Abstract
Purpose
The objective of this study was to evaluate whether 68Ga-PSMA PET/CT whole-body tumor burden (PSMAwbtb) is associated with clinical parameters and laboratory parameters in prostate cancer patients.
Methods
We retrospectively evaluated prostate cancer patients submitted to PSMA PET/CT for primary staging purposes or due to biochemical recurrence (BR). PSMAwbtb metrics (total volume of PSMA-avid tumor (PSMA-TV)) and total uptake of PSMA-avid lesions (PSMA-TL) were calculated semi-automatically. Spearman’s rank correlations between PSMAwbtb metrics and clinical, laboratory parameters (age, time-to-BR, years of diagnosis of prostate cancer, free and total serum PSA levels, and the Gleason score) and with the highest SUVmax of a lesion (hSUVmax) were analyzed.
Results
Among the 257 PSMA PET/CT studies, there were 46 scans (17.9%) performed for primary staging and 211 (82.1%) for BR. PSMA-TV and PSMA-TL were calculated for the 157 positive scans (58.8%), which were 43 patients (93.5%) in the primary staging group and 114 patients (54.0%) in the BR group. In the primary staging group, we observed a significant correlation between PSMA-TL and hSUVmax (p = 0.0021). In the BR group, there was a significant direct correlation between PSMA-TL and the variables age (p = 0.0031), total serum PSA values (p = < 0.0001), free serum PSA values (p = < 0.0001), and the hSUVmax (p = < 0.0001). Similar results were obtained for PSMA-TV.
Conclusion
PSMAwbtb has a direct and positive correlation with serum PSA values and age in prostate cancer patients with BR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the second most frequent malignant neoplasm in men, being the sixth cause of death according to the World Health Organization [1]. Advances in diagnostic imaging, surgeries, radiotherapies, secondary hormonal therapies, and chemotherapies have increased both diagnostic and therapeutic efficacy, facilitating the management of patients with prostate cancer and improving quality of life [2,3,4]. In spite of this, more than 50% of high-risk prostate cancer patients develop biochemical recurrence (BR), after initial therapy [5].
Imaging plays an essential role in the evaluation of intermediate and high-risk prostate cancer patients and gallium-labeled prostate membrane-specific antigen (68Ga-PSMA) performed on positron emission computed tomography combined with computed tomography (PET/CT) scanners has gained widespread use as it essentially translates tumor molecular biology into clinical reality. 68Ga-PSMA PET/CT is highly sensitive to identify the underlying cause of BR (including patients with PSA levels below 0.5 ng/mL), and also in the primary staging of high-risk prostate cancer patients [6,7,8,9]. Moreover, 68Ga-PSMA PET/CT is a reliable tool in prostate cancer staging, which leads to proper changes in patient management compared with the traditional staging methods, as demonstrated by Hofman et al. [10]
In several tumors, PET/CT whole-body tumor burden with 18F-FDG and with 18F-sodium fluoride has shown to correlate directly with their aggressiveness [11]. Specifically, in prostate cancer, a clear understanding of the role of 68Ga-PSMA PET/CT whole-body tumor burden (PSMAwbtb) is an unmet need that may help to better define prostate cancer treatment approach. Therefore, the purpose of this investigation was to evaluate if PSMAwbtb correlates with clinical and laboratory parameters in prostate cancer patients.
Patients and methods
The Committee for Ethics in the Research of Faculty of Medical Sciences of Campinas University - UNICAMP (CAEE # 89384418.1.0000.5404) approved this retrospective study and waived the need of informed consent.
Patients with a diagnosis of adenocarcinoma of the prostate that underwent 68Ga-PSMA PET/CT due to primary staging and to biochemical recurrence (BR) between April 2017 and December 2018 were consecutively included.
A primary staging 68Ga-PSMA PET/CT scan was requested in patients with suspicion of intermediate or high-risk cancer, defined by at least one of the following characteristics: prostate specific antigen (PSA) levels above 10 ng/mL (in at least two consecutive measures); Gleason score superior or equal to 7; and tumors classified as T2b or above.
