Abstract
Background
68 Ga-PSMA-PET has an increasing importance in the evaluation of prostate cancer patients due to its high sensitivity and specificity in identifying neoplastic lesions in the clinical setting of elevated prostate-specific antigen (PSA). The objective of this study was to calculate the whole-body tumor burden using volumetric quantification of lesions detected in 68Ga-PSMA-PET of prostate cancer patients with biochemical recurrence and correlate these findings with clinical and image parameters.
Methods
Each patient had their 68Ga-PSMA-PET analyzed for the presence of neoplastic lesions. Their PSA levels and clinical information were recorded. In positive cases, the tumor burden (TL-PSMA) was calculated with a semi-automatic software and manually, and the results are analyzed and tested.
Results
We analyzed 100 prostate cancer patients, mean age of 69.9 ± 9.7 years and a median PSA of 1.73 ng/dL. 68Ga-PSMA-PET identified neoplastic lesions in 72% of them. The median TL-PSMA was 55.95 ml (1.1–28,080 ml). TL-PSMA and PSA were strongly correlated (rho = 0.71, p < 0.0001, 95% CI 0.60–0.80). TL-PSMA and PSA levels groups had a significant correlation and TL-PSMA and Gleason score were independent variables associated with PSA levels (p < 0.05).
Conclusion
TL-PSMA strongly and independently correlates with PSA levels in prostate cancer patients and could be used as a biomarker to separate them into groups with high or low tumor burden, instead of considering only the number of lesions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prostate cancer has a high incidence and prevalence in the male population around the world. Recurrence is relatively common after primary treatment and is usually diagnosed by an increase of the serum prostate-specific antigen (PSA), which is called biochemical recurrence (BR). Characterization of BR depends on the clinical setting: after prostatectomy, a patient is considered to have BR if he presents with two consecutive PSA levels above 0.2 ng/ml; after radiation therapy, BR is defined by any PSA level > 2 ng/ml above the nadir value [1]. However, with new ways of detecting PSA levels by ultra-sensitive methods, capable of detecting values smaller than < 0.001 ng/ml, lower values could be useful in the early diagnosis of cancer recurrence [2].
PET/CT with 68Ga-PSMA (68Ga-PSMA-PET) is a new diagnostic method which is being increasingly indicated in patients with prostate cancer because of its high sensitivity and specificity, especially in the setting of BR [3] after conventional investigation with bone scintigraphy, computed tomography (CT) and/or magnetic resonance imaging. Usually, 68Ga-PSMA-PET reports describe the presence or absence of neoplastic disease with the number, location and maximum standardized uptake value (SUVmax) of neoplastic lesions.
However, it is not clear whether reporting only these parameters is enough and if they correctly identify disease volume and prognosis. Some studies report the calculation of whole-body tumor burden for this purpose using various PET/CT radiopharmaceuticals. For instance, quantification methods for 18F-FDG-PET/CT such as the metabolic tumor volume (MTV) and the total tumor burden, called total lesion glycolysis (TLG), are already well established for several cancers. They have been shown to be able to objectively assess response and to be better prognostic indicators for lymphoma [4], as well as to predict survival in breast cancer [5, 6] and non-small cell lung cancer [7]. Furthermore, in prostate cancer, volumetric quantification of 18F-Fluoride-PET/CT (capable of detecting exclusively metastatic bone lesions) has already been shown to correlate with prognosis [8, 9]. Despite this, there are few studies using volumetric quantification with 68Ga-PSMA-PET in prostate cancer patients [10, 11], and whole-body tumor burden is still not widely used in clinical practice, mainly because it is laborious when done manually.
Considering this scenario, this study aims to calculate the whole-body tumor burden of 68Ga-PSMA-PET in prostate cancer patients with BR using a semi-automatic volumetric quantification tool and to correlate it with their clinical and image parameters.
Materials and methods
Patients
We included prostate cancer patients who performed 68Ga-PSMA-PET in our institution from November 2016 to December 2017 due to BR according to the clinical indication of the attending physician. For each patient, clinical data such as PSA value, primary Gleason score, age, time of disease and prostatectomy status were recorded when available.
This work is a retrospective study performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and approved by the local Institutional Review Board (CAAE 76305317.4.0000.5199).
PET/CT with 68Ga-PSMA acquisition
68Ga-PSMA was obtained from a 68Ge/68Ga generator system and a semi-automated synthesizer (itG and iQS® Ga-68 Fluidic Labeling Module, GmbH, Germany). PET/CT studies were performed 60 min after intravenous injection of 1.8–2.2 MBq/kg of 68Ga-PSMA. An intravenous diuretic was injected following the tracer administration.
