKDC) Subjective Knee Form and the visual analogue scale (VAS) at rest and during activity were used. Both scores are valid, reliable [6, 15] and have been frequently applied in various studies analysing autologous chondrocyte implantation in the knee joint with ACI [27, 32, 33].
Several studies address the second-generation ACI in the knee joint [36]. These studies have shown an increased failure rate in the second-line ACI group. In the study by Pestka et al., 28 patients with second-line ACI were analysed after failed microfracturing [36] with matched pair analysis. They also observed an increased failure rate and significant reduced clinical scores in the second-line ACI group. Jungmann et al. [17] shows that patients with second-line ACI after previous BMS, for the most part with microfracturing, have an increased failure risk. There were no specific data about the third-generation ACI mentioned. In the present study, we also found a significantly higher rate of failure in the second-line ACI Group II of 30% in cases with third-generation ACI.
In the study by Zaslav et al., first-generation ACI was analysed without a control group. They showed that patients with second-line ACI after failed prior cartilage treatments can expect significant clinical and long-lasting improvements in pain and knee function [46]. A comparison between patients with ACI as first-line therapy was not performed in this study. Minas et al. [24] reported an increased failure rate of second-line ACI after treatment with the previous bone marrow stimulation (BMS) techniques in cases with first-generation ACI. The failure rates of second-line ACI after drilling was 28%, abrasion arthroplasty 27% and microfracture 20%. No further assessment regarding clinical knee function after ACI was investigated in this study.
In a further study, Minas et al. described the survivorship of first-generation ACI in a large patient cohort over 10 years [25]. The survivorship of first-generation ACI grafts was significantly decreased in patients with prior microfracturing (44%) in comparison to patients with first-line ACI (77%). Interestingly, there was no significant difference in clinical outcome scores between second-line ACI after failed marrow stimulation and first-line ACI with periosteal flap.
In the present study, it could be demonstrated that bone marrow stimulation (BMS) (e.g. microfracturing) has a negative effect on subsequent cartilage repair with third-generation ACI. A possible explanation for these findings can be a thickening and alteration of the subchondral plate after microfracturing. Microfracturing and microcracks could be responsible for initiating the secondary ossification centre [8]. With regard to that, the overlying articular cartilage becomes more vulnerable to damage from shear forces [4, 11, 22]. This mechanism results in thickening of the subchondral bone and corresponding thinning of the overlying cartilage, which is then more susceptible to damage and further degeneration [2]. Similar changes are found in osteoarthritis and chronic chondral defects, which have demonstrated worse outcomes with cartilage repair procedures [9].
A deterioration of the regenerated cartilage which appears several years after BMS techniques [20] was observed. The reason for this could be that the new regenerated fibrocartilage tissue induced by bone marrow-stimulating techniques seems to be inferior in its histological-structural quality in direct comparison with hyaline articular cartilage [39]. Although the mechanism of this degeneration has not been conclusively proven, changes in the subchondral bone could be potentially seen as an explanation for the deterioration of BMS and subsequent second-line ACI therapy.
Limitations of the present study are the relatively small number of patients—40, with 20 in each group and the relatively short follow-up of 3 years. A larger study population would be helpful for analysing the subgroups to identify the risk factors for the poorer outcomes of the group with previous BMS therapy. Additional research is needed to identify the exact cause of worse outcomes in cases with second-line ACI. A possible explanation is the influence of the damaged subchondral plate with increased mechanical stiffness. Therefore, an extensive analysis of the subchondral plate in patients with cartilage therapy is needed.
Based on the results of this study, the autologous chondrocyte implantation provides clinical benefits in both first-line cartilage therapy and second-line therapy. In addition, our data demonstrate that BMS has a negative effect on subsequent cartilage repair with autologous chondrocyte implantation in the knee joint. Therefore, the choice of which primary cartilage therapy to perform should be made very carefully. This study has shown that first-line ACI leads to superior clinical results compared with the results after second-line ACI.
Conclusion
This matched pair study analysed the effect of previous bone marrow stimulation on subsequent second-line third-generation ACI. This study showed that third-generation autologous chondrocyte implantation is a suitable method for treatment of full-thickness cartilage defects, including for patients with prior BMS. However, the outcome of ACI patients as second-line therapy was worse than that of the first-line ACI. These results should be considered when choosing the appropriate therapy in cases with large full-thickness cartilage defects.