In the BR group, the patients included were those defined using current guidelines [12]: PSA levels above 0.2 ng/mL (in at least two consecutive measures) after radical prostatectomy (RP) and rise by 2 ng/mL or more above the nadir PSA after radiation therapy with or without concurrent androgen deprivation therapy. In the BR group, also included were patients that presented with rising serum PSA levels after achieving undetectable values.
Patient treatments consisted of one or more of the following during the course of the disease: radical prostatectomy (RP), radiotherapy (RT), chemotherapy, and androgen deprivation therapy (ADT).
The PSMAwbtb metrics on 68Ga-PSMA PET/CT studies were determined for all patients and compared with the highest maximum standardized uptake value SUVmax (hSUVmax) on PSMA PET/CT and to the following clinical and laboratory parameters: age, years of diagnosis of prostate cancer; Gleason score, time-to-BR, and serum free and total PSA values obtained at the time of the 68Ga-PSMA PET/CT study.
Patients with missing histopathology data and Gleason score were excluded.
Conventional and metabolic images prior to 68Ga-PSMA PET/CT
All patients imaged for primary staging or BR of prostate cancer had negative conventional images, which consisted of pelvic ultrasound, bone scintigraphy (all of them with single photon emission computed tomography (SPECT/CT) using 99mTc-MDP), pelvic magnetic resonance imaging (MRI), and CT of the abdomen. Some patients also underwent PET/CT images performed with one or more of the following radiotracers: 18F-FDG, 11C-Choline, and/or 18F-Fluoride which did not detect the location of the metastases in patients with BR.
68Ga-PSMA PET/CT
Image acquisition and processing
All patients underwent a whole-body PET/CT scan (Siemens Biograph, Siemens ® Healthcare, USA) 60 min after intravenous injection of 185 MBq of 68Ga-PSMA (grupoRPH®, Brazil). Moreover, if the first scan lead to equivocal findings, delayed pelvic images after diuretics and voiding were acquired after (at least) 30 min of the 1st set of images to improve detection of lesions adjacent to the bladder or small lesions with lower expression of PSMA and/or slower tracer uptake.
CT parameters included 5-mm axial reconstruction and 120 kV or dose care kV tube voltage. PET images were acquired in 3-dimensional mode using 90s/bed position.
PET/CT images were processed in a dedicated workstation equipped with a commercial software package (Syngovia VB20; Siemens Medical Solutions, Chicago, IL), allowing simultaneous and fused review of PET and CT data.
68Ga-PSMA PET/CT interpretation
Two Nuclear Medicine physicians, with over 17 years of experience (EE and AS) with PET/CT, blindly interpreted all 68Ga-PSMA PET/CT images.
Focal uptake of 68Ga-PSMA higher than background was reported as suspicious for malignancy. Images were evaluated for detection of the primary tumor or local recurrence (including the seminal vesicles as local recurrence), locoregional and extra-pelvic lymph node metastases, bone metastases, and visceral metastases. Furthermore, the lesion with the highest maximum standardized uptake value SUVmax (hSUVmax) on 68Ga-PSMA PET/CT was recorded.
Semi-automatic calculation of PSMAwbtb
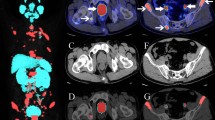
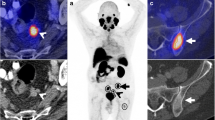
PET/CT images were quantified semi-automatically in a dedicated workstation (Syngovia VB20; Siemens Medical Solutions, Chicago, IL). We obtained the PSMAwbtb parameters by drawing a rectangular isocontour volume of interest (VOI) surrounding the entire body of the patient on the coronal axis and, if necessary, adjusted on the axial and sagittal planes. The SUVmax lower limit established prior to delineation of the VOIs was 2.5. Afterwards, all lesions were automatically delineated with a 41% VOI isocontour threshold of the SUVmax [13, 14] (Fig. 1). We excluded all VOIs not related to metastatic sites, such as areas of physiologic uptake (kidneys, bladder, ureters, etc.) as well as benign lesions. Furthermore, we also included the metastatic foci with uptake below the established SUVmax cutoff of 2.5 (initially excluded by the software) such as small lymph nodes with mild uptake.
