Abstract
Tumor-infiltrating lymphocytes are an important prognostic factor after neoadjuvant chemotherapy (NAC) in patients with breast cancer. Natural killer (NK) cells play critical roles in antitumor immune surveillance. Here, we assessed the relationship between peripheral natural killer (pNK) cell activity, tumor microenvironmental factors (TMEFs), and the therapeutic efficacy of preoperative chemotherapy in patients with breast cancer. In a cohort of 39 patients diagnosed with stage II–IV breast cancer who received NAC, we measured pNK cell activity by chromium release assay and assessed TMEF levels by next-generation sequencing. Following NAC, pNK cell activity was increased in 24/39 patients but decreased in 15/39 patients. Increased pNK cell activity following preoperative chemotherapy was associated significantly with the disappearance of axillary lymph node metastasis (Ax+; p = 0.0235). Increased pNK cell activity remained significantly associated with the disappearance of Ax+ in multivariate logistic regression analysis (OR 5.41, 95% CI 1.19–24.52, p = 0.0283). A Grade 2 or higher effect of NAC was associated with high pre-NAC cytotoxic T lymphocyte-associated protein 4 (CTLA-4) levels (p = 0.0281) and elevated post-NAC NK (p = 0.0005) cells and transforming growth factor-beta (TGF-β; p = 0.0350) levels. The disappearance of Ax+ was associated with high pre-NAC CTLA-4 levels (p = 0.0278) and elevated CD4 levels after NAC (p = 0.0250). The systemic activation of pNK cells after NAC may improve metastatic tumor elimination in patients with breast cancer owing to a release from local immunosuppression, and immune activation in the tumor microenvironment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tumor-infiltrating lymphocytes, including cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells, play a key role in the development of a pathologically complete response to neoadjuvant chemotherapy (NAC) in patients with breast cancer. The prognostic value of a pathological tumor response differs by tumor subtype, being predictive of good outcomes in patients with human epidermal growth factor receptor-2 (HER-2) positive or triple negative (TN) breast cancer, but not in patients with luminal type cancer [1].
NK cells are critical for the innate immunity-mediated eradication of tumor cells in a way that involves a cooperative relationship with T-cell amplified adaptive immunity [2]. The presence of tumor-infiltrating lymphocytes within primary lesions, and more specifically the infiltration of CD8+ CTLs, is associated with NAC efficacy in terms of the posttreatment reduction of tumor cells [3]. However, the precise role of NK cells in human breast cancer remains unclear, and the behavior of NK cells following NAC has yet to be clarified. The observation that NK cells have a substantial presence peritumorally, while infiltrating the tumor proper only rarely [4], suggests that peritumor-infiltrating NK cells may have a functional role in killing tumor cells in addition to the CD8+ T-cell subpopulation of stromal tumor-infiltrating lymphocytes. Providing additional support for this scenario, increased numbers of peripheral NK (pNK) cells and increased pNK activity levels were found to be associated with good pathological therapeutic efficacy of NAC in patients with breast cancer [5].
The aim of the present study was to investigate the functional role of NK cells in peripheral blood in terms of a systemic immune response to NAC in patients with breast cancer. Toward this aim, we assessed pNK cell activity and analyzed whether it was associated with the pathologic therapeutic efficacy of NAC and a specific tumor subtype. To assess the relationship between systemic and local immune responses to NAC, tumor microenvironmental factors (TMEFs) were evaluated.