References
Alford JW, Cole BJ (2005) Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med 33:295–306
Armstrong CG (1986) An analysis of the stresses in a thin layer of articular cartilage in a synovial joint. Eng Med 15:55–61
Basad E, Ishaque B, Bachmann G, Sturz H, Steinmeyer J (2010) Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc 18:519–527
Brandt KD, Radin EL, Dieppe PA, van de Putte L (2006) Yet more evidence that osteoarthritis is not a cartilage disease. Ann Rheum Dis 65:1261–1264
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331:889–895
Brittberg M, Winalski CS (2003) Evaluation of cartilage injuries and repair. J Bone Joint Surg Am 85-A Suppl 2:58–69
Buckwalter JA, Mankin HJ (1998) Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect 47:487–504
Burr DB, Radin EL (2003) Microfractures and microcracks in subchondral bone: are they relevant to osteoarthrosis? Rheum Dis Clin North Am 29:675–685
Gillogly SD (2003) Treatment of large full-thickness chondral defects of the knee with autologous chondrocyte implantation. Arthroscopy 19(Suppl 1):147–153
Gomoll AH, Gillogly SD, Cole BJ, Farr J, Arnold R, Hussey K et al (2014) Autologous chondrocyte implantation in the patella: a multicenter experience. Am J Sports Med 42:1074–1081
Gomoll AH, Madry H, Knutsen G, van Dijk N, Seil R, Brittberg M et al (2010) The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc 18:434–447
Goyal D, Goyal A, Keyhani S, Lee EH, Hui JH (2013) Evidence-based status of second- and third-generation autologous chondrocyte implantation over first generation: a systematic review of level I and II studies. Arthroscopy 29:1872–1878
Goyal D, Keyhani S, Lee EH, Hui JH (2013) Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy 29:1579–1588
Harris JD, Siston RA, Pan X, Flanigan DC (2010) Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am 92:2220–2233
Higgins LD, Taylor MK, Park D, Ghodadra N, Marchant M, Pietrobon R et al (2007) Reliability and validity of the International Knee Documentation Committee (IKDC) Subjective Knee Form. Joint Bone Spine 74:594–599
Hunter W (1743) On the structure and disease of articular cartilage. . Philos Trans R Soc London Biol 514–521
Jungmann PM, Salzmann GM, Schmal H, Pestka JM, Sudkamp NP, Niemeyer P (2012) Autologous chondrocyte implantation for treatment of cartilage defects of the knee: what predicts the need for reintervention? Am J Sports Med 40:58–67
Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC et al (2007) A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am 89:2105–2112
Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grontvedt T, Solheim E et al (2004) Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am 86:455–464
Kon E, Gobbi A, Filardo G, Delcogliano M, Zaffagnini S, Marcacci M (2009) Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med 37:33–41
Kreuz PC, Muller S, von Keudell A, Tischer T, Kaps C, Niemeyer P et al (2013) Influence of sex on the outcome of autologous chondrocyte implantation in chondral defects of the knee. Am J Sports Med 41:1541–1548
Madry H (2010) The subchondral bone: a new frontier in articular cartilage repair. Knee Surg Sports Traumatol Arthrosc 18:417–418
Mall NA, Harris JD, Cole BJ (2015) Clinical evaluation and preoperative planning of articular cartilage lesions of the knee. J Am Acad Orthop Surg 23:633–640
Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T (2009) Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med 37:902–908
Minas T, Von Keudell A, Bryant T, Gomoll AH (2014) The John Insall Award: a minimum 10-year outcome study of autologous chondrocyte implantation. Clin Orthop Relat Res 472:41–51
Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR (2009) Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med 37:2053–2063
Muller S, Hirschmuller A, Erggelet C, Beckmann NA, Kreuz PC (2015) Significantly worse isokinetic hamstring-quadriceps ratio in patellofemoral compared to condylar defects 4 years after autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc 23:2151–2158
Mundi R, Bedi A, Chow L, Crouch S, Simunovic N, Sibilsky Enselman E et al (2016) Cartilage restoration of the knee: a systematic review and meta-analysis of level 1 studies. Am J Sports Med 44:1888–1895
Nawaz SZ, Bentley G, Briggs TW, Carrington RW, Skinner JA, Gallagher KR et al (2014) Autologous chondrocyte implantation in the knee: mid-term to long-term results. J Bone Joint Surg Am 96:824–830