Example of PSMAwbtb quantification with the pre-established configurations (SUVmax lower limit = 2.5; isocontour threshold SUVmax = 41%VOI). After a rectangular isocontour VOI surrounding the entire body of the patient on the coronal axis is drawn, all lesions are automatically delineated. a The MIP images display the VOIs that automatically surrounded all the metastatic sites. b The fused PET/CT image demonstrates the VOIs in the pelvic lymph node metastases. Careful evaluation of the images is necessary to exclude all VOIs not related to metastatic sites, such as areas of physiologic uptake (bladder, ureters, etc.). Afterwards, the software automatically generated the total tumor volume (PSMA-TV) and the total PSMA uptake in lesions (PSMA-TL)
Afterwards, the software automatically generated the total tumor volume (PSMA-TV) and the total PSMA uptake in lesions (PSMA-TL). To overcome any potential subjectivity of the adjustments, the analysis of the semi-automatic was performed by at least two investigators, and the final adjustment was based on consensus.
Statistical analyses
Exploratory data analysis was performed through summary measures (mean, standard deviation, minimum, median, maximum, frequency, and percentage). We correlated the clinical and laboratory parameters (PSA levels, Gleason score, and the lesion with the highest SUVmax) to the volumetric parameters (PSMA-TV and PSMA-TL), using the Spearman’s correlation coefficient for numerical variables. A p value of 0.05 was considered statistically significant. The Statistical Analysis System (SAS for Windows version 9.4) (SAS Institute Inc., 2002–2012, Cary, NC, USA) was used for calculations.
Results
A total of 279 PSMA PET/CT scans were evaluated. Twenty-two PSMA PET/CT scans were excluded due to incomplete data, and therefore, 257 patients with PSMA PET/CT studies were available for analysis.
Among these 257 patients with PSMA PET/CT studies, 46 (17.9%) were performed for primary staging, and 211 (82.1%) were requested due to BR. The mean age of the total patient population was 67.4 ± 8.8 years.
The total serum PSA values obtained immediately prior to the PSMA PET/CT studies were available for review in 244 (94.9%) patients. The serum total PSA values ranged from 0.03 to 227.8 ng/dL. In the subgroup of patients with a positive PSMA PET/CT study that underwent study due to BR (n = 114), the total PSA values were higher (mean = 17.3 ng/dL; median = 3.51 ng/dL) than in patients with a negative PSMA PET/CT (mean = 1.04 ng/dL; median = 0.43 ng/dL). Likewise, the free serum PSA values obtained immediately prior to the PSMA PET/CT in this group followed the same pattern. The free serum PSA values in patients with a positive PSMA PET/CT scan were higher (mean = 1.79 ng/dL; median = 0.49 ng/dL) than in patients with a negative scan (mean = 0.11 ng/dL; median = 0.03 ng/dL). Table 1 displays the demographic data of all patients and stratified according to primary staging and BR groups.
The Gleason score is described in Table 2. The majority of these prostate cancer patients had a Gleason 7 (40.3%) at initial diagnosis; this was also true in both the primary staging (43.2%) and the BR (29.7%) groups.
Treatment strategies prior to PSMA PET/CT imaging consisted of prostatectomy, chemotherapy, radiotherapy, or androgen deprivation therapy (ADT) but varied among both groups. In the group of patients that underwent PSMA PET/CT for primary staging, three patients (6.5%) were submitted to prostatectomy prior to imaging. These three patients were scanned shortly after prostatectomy and before any other treatment was introduced as pathological surgical findings resulted in increased risk for distant metastasis when compared with pre-surgical data. No patient in this group was submitted to chemotherapy, radiotherapy, or ADT prior to the PSMA PET/CT scans.
In the BR group, the majority (81.6%) of the patients underwent prostatectomy but were also submitted to one or more treatment regimens. Table 3 displays the treatment regimens applied to the patients and stratified according to groups (primary staging and BR).