First, a non-contrast-enhanced CT acquisition (120 kV, slice thickness of 5 mm) was performed to be used for attenuation correction and anatomical correlation. Then, PET images from skull to thighs were obtained on a PET scanner (Biograph 16, Siemens Healthcare, USA) using 180 s/bed position. PET images were reconstructed with an iterative reconstruction algorithm (two iterations, eight subsets, Gaussian filter).
Image analysis and quantification
All PET/CT scans were analyzed by two nuclear physicians and one radiologist. In positive cases for neoplastic disease, the number and location of lesions, and the mean SUV (SUVmean) and the maximum SUV (SUVmax) of all lesions were recorded. Also, the volumetric quantification of each patient was performed using a semi-automatic software called METAVOL® [12], which provided values of whole-body PSMA tumor volume (PSMA-TV) and whole-body total lesions uptake (TL-PSMA). PSMA-TV consists of the total volume of metastatic lesions in milliliters and TL-PSMA is the sum of the volume in milliliters of each lesion multiplied by their SUVmean for each patient. Thus, the final parameter of a volumetric quantification is the TL-PSMA, since it provides the whole-body tumor burden represented by both volume and uptake. Therefore, only TL-PSMA was used for correlation with clinical and image parameters.
The minimum SUVmax of all neoplastic lesions of all patients was used as the SUVmax threshold in the semi-automatic quantification to avoid exclusion of any lesion. After the selection of neoplastic lesions, the software automatically provided PSMA-TV and TL-PSMA. Examples of semi-automatic quantifications can be found in Figs. 1 and 2.
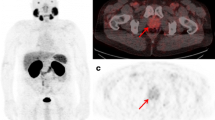
Example of semi-automatic quantification in a male patient, 76 years old, with biochemical recurrence (PSA = 0.83 ng/dL). 68Ga-PSMA-PET (a) showed one small lymph node measuring 0.3 cm (b) with radiotracer uptake in the left pelvis (SUVmax = 3.4). The lymph node is marked in red (c) and all blue marks correspond to physiologic uptake and were not included in the quantification (d). TL-PSMA was 2.2
Example of semi-automatic quantification of a male patient, 63 years old, with biochemical recurrence (PSA = 63.8 ng/dL). 68Ga-PSMA-PET showed disseminated lymph node disease (a). The right kidney had to be excluded and is marked in brown (b). All neoplastic lesions are marked in red and TL-PSMA was 18,241.43. Other physiologic uptakes are marked in blue (c)
For comparison purposes, all patients were also submitted to manual quantification using an isocontour threshold of 41% of the SUVmax in the volume of interest (VOI) of each malignant lesion.
Statistical analyses
The level of significance in this study is 5% and the statistical analyses were performed using the MedCalc software. Categoric variables are presented with frequencies and continuous variables are presented with the mean value ± standard variation and range. Pearson’s correlation test was used to correlate the quantitative parameters and stepwise multiple-regression analysis was performed to identify independent variables for TL-PSMA. Spearman’s coefficient and Kruskal–Wallis test were used to evaluate the correlation between the volumetric parameters and PSA levels.
Results
One hundred male patients in BR investigation were included in this study, with a mean age of 69.9 ± 9.7 years (44–92 years) and a median PSA of 1.73 ng/ml, ranging from 0.02 to 163.3 ng/ml. 69% of them underwent radical prostatectomy as primary treatment.
68Ga-PSMA-PET identified neoplastic lesions in 72 patients, having an overall detection rate of 72%. The mean SUVmax of all patients was 11.5 ± 22.8 (1.5–148.6). Amongst the positive scans, twenty-four patients (33%) had four or more neoplastic lesions and most patients showed recurrence in lymph nodes (45.8%), bone (44%) or in the prostate or surgical bed (37.5%). The patients’ clinical characteristics and other 68Ga-PSMA-PET findings are presented in Table 1.
The group of patients with negative 68Ga-PSMA-PET had a median PSA level of 0.46 ng/ml (0.02–2.64 ng/ml) and the positive patients had a median of 3.55 ng/ml (0.04–163.3 ng/ml) (p < 0.0001, 95% CI 1.16–6.53). The detection rates separated by PSA level groups can be seen in Fig. 3.
In the semi-automatic quantification, the median PSMA-TV was 13.98 ml (0.7–4,689 ml) and the median TL-PSMA was 55.95 ml (1.1–28,080 ml). PSMA-TV and TL-PSMA showed a strong correlation (rho = 0.93, p < 0.0001, 95% CI 0.89–0.95). Thus, only TL-PSMA was used in the following analysis. Also, the semi-automatic values had strong correlation with the manual values (rho = 0.95, p < 0.0001, 95% CI 0.94–0.97).