Materials and methods
Patients
Thirty-nine patients with stage II–IV breast cancer who received NAC between July 2012 and August 2017 at our clinic were enrolled in this study. Their disease stages were diagnosed according to the tumor–node–metastasis (TNM) classification scheme recommended by the Union for International Cancer Control [6]. Therapy effects were evaluated according to the histopathological criteria for the assessment of therapeutic effects in breast cancer indicated by the Japanese Breast Cancer Society [7]. Pathological therapeutic efficacy in intramammary lesions was graded as follows: Grade 0 (G0), no or negligible change in cancer cells; Grade 1a, mild changes in cancer cells regardless of area, or marked changes in < 1/3 of cancer cells; Grade 1b, marked changes in ≥ 1/3 but < 2/3 of cancer cells; Grade 2a, marked changes in ≥ 2/3 of cancer cells; Grade 2b, disappearance of almost all cancer cells; and Grade 3, apparent disappearance of all cancer cells. Efficacy grades were classified as involving breast ducts and/or axillary lymph nodes. When observed, the disappearance of lymph node metastasis was noted. The post-NAC disappearance of cancer cells from all breast tissue and axillary lymph nodes was considered a complete response, defined as ypT0 ypN0, since it has been suggested that axillary status is a better prognostic factor than the response of the primary tumor to NAC [8,9,10]. The disappearance of axillary lymph node metastasis (Ax+) was confirmed by the pathological analysis of surgically dissected axillary lymph nodes.
Measurement of pNK cell activity
Peripheral NK cell activity was measured immediately before and 3 weeks after NAC by chromium release assay conducted by SRL, Inc. (Tokyo, Japan). Peripheral NK cells were assayed for cytotoxic activity against 51Cr-labeled target cells. To isolate lymphocytes, 5-mL blood samples were centrifuged for 20 min at 1800 rpm and then washed twice in phosphate buffered saline at 2000 rpm for 5 min. Effector (E) and target (T) cells (K-562) were adjusted to 1 × 106/mL and 10 µL of target cells were transferred to plates. Then, 200 µL of effector cells (desired E:T ratio, 20:1) were plated and incubated at 37 °C for 3.5 h in a CO2 incubator. The cells were then centrifuged, collected, and counted in a gamma counter. The percentage of pNK cell activity was calculated as follows: pNK cell activity (%) = experimental group release − background release/maximal release − background release × 100.
Assessment of TMEFs
TMEFs were subjected to next-generation sequencing for CD4, CD8, NK, forkhead box P3 (FOXP3), cytotoxic T lymphocyte-associated protein 4 (CTLA-4), programmed death 1 (PD-1), programmed death-ligand 1 (PD-L1), interleukin (IL)-2, IL-6, IL-12, interferon (IFN)-γ, IL-10, transforming growth factor (TGF)-β, and vascular endothelial growth factor (VEGF) in formalin-fixed paraffin embedded (FFPE) sections collected from preoperative vacuum-associated biopsy samples and surgical specimens.
Purification of total RNA from FFPE tissue sections
FFPE samples were prepared in an automated fashion with the RNeasy® FFPE kit (Qiagen, Venlo, Netherlands). Paraffin was eliminated from freshly cut FFPE tissue sections using a deparaffinization solution. The samples were incubated in an optimized lysis buffer containing proteinase K to release RNA from the sections and then heated briefly to partially reverse formalin crosslinking of released nucleic acids, improving RNA yield and quality.
Targeted RNA sequencing
Complementary DNA was synthesized by reverse transcriptase and random primers. A TruSeq targeted RNA expression assay kit (Illumina, San Diego, CA, USA) was then used to profile gene expression. This kit allows up to 15,000 gene expression results to be examined with 50 bp of sequencing reads targeting specific exons and splice junctions. An oligo pool containing upstream and downstream oligos specific to targeted regions was hybridized, wherein they were bound to paramagnetic streptavidin beads. After combining up to two targeted oligo pools, the extension-ligation product was amplified, and index 1 (i7) and index 2 (i5) adapters were added with the sequences required for cluster formation to produce cDNA libraries. The libraries were cleaned with AMPure XP beads, which purify polymerase chain reaction products from other reaction components. The cDNA libraries were combined, quantified, denatured, and diluted in equal volumes in hybridization buffer for pool and quantification libraries. Libraries prepared with TruSeq kit components were used with the MiniSeq system (Illumina). Final TMEF levels were determined relative to glyceraldehyde 3-phosphate dehydrogenase expression. TMEF levels were reported as medians and ranges of targeting genes from several immune response amplicon sequences in the tumor microenvironment.