PSMA whole-body tumor burden metrics versus clinical and laboratory characteristics
Positive PSMA PET/CT studies were quantified to determine the PSMAwbtb metrics (PMSA-TV and PSMA-TL) and the hSUVmax. Among the 46 patients imaged for primary staging purposes, 43 patients (93.5%) had positive PSMA PET/CT studies. Likewise, among the 211 patients imaged due to BR, 114 patients (54%) had positive 68Ga-PSMA PET/CT studies. Thus, a total of 157 PSMA PET/CTs were quantified.
Overall, the hSUVmax ranged from 1 to 170 (Table 4). In the patients that underwent a primary staging PSMA PET/CT, hSUVmax values were located in the majority (82.9%) as expected in the prostate bed, and in the BR group, the majority of the locations with the highest uptake were related to local recurrence (40.5%) followed by locoregional lymph node metastases.
Specifically evaluating the 33 patients that developed BR up to 1 year after prostatectomy, PSMA PET/CT was negative in 44% of patients. In the remaining 56% positive PSMA PET/CT scans, 18% were due to local recurrence, 25% had locoregional lymph node metastases, and 13% had distant metastases (including bone metastases in 50%).
PSMAwbtb was correlated to clinical and laboratory parameters for the entire group with positive scans (n = 157) and also correlated to the variables stratified by groups: staging (n = 43) and BR (n = 211). Overall, there was a significant correlation between the PSMA tumor burden metrics and the following variables: age (PSMA-TV: p = 0.0056; PSMA-TL: p = 0.0004), total serum PSA values (PSMA-TV: p = < 0.0001; PSMA-TL: p = < 0.0001), free serum PSA values (PSMA-TV: p = 0.0002; PSMA-TL: p = < 0.0001), the hSUVmax (PSMA-TV: p = 0.0195; PSMA-TL: p = <0.0001), and the Gleason score (PSMA-TV: p = 0.0462) (Table 5). Years of diagnosis of prostate cancer and the time-to-BR were not significantly associated with increased PSMAwbtb. However, when stratifying by groups, in the primary staging group, the only significant correlation with the PSMAwbtb was with the hSUVmax (PSMA-TL: p = 0.0021). On the other hand, in the BR group, there was a significant direct correlation between PSMAwbtb and the variables age (PSMA-TV: p = 0.0334; PSMA-TL: p = 0.0031), total serum PSA values (PSMA-TV: p < 0.0001; PSMA-TL: p < 0.0001), free serum PSA values (PSMA-TV: p = 0.0100; PSMA-TL: p < 0.0001), and the hSUVmax (PSMA-TL: p < 0.0001) (Table 5).
Discussion
PSMAwbtb has a strong correlation to the age and serum PSA levels in prostate cancer patients with BR. To our knowledge, this is the largest study performing PSMAwbtb semi-automatic quantitative analysis in prostate cancer patients (n = 257) being the largest evaluating patients with BR (n = 211) and the first to evaluate primary staging (n = 43). Similar studies performing PSMAwbtb semi-automatic quantification in patients with BR [15,16,17] also demonstrated that PSMAwbtb bears a strong correlation with PSA levels. However, PSMAwbtb did not correlate with PSA levels of the primary staging group indicating that it is probably helpless in such setting. The hSUVmax values were noted in the prostate (primary staging group) and in bone and distant lymph node metastases (BR group) with SUVmax values similar to those described in the literature [18,19,20].
Although tumor aggressiveness has been demonstrated to be more prevalent in younger patients, regardless of Gleason score [20], PSMAwbtb was significantly and directly correlated to patient age (> 75 years old). However, our results may be related to confounding aspects and not to the age per se, such as a delay in restaging (some cases up to 13 months) in older patients. This may also explain why no correlation was noted between the PSMAwbtb and the years of diagnosis of prostate cancer. Based on our findings, age should not be the reason for postponing restaging as it may result in larger tumor burden and more advanced recurrent disease at PSMA PET/CT. Similar to other studies [21, 22], we found that Gleason score had no significant correlation with SUV or positivity of PSMA PET/CT but did have a correlation with the PSMAwbtb.