Niemeyer P, Andereya S, Angele P, Ateschrang A, Aurich M, Baumann M et al (2013) Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: a guideline by the working group "Tissue Regeneration" of the German Society of Orthopaedic Surgery and Traumatology (DGOU). Z Orthop Unfall 151:38–47
Niemeyer P, Pestka JM, Salzmann GM, Sudkamp NP, Schmal H (2012) Influence of cell quality on clinical outcome after autologous chondrocyte implantation. Am J Sports Med 40:556–561
Niemeyer P, Salzmann G, Feucht M, Pestka J, Porichis S, Ogon P et al (2014) First-generation versus second-generation autologous chondrocyte implantation for treatment of cartilage defects of the knee: a matched-pair analysis on long-term clinical outcome. Int Orthop 38:2065–2070
Niethammer TR, Safi E, Ficklscherer A, Horng A, Feist M, Feist-Pagenstert I et al (2014) Graft maturation of autologous chondrocyte implantation: magnetic resonance investigation with T2 mapping. Am J Sports Med 42:2199–2204
Niethammer TR, Valentin S, Ficklscherer A, Gulecyuz MF, Pietschmann MF, Muller PE (2015) Revision surgery after third generation autologous chondrocyte implantation in the knee. Int Orthop 39:1615–1622
Pestka JM, Bode G, Salzmann G, Steinwachs M, Schmal H, Sudkamp NP et al (2014) Clinical outcomes after cell-seeded autologous chondrocyte implantation of the knee: when can success or failure be predicted? Am J Sports Med 42:208–215
Pestka JM, Bode G, Salzmann G, Sudkamp NP, Niemeyer P (2012) Clinical outcome of autologous chondrocyte implantation for failed microfracture treatment of full-thickness cartilage defects of the knee joint. Am J Sports Med 40:325–331
Richter DL, Schenck RC Jr, Wascher DC, Treme G (2016) Knee articular cartilage repair and restoration techniques: a review of the literature. Sports Health 8:153–160
Saris DB, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J et al (2009) Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med 37(Suppl 1):10S–19S
Saris DB, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y et al (2008) Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med 36:235–246
Steadman JR, Miller BS, Karas SG, Schlegel TF, Briggs KK, Hawkins RJ (2003) The microfracture technique in the treatment of full-thickness chondral lesions of the knee in National Football League players. J Knee Surg 16:83–86
Steadman JR, Rodkey WG, Rodrigo JJ (2001) Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res 391(Suppl):S362–369
Vanlauwe J, Saris DB, Victor J, Almqvist KF, Bellemans J, Luyten FP et al (2011) Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med 39:2566–2574
Vasiliadis HS, Wasiak J, Salanti G (2010) Autologous chondrocyte implantation for the treatment of cartilage lesions of the knee: a systematic review of randomized studies. Knee Surg Sports Traumatol Arthrosc 18:1645–1655
Vavken P, Samartzis D (2010) Effectiveness of autologous chondrocyte implantation in cartilage repair of the knee: a systematic review of controlled trials. Osteoarthritis Cartilage 18:857–863
Weber AE, Locker PH, Mayer EN, Cvetanovich GL, Tilton AK, Erickson BJ et al (2018) Clinical outcomes after microfracture of the knee: midterm follow-up. Orthop J Sports Med 6:2325967117753572
Zaslav K, Cole B, Brewster R, DeBerardino T, Farr J, Fowler P et al (2009) A prospective study of autologous chondrocyte implantation in patients with failed prior treatment for articular cartilage defect of the knee: results of the Study of the Treatment of Articular Repair (STAR) clinical trial. Am J Sports Med 37:42–55
Acknowledgements
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution. Prof. Müller and David Gallik have contributed equally to this article and share the first authorship.
Funding
No external funding was used.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures were performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1864 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Müller, P.E., Gallik, D., Hammerschmid, F. et al. Third-generation autologous chondrocyte implantation after failed bone marrow stimulation leads to inferior clinical results. Knee Surg Sports Traumatol Arthrosc 28, 470–477 (2020). https://doi.org/10.1007/s00167-019-05661-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-019-05661-6