Statistical analysis
All data were recorded in a spreadsheet and analyzed in Statcel 4 (2015). Continuous and independent variables were analyzed using Mann–Whitney tests. Categorical variables were analyzed using Chi-square and Fisher’s exact tests. Univariate and multivariate analyses were performed to evaluate associations between clinicopathological factors, pNK cell activity, and TMEF levels. In multivariate analysis, we used a logistic regression model to determine statistical relationships between each dependent variable and independent variables. Odds ratios (ORs) are reported with 95% confidence intervals (CIs). A p value < 0.05 was considered significant.
Results
Patient characteristics and therapeutic efficacy
The cohort of 39 patients had a median age of 53 years (range 27–69) and the distribution of their TNM stages was as follows: II (N = 18), III (N = 16), and IV (N = 5). The tumor subtypes represented included luminal (N = 23), HER-2 positive (N = 12), and TN (N = 4). In general, the five stage IV patients did not undertake NAC. In this study, locoregional treatment was considered as palliative surgery in patients with chemotherapy effectively downstaging primary breast cancer, although the role of breast surgery in stage IV patients remains unresolved. The clinical characteristics of these five patients were as follows: T2N1M1 (bone); T3N3M1 (distant lymph node); T4bN1M1 (bone); T2N3M1 (bone); and T2N3M1 (lung/bone). The treatment regimens employed included nanoparticle albumin-bound-paclitaxel (Nab-PTX)/5-fluorouracil/epirubicin/cyclophosphamide (FEC) (N = 11; + trastuzumab: N = 2), docetaxel (DTX)/FEC (N = 3), FEC/Nab-PTX (N = 16; + trastuzumab: N = 9), and FEC/DTX (N = 3; + trastuzumab: N = 1), FEC/PTX (N = 2), FEC/Nab-PTX (N = 1), epirubicin/cyclophosphamide (EC)/DTX (N = 2), and EC/PTX (N = 1); trastuzumab (Tz) was used for cases with HER-2 positivity. According to histopathological criteria, pathological therapeutic outcomes were G1a in 8 cases, G1b in 13 cases, G2a in 7 cases, G2b in 4 cases, and G3 (i.e., complete) in 7 cases. Thus, the pathological complete response rate was 17.9%.
Post-NAC pNK cell activity changes and relationship with pathological therapeutic effect
Relative to pre-NAC levels, pNK cell activity was altered significantly following NAC (p = 0.0005), with 24/39 patients showing an increase and 15/39 patients showing a decrease. The results of our univariate analyses of increased vs. decreased post-NAC pNK cell activity groups in relation to clinicopathological factors are shown in Table 1. Briefly, these two groups did not differ significantly in terms of age, tumor type (luminal, HER-2 positive, or TN) distribution, TNM stage (II, III, or IV) distribution, administration of trastuzumab treatment, nuclear grade (1, 2, or 3) distribution, Ki-67 positivity (< 15%, 15–35%, or > 35%) distribution, or proportion of patients with a G2 or better response. However, increased pNK cell activity was observed more frequently in patients who exhibited a disappearance of Ax+ relative to those who did not (Table 1). Further, based on univariate analyses, multivariate logistic regression analysis concerning the relationship between pNK cell activity as a dependent variable and the clinicopathological factors of Ax+ disappearance and a G2 or better response as independent variables confirmed that increased pNK cell activity following NAC was associated with the disappearance of Ax+ (OR 5.41; 95% CI 1.19–24.52; p = 0.0283; Table 2). Similarly, multivariate logistic regression analysis of the relationship between pNK cell activity and tumor subtype showed that increased pNK cell activity did not associate significantly with a luminal, HER-2 positive, or TN tumor status (Table 2).