In our study, 54% of patients with BR had a positive PSMA PET/CT study, a percentage slightly lower than published by other groups, which may be attributable to various factors. Firstly, because of the lower serum total PSA levels prior to scanning, compared with other studies [23,24,25], we found higher median PSA values in patients with a positive PSMA PET/CT compared with patients with a negative scan (3.51 ng/dL versus 0.43 ng/dL, respectively). Secondly, only patients in which all conventional imaging were negative were submitted to PSMA PET/CT as this modality is not routinely performed in our country due to its high cost. If the source of BR can be identified by cheaper, widely available imaging modalities or if in primary staging metastases are already diagnosed, then PSMA PET/CT would be rarely performed. Also, conventional imaging and other PET-tracer imaging are considerably less sensitive than PSMA PET/CT, especially in patients with low PSA levels as observed in our cohort. So, it is not surprising that we only detected bone metastasis by PMSA PET/CT.
Interestingly, among the 33 patients that developed BR up to 1 year after prostatectomy, 38% of them had BR due to distant metastases. Even assuming that the patients with local recurrence were those with a negative PSMA PET/CT (44%) and those with a positive PSMA uptake in the prostate bed (18%), then only 62% of the patients were likely to have local recurrence. This fact reinforces the idea that PSMA PET/CT is warranted prior to indicating radiotherapy for patients with BR even if within 1 year after surgery.
In the BR group, PSMAwbtb had a significant and direct correlation with PSA values, especially with PSA > 15 ng/mL. Likewise, studies using manual quantification of PSMAwbtb found positive direct relationships with PSA values and no correlation with Gleason scores [11, 26]; with PSA > 4 ng/mL, there seems to be a nearly linear progression of PSMA tumor burden, with a detection rate > 95% [26]. In opposite, we did not find PSMAwbtb to have any correlation with serum PSA values in the primary staging group; highest uptakes were located in the prostate in 83% of patients.
Our study had some limitations. First, it was a retrospective study and included patients in different stages of disease and submitted to heterogenous previous treatment modalities. Secondly, the number of patients in the primary staging group (N = 46) was insufficient to determine if PSMAwbtb metrics would correlate with clinical and laboratory values because we collected these patients’ data from 2018 since publications concerning the benefit of PSMA PET/CT scan for primary staging only began to increase after the year 2018 and with no guidelines to suggest PSMA PET/CT for primary staging of high and intermediate risk prostate cancer [27,28,29,30,31,32].
In conclusion, PSMAwbtb has a strong correlation to patient age and serum PSA in patients with BR; however, determining PSMAwbtb may not be useful in the primary staging setting. PSMAwbtb could potentially be helpful as a prognostic indicator and to objectively predict both the likelihood of a positive scan according to the PSMA values and outcome after therapy.
References
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–53. https://doi.org/10.1002/ijc.31937.
Henderickx MMEL, Brits T, Muilwijk T, Adams T, Vandeursen H. Localized prostate cancer and robot-assisted laparoscopic radical prostatectomy: a retrospective, comparative study between pre- and post-operative Gleason scores. Acta Chir Belg. 2017. https://doi.org/10.1080/00015458.2017.1353234.
Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378:2465–74. https://doi.org/10.1056/NEJMoa1800536.
Naccarato AMEP, Consuelo Souto S, Matheus WE, Ferreira U, Denardi F. Quality of life and sexual health in men with prostate cancer undergoing radical prostatectomy. Aging Male. 2018:1–8. https://doi.org/10.1080/13685538.2018.1486397.
Choueiri TK, Dreicer R, Paciorek A, Carroll PR, Konety B. A model that predicts the probability of positive imaging in prostate cancer cases with biochemical failure after initial definitive local therapy. J Urol. 2008;179:906–10. https://doi.org/10.1016/j.juro.2007.10.059.
Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:197–209. https://doi.org/10.1007/s00259-014-2949-6.
Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44:1258–68. https://doi.org/10.1007/s00259-017-3711-7.
Petersen LJ, Nielsen JB, Langkilde NC, Petersen A, Afshar-Oromieh A, De Souza NM, et al. 68Ga-PSMA PET/CT compared with MRI/CT and diffusion-weighted MRI for primary lymph node staging prior to definitive radiotherapy in prostate cancer: a prospective diagnostic test accuracy study. World J Urol. 2020;38:939–48. https://doi.org/10.1007/s00345-019-02846-z.
Öbek C, Doğanca T, Demirci E, Ocak M, Kural AR, Yıldırım A, Yücetaş U, Demirdağ Ç, Erdoğan SM, Kabasakal L; Members of Urooncology Association, Turkey. The accuracy of 68Ga-PSMA PET/CT in primary lymph node staging in high-risk prostate cancer. Eur J Nucl Med Mol Imaging 2017; 44: 1806–1812. doi: https://doi.org/10.1007/s00259-017-3752-y.
Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET- CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multi-centre study. The Lancet. 2020;11(395):1208–16. https://doi.org/10.1016/S0140-6736(20)30314-7.
Schmidkonz C, Cordes M, Schmidt D, Bäuerle T, Goetz TI, Beck M, et al. 68Ga-PSMA-11 PET/CT-derived metabolic parameters for determination of whole-body tumor burden and treatment response in prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:1862–72. https://doi.org/10.1007/s00259-018-4042-z.
Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part II: recommended approaches and details of specific care options. J Urol. 2018;199:990–7. https://doi.org/10.1016/j.juro.2018.01.002.
Boellaard R, O’Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010;37:181–200. https://doi.org/10.1007/s00259-009-1297-4.
Burger IA, Vargas HA, Apte A, Beattie BJ, Humm JL, Gonen M, et al. PET quantification with a histogram derived total activity metric: superior quantitative consistency compared to total lesion glycolysis with absolute or relative SUV thresholds in phantoms and lung cancer patients. Nucl Med Biol. 2014;41:410–8. https://doi.org/10.1016/j.nucmedbio.2014.02.006.
Gafita A, BiethM KM, Tetteh G, Navarro F, Wang H, et al. qPSMA: Semiautomatic software for whole-body tumor burden assessment in prostate cancer using 68Ga-PSMA11 PET/CT. J Nucl Med. 2019;60:1277–83. https://doi.org/10.2967/jnumed.118.224055.
Brito AET, Mourato FA, de Oliveira RPM, Leal ALG, Filho PJA, de Filho JLL. Evaluation of whole-body tumor burden with 68Ga-PSMA PET/CT in the biochemical recurrence of prostate cancer. Ann Nucl Med. 2019;35:344–50. https://doi.org/10.1007/s12149-019-01342-z.
Schmuck S, von Klot C, Henkenberens C, Sohns M, Christiansen H, Wester HJ, et al. Initial experience with volumetric 68Ga-PSMA I&T PET/CT for assessment of whole-body tumor burden as a quantitative imaging biomarker in patients with prostate cancer. J Nucl Med. 2017;58:1962–8. https://doi.org/10.2967/jnumed.117.193581.
Basha MAA, Hamed MAG, Hussein O, El-Diasty T, Abdelkhalek YI, Hussein YO, et al. 68Ga-PSMA-11 PET/CT in newly diagnosed prostate cancer: diagnostic sensitivity and interobserver agreement. Abdom Radiol. 2019;44:2545–56. https://doi.org/10.1007/s00261-019-02006-2.
Fendler WP, Calais J, Allen-Auerbach M, Bluemel C, Eberhardt N, Emmett L, et al. 68Ga-PSMA-11 PET/CT Interobserver agreement for prostate cancer assessments: an international multicenter prospective study. J Nucl Med. 2017;58:1617–23. https://doi.org/10.2967/jnumed.117.190827.
Yilmaz U, Komek H, Can C, Altindag S. The role of (68Ga) PSMA I&T in biochemical recurrence after radical prostatectomy: detection rate and the correlation between the level of PSA, Gleason score, and the SUVmax. Ann Nucl Med. 2019;33:545–53. https://doi.org/10.1007/s12149-019-01360-x.