Involvement of clinical factors and TMEFs in therapeutic effect of NAC
A subgroup (28/39 patients in the study) was subjected to univariate analyses of clinical factors and TMEF levels in relation to NAC response (G1 vs. G2–3), the results of which are shown in Table 3. In this subgroup, we found that a G2 or better outcome was positively associated with a HER-2 positive tumor status and the use of trastuzumab-containing regimens (Table 3). Increased pNK cell activity tended to be associated with decreased PD-L1 after NAC (data not shown). With respect to pretreatment TMEFs, a G2 or better therapeutic effect was significantly associated with high pre-NAC levels of CTLA-4, but not with pre-NAC levels of CD4. Additionally, we observed nonsignificant trends with respect to pre-NAC IL-12 and TGF-β levels potentially being related to a G2 or better therapeutic effect (Table 3). With respect to post-NAC TMEFs, a G2 or better therapeutic effect was significantly associated with post-NAC NK and TGF-β levels, but not with post-NAC levels of CTLA-4 or IL-12 (Table 3). With respect to TMEF level changes from before to after NAC, it is interesting to note that the patients who had G1 vs. G2 or better outcomes tended to exhibit opposite respective directionalities of changes in CD4 (decrease vs. increase), IL-12 (increase vs. decrease), and TGF-β (decrease vs. increase). Further, multivariate logistic regression analysis showed nonsignificant trends suggestive of a G2 or better effect potentially being associated with higher NK after NAC (OR 1.16; 95% CI 0.99–1.36; p = 0.0625) and increased TGF-β level before chemotherapy (OR 0.99; 95% CI 0.99–1.00; p = 0.0739; Table 2).
Factors related to disappearance of Ax+
A subgroup (23/39 patients in the study) was subjected to univariate analyses of TMEF levels in relation to Ax+ disappearance (not disappeared vs. disappeared), the results of which are reported in Table 4. In this subgroup, we found a significant relationship between tumor subtype and Ax+ disappearance, with HER-2 positive tumors clustering in the Ax+ disappearance group (8/10), and both of only two patients with TN tumors exhibiting the disappearance of Ax+. We also observed a significant relationship of increased pNK cell activity following NAC and the disappearance of Ax+ in this subgroup (Table 4). Further, multivariate logistic regression analysis showed that the disappearance of Ax+ was significantly associated with increased pNK cell activity (OR 8.47; 95% CI 1.41–50.72; p = 0.0019), but not tumor subtype (Table 2). With respect to TMEFs, univariate analyses indicated that the disappearance of Ax+ was significantly associated with high pre-NAC CTLA-4 levels and elevated post-NAC CD4 levels (Table 4). With respect to TMEF level changes before to after NAC, it is interesting to note that the patients who exhibited a disappearance of Ax+ tended to show increases in CD4 and decreases in CTLA-4 from pre- to post-NAC, whereas those who did not show a disappearance of Ax+ displayed the opposite tendencies (decreased CD4 and increased CTLA-4).
Discussion
In the present study, increased pNK cell activity was found to be associated with the disappearance of Ax+ following NAC, irrespective of tumor subtype, in patients with breast cancer. Because NK cells play a key role in innate antitumor immune surveillance, pNK cell activation has the potential to eradicate metastatic cells in lymph nodes, separate from shrinkage of the primary tumor. The mechanism underlying elevated pre-NAC pNK cell activity in treatment responders, compared to that in non-responders, remains to be determined. However, our data are consistent with the idea that increased pNK cell activity may facilitate the elimination of metastatic tumor cells. This is further supported by prior tumor model experiments showing that NK cell deficiency was associated with accelerated metastasis, independent of primary tumor growth [11].