Sanli Y, Kuyumcu S, Sanli O, Buyukkaya F, İribaş A, Alcin G, et al. Relationships between serum PSA levels, Gleason scores and results of 68Ga-PSMAPET/CT in patients with recurrent prostate cancer. Ann Nucl Med. 2017;31:709–17. https://doi.org/10.1007/s12149-017-1207-y.
Liu C, Liu T, Zhang N, Liu Y, Li N, Du P, et al. 68Ga-PSMA-617 PET/CT: a promising new technique for predicting risk stratification and metastatic risk of prostate cancer patients. Eur J Nucl Med Mol Imaging. 2018;45:1852–61. https://doi.org/10.1007/s00259-018-4037-9.
Ceci F, Uprimny C, Nilica B, Geraldo L, Kendler D, Kroiss A, et al. (68)Ga-PSMA PET/CT for restaging recurrent prostate cancer: which factors are associated with PET/CT detection rate? Eur J Nucl Med Mol Imaging. 2015;42:1284–94. https://doi.org/10.1007/s00259-015-3078-6.
Gauthé M, Belissant O, Girard A, Zhang Yin J, Ohnona J, Cottereau AS, et al. PET/CT and biochemical recurrence of prostate adenocarcinoma: Added value of 68Ga-PSMA-11 when 18F-fluorocholine is non-contributive. Prog Urol. 2017;27:474–81. https://doi.org/10.1016/j.purol.2017.04.004.
Calais J, Ceci F, Eiber M, et al. 18F-Fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019;20:1286–94.
Sevastián SMO, Francisco OGP, Norma YHP, Angélica EAZ, Blanca LAL. Correlation between molecular tumor volume evaluated with 68 Ga-PSMA PET/CT and prostatic specific antigen (PSA) levels. Revista Española de Medicina Nuclear e Imagen Molecular (English Edition) 2018; 37: 223–228. doi:https://doi.org/10.1016/j.remnie.2017.10.023.
Zang S, Shao G, Cui C, Li TN, Huang Y, Yao X, et al. 68Ga-PSMA-11 PET/CT for prostate cancer staging and risk stratification in Chinese patients. Oncotarget. 2017, 8:12247–58. https://doi.org/10.18632/oncotarget.14691.
Zaman MU, Fatima N, Zaman A, Sajid M, Zaman U, Zaman S. Diagnostic challenges in prostate cancer and 68Ga-PSMA PET imaging: a game changer? Asian Pac J Cancer Prev. 2017;18:2625–8.
Keidar Z, Gill R, Goshen E, Israel O, Davidson T, Morgulis M, et al. 68Ga-PSMA PET/CT in prostate cancer patients - patterns of disease, benign findings and pitfalls. Cancer Imaging. 2018;18:39. https://doi.org/10.1186/s40644-018-0175-3.
Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S, et al. 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017;44:1014–24. https://doi.org/10.1007/s00259-017-3670-z.
Jędroszka D, Orzechowska M, Hamouz R, Górniak K, Bednarek AK. Markers of epithelial-to-mesenchymal transition reflect tumor biology according to patient age and Gleason score in prostate cancer. PLoS One. 2017;12:e0188842. https://doi.org/10.1371/journal.pone.0188842.
Grubmüller B, Baltzer P, D’Andrea D, Korn S, Haug AR, Hacker M, et al. 68Ga-PSMA 11 ligand PET imaging in patients with biochemical recurrence after radical prostatectomy - diagnostic performance and impact on therapeutic decision-making. Eur J Nucl Med Mol Imaging. 2018;45:235–42. https://doi.org/10.1007/s00259-017-3858-2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was waived for this retrospective manuscript.
Additional information
Declarations
We declare that this manuscript has not been published before and is not under consideration for publication anywhere else, and this publication has been approved by all co-authors.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology - Genitourinary
Rights and permissions
About this article
Cite this article
Santos, A., Mattiolli, A., Carvalheira, J.B. et al. PSMA whole-body tumor burden in primary staging and biochemical recurrence of prostate cancer. Eur J Nucl Med Mol Imaging 48, 493–500 (2021). https://doi.org/10.1007/s00259-020-04981-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04981-x