According to our univariate analysis, the disappearance of Ax+ was more frequent in HER-2 positive and TN breast cancer cases than in luminal type cancer cases, and was associated with increased pNK cell activity. Although it is possible that trastuzumab-induced antibody-dependent cellular cytotoxicity may promote pNK cell activity, multivariate logistic regression analysis showed that the disappearance of Ax+ was associated with pNK cell activity, but not tumor subtype, suggesting that other factors are likely to be involved in increasing pNK cell activity following preoperative NAC in patients with breast cancer.
Stromal and/or tumor-derived immunosuppressive cytokines, such as TGF-β and IL-10, have been found to mediate reductions in NK-cell function [12]. Here, we found that post-NAC increases in pNK cell activity tended to be associated with post-NAC reductions in PD-L1 levels. Notably, we found that elevated CTLA-4 levels prior to NAC were associated with both good NAC outcomes (G2 or better) and the disappearance of Ax+. These positive treatment responses appear to reflect a release from tumor cell PD-L1-generated immunosuppression, perhaps owing to the effects of CTLA-4 in regulatory T (Treg) cells.
The present findings of a G2 or better NAC effect being associated with relatively low pre-NAC TGF-β levels and of the disappearance of Ax+ being associated with high post-NAC CD4 levels suggest that effective NAC involves the co-activation of antitumor immunity at both the primary tumor site and in metastatic lymph nodes. CD8 levels were not significantly related to pNK cell activity, grade of the NAC effect (G2 or better), or the disappearance of Ax+ in this analysis. Of interest, although the immunosuppressive cytokine effects of TGF-β are well known [13], a good therapeutic effect at the primary tumor site was associated with relatively high post-NAC levels of TGF-β in this study, suggesting that NAC-induced stress on tumor cells may trigger these to signal for protective increases by further augmenting the immunosuppressive characteristics of the tumor microenvironment.
Although the present study had the limitation of having only a small number of cases, in the subgroup analyses of 28 cases for therapeutic outcome and 23 cases for Ax+ disappearance, a statistically significant difference in TMEF immune response between responders and non-responders was observed. Further examination is necessary to obtain more information in relation to TMEFs and the pathological therapeutic effects of NAC. In addition, elucidating how NK cell activity is regulated is of the utmost importance. Recent studies have indicated that NK-cell proliferation, accumulation, and activation are under the control of Treg cells via a Treg-NK cell interaction [14]. More specifically, Tregs have been shown to inhibit NK cell function in a cell contact-dependent, TGF-β-mediated process [15].
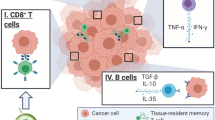
Our findings of increased pNK cell activity following NAC being associated with the disappearance of Ax+, but not with the grade of the pathological therapeutic effect at the primary tumor site raises a key question regarding what factors are involved in pNK activation—that is, which systemic immune factors distinguish responders from non-responders. It is our view that the systemic immune response may work in cooperation with the local immune response, which is reflected by TMEFs including tumor-infiltrating lymphocytes, cytokines, and other immune-related factors. A recent study indicated that systemic immunity, such as a peripheral CD4 T-cell response, was required for tumor eradication as well as local immunity within the tumor in immunotherapy models [16]. As shown in Fig. 1, local immunosuppression may be formed by the upregulation of both PD-L1 in tumor cells and CTLA-4 in Treg cells prior to chemotherapy. After NAC, both PD-L1 and CTLA-4 were downregulated, perhaps leading to a release from local immunosuppression. The presence of IL-12 may upregulate CD4 levels and NK cells, leading to the activation of a local antitumor immune response. Such local immunity may cooperate with systemic immunity, such as pNK cells, to eradicate tumor cells in responders.
A hypothesis of systemic and local immune activation interactions in the therapeutic effect induced by neoadjuvant chemotherapy (NAC) in patients with breast cancer. According to this model, in NAC responders, elevated peripheral natural killer (pNK) cell activity works cooperatively with not only a release from local suppression of antitumor immunity (imposed by the tumor cell expression of programmed death-ligand 1 [PD-L1]) and decreased cytotoxic T lymphocyte-associated protein 4 (CTLA-4) expression in regulatory T (Treg) cells, but also with the local immune activation of CD4-expressing and natural killer (NK) cells in the presence of interleukin (IL)-12 in the tumor microenvironment. Increased transforming growth factor (TGF)-β may contribute to the inhibitory functions of tumor immunity. NK natural killer, Ax+ axillary lymph node metastasis
Conclusions
Our findings suggest that the presence of CD4 and NK cells within tumor-infiltrating lymphocyte population profiles may favor NAC-induced antitumor immunity in patients with breast cancer. Although the immunosuppressive network derived from the presence of tumor-derived soluble immunosuppressive factors remains to be delineated, the present findings implicating PD-L1 and CTLA-4 in the formation of the immunosuppressive tumor microenvironment suggest that immune checkpoint inhibitors targeting PD-L1 and CTLA-4 may improve NAC efficacy for breast cancer. Good NAC treatment responders were found to exhibit post-NAC TGF-β increases, though it is unclear whether attempts by tumor cells to further suppress immunity were overwhelmed. Further characterization of functional alterations in pNK cells and pNK subpopulations with respect to NAC responder status in patients with breast cancer is required.
Abbreviations
- Ax+:
-
Axillary lymph node metastasis
- CI:
-
Confidence interval
- CTLA-4:
-
Cytotoxic T lymphocyte-associated protein 4
- DTX:
-
Docetaxel
- FEC:
-
5-Fluorouracil/epirubicin/cyclophosphamide
- FFPE:
-
Formalin-fixed paraffin embedded
- HER-2:
-
Human epidermal growth factor receptor-2
- Nab-PTX:
-
Nanoparticle albumin-bound-paclitaxel
- NAC:
-
Neoadjuvant chemotherapy
- OR:
-
Odds ratio
- pNK:
-
Peripheral natural killer
- TMEF:
-
Tumor microenvironmental factor
- TN:
-
Triple negative
- Tz:
-
Trastuzumab
References
Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J, Huober J, Klauschen F, Furlanetto J, Schmitt WD, Blohmer JU, Karn T, Pfitzner BM, Kümmel S, Engels K, Schneeweiss A, Hartmann A, Noske A, Fasching PA, Jackisch C, van Mackelenbergh M, Sinn P, Schem C, Hanusch C, Untch M, Loibl S (2018) Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 19:40–50. https://doi.org/10.1016/S1470-2045(17)30904-X
Shanker A, Marincola FM (2011) Cooperativity of adaptive and innate immunity: implications for cancer therapy. Cancer Immunol Immunother 60:1061–1074. https://doi.org/10.1186/1479-5876-11-145
Seo AN, Lee HJ, Kim EJ, Kim HJ, Jang MH, Lee HE, Kim YJ, Kim JH, Park SY (2013) Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer 109:2705–2713. https://doi.org/10.1038/bjc.2013.634
Verma C, Kaewkangsadan V, Eremin JM, Cowley GP, Ilyas M, El-Sheemy MA, Eremin O (2015) Natural killer (NK) cell profiles in blood and tumour in women with large and locally advanced breast cancer (LLABC) and their contribution to a pathological complete response (PCR) in the tumour following neoadjuvant chemotherapy (NAC): differential restoration of blood profiles by NAC and surgery. J Transl Med 13:180. https://doi.org/10.1186/s12967-015-0535-8
Muraro E, Comaro E, Talamini R, Turchet E, Miolo G, Scalone S, Militello L, Lombardi D, Spazzapan S, Perin T, Massarut S, Crivellari D, Dolcetti R, Martorelli D (2015) Improved Natural Killer cell activity and retained anti-tumor CD8(+) T cell responses contribute to the induction of a pathological complete response in HER2-positive breast cancer patients undergoing neoadjuvant chemotherapy. J Transl Med 13:204. https://doi.org/10.1186/s12967-015-0567-0
Brierley JD, Gospodarowicz MK, Wittekind C (eds) (2017) TNM classification of malignant tumours, 8th edn. Wiley-Blackwell, Hoboken
Kurosumi M (2006) Significance and problems in evaluations of pathological responses to neoadjuvant therapy for breast cancer. Breast Cancer 13:254–259. https://doi.org/10.2325/jbcs.13.254
Rouzier R, Extra JM, Klijanienko J, Falcou MC, Asselain B, Vincent-Salomon A, Vielh P, Bourstyn E (2002) Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J Clin Oncol 20:1304–1310
Diaz-Botero S, Espinosa-Bravo M, Gonçalves VR, Esgueva-Colmenarejo A, Peg V, Perez J, Cortes J, Rubio IT (2016) Different prognostic implications of residual disease after neoadjuvant treatment: Impact of Ki 67 and site of response. Ann Surg Oncol 23:3831–3837
Hayashi N, Takahashi Y, Matsuda N, Tsunoda H, Yoshida A, Suzuki K, Nakamura S, Yamauchi H (2018) The prognostic effect of changes in tumor stage and nodal status after neoadjuvant chemotherapy in each primary breast cancer subtype. Clin Breast Cancer 18:e219–e229. https://doi.org/10.1016/j.clbc.2017.09.013
Slaney CY, Rautela J, Parker BS (2013) The emerging role of immunosurveillance in dictating metastatic spread in breast cancer. Cancer Res 73:5852–5857. https://doi.org/10.1158/0008-5472.CAN-13-1642
Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, Gonçalves A, André P, Romagné F, Thibault G, Viens P, Birnbaum D, Bertucci F, Moretta A, Olive D (2011) Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest 121:3609–3622. https://doi.org/10.1172/JCI45816
Pickup M, Novitskiy S, Moses HL (2013) The roles of TGFβ in the tumour microenvironment. Nat Rev Cancer 13:788–799. https://doi.org/10.1038/nrc3603
Kerdiles Y, Ugolini S, Vivier E (2013) T cell regulation of natural killer cells. J Exp Med 210:1065–1068. https://doi.org/10.1084/jem.20130960
Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, Lecesne A, Robert C, Blay JY, Bernard J, Caillat-Zucman S, Freitas A, Tursz T, Wagner-Ballon O, Capron C, Vainchencker W, Martin F, Zitvogel L (2005) CD4+ CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med 202:1075–1085
Spitzer MH, Carmi Y, Reticker-Flynn NE, Kwek SS, Madhireddy D, Martins MM, Gherardini PF, Prestwood TR, Chabon J, Bendall SC, Fong L, Nolan GP, Engleman EG (2017) Systemic immunity is required for effective cancer immunotherapy. Cell 168:487–502. https://doi.org/10.1016/j.cell.2016.12.022
Acknowledgements
The authors thank the patients and their families for their participation in the study. The authors also thank SRL. Inc. (Tokyo, Japan) for the measurement of pNK cell activity.
Funding
No specific funding was received for this study.
Author information
Authors and Affiliations
Contributions
Conception and design: RK. Collection and assembly of data: RK, AK, MW, YF, NY, MH, YM, SO, MI, KA. Data analysis and interpretation: RK, AK, MH, YM. Manuscript writing: RK, YM. Final approval of manuscript: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was approved by the research ethics committee of the Hiroshima Mark Clinic on July 1, 2012. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kim, R., Kawai, A., Wakisaka, M. et al. A potential role for peripheral natural killer cell activity induced by preoperative chemotherapy in breast cancer patients. Cancer Immunol Immunother 68, 577–585 (2019). https://doi.org/10.1007/s00262-019-02305-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-019-02305-z