TL-PSMA and PSA were strongly correlated (rho = 0.73, p < 0.0001, 95% CI 0.59–0.83) and Kruskal–Wallis test showed a significant correlation between TL-PSMA and the groups of PSA levels (p = 0.000006) (Fig. 4). When analyzing the variables related to PSA levels in these patients, TL-PSMA (rpartial = 0.33, p = 0.03) and Gleason score (rpartial = 0.46, p = 0.002) were independently associated. The other variables (age, time of disease, number of lesions and SUVmax) showed no statistically significant correlation (p > 0.05).
TL-PSMA demonstrated only moderate correlation with the number of neoplastic lesions (rho = 0.58, p < 0.0001, 95% CI 0.406–0.721). Patients with less than four lesions presented a mean TL-PSMA of 75.9 ± 132.8 ml (1.1–761.9 ml). In this group, the patient with the highest TL-PSMA presented only one lesion. The patients with four or more lesions had a mean TL-PSMA of 4176.5 ± 6914.7 ml (5.5–28,080 ml) and 12 of them had TL-PSMA of less than 1000 ml.
Discussion
Our study performed a semi-automatic quantification of whole-body tumor burden in patients with prostate cancer who underwent 68Ga-PSMA-PET for investigation of BR, including in patients with high disease volume. We found a strong and independent correlation of TL-PSMA with their PSA levels, confirming the belief that a higher PSA level is associated with a greater tumor burden. Although this correlation was expected empirically, the volumetric quantification methodology allowed confirming and demonstrating it.
Two previous studies found similar results, with a significant correlation between TL-PSMA and PSA levels in BR of prostate cancer [10, 11]. However, they both performed manual quantification instead of semi-automatic. As a result, one of these studies reported the need to exclude all patients with more than ten lesions due to the difficulty in performing non-automated measurement of tumor volume in them, which would result in an exhaustive and time-consuming process [11]. Limiting the number of lesions in patients with prostate cancer may not represent the true characteristics of metastatic disease, which is usually present in many sites with up to 36% of patients having multiple metastases [13]. In our population, we found a similar rate of patients with multiple metastases. With this higher number of lesions, it would be slow and laborious to perform the quantification manually, since it is necessary to multiply the SUVmean of each lesion by their volume. Because of that, volumetric quantification is not routinely used in clinical practice. In our study, the software performed it semi-automatically, making this process feasible even in patients with multiple lesions.
Although there are already semi-automatic quantification studies for prostate cancer patients using 18F-Fluoride-PET/CT [14], the quantification with 68Ga-PSMA-PET became clearly superior as it has the same accuracy of 18F-Fluoride-PET/CT for detecting bone lesions [15, 16], but also detects lymph node and soft tissue lesions.
Usually, in the absence of a specific biomarker, clinical trials use the number and location of metastatic lesions to classify patients into high or low-volume disease and to guide therapeutic approach. The long-term survival analysis of the CHAARTED trial, for example, revealed that patients with low-volume disease had no overall survival benefit with docetaxel added to androgen-deprivation therapy [17]. However, this study considered as low-volume disease cases without visceral metastases and with less than four skeletal lesions, instead of tumor burden. In our series, the TL-PSMA demonstrated only moderate correlation with the number of neoplastic lesions. If we had employed these criteria in our population to separate these groups, at least 13 patients would have been classified in a different group from that found considering their TL-PSMA.
Considering that quantitative parameters have been shown to be associated with survival in patients with prostate cancer using other PET/CT tracers [14], the use of TL-PSMA could be more appropriate than the number of lesions alone to separate patients into groups of high or low tumor burden, making the separation of groups more accurate and objective.
68Ga-PSMA has renal excretion [18], which is a limitation for the quantification process of lesions adjacent to the excretory system, very commonly found in prostate cancer. In our population, 32% of patients had pelvic lesions in the prostate, seminal vesicles or prostatic bed. In addition, it is common for BR to occur in pelvic lymph nodes and in the hip bones. To reduce this interference, the injection of a diuretic to stimulate urinary elimination is done concomitantly with the administration of the tracer. In cases in which the lesions remain close to physiological uptake sites, manual adjustments can be made on the software to separate them. This problem may not occur or occur in a lesser extent when using 18F-PSMA tracer, as it does not exhibit significant urinary excretion [19]. To date, we have not found volumetric quantification studies with 18F-PSMA.
A meta-analysis including nine studies that used 68Ga-PSMA-PET in prostate cancer patients with BR found a median PSA level of 2.3 ng/ml [13], similar to the value found in our population (1.73 ng/ml). Other expected similarity to this meta-analysis was that 68Ga-PSMA-PET-positive patients had higher mean PSA values than the negative ones. Unfortunately, we had a lower overall detection rate (72%) compared to this meta-analysis (81%), despite similar PSA level rates between 0.5 and 1.0 ng/ml (50% versus 53%).
To perform a semi-automatic quantification, it is necessary to choose a SUVmax threshold. In our study, we used a fixed SUVmax threshold of 1.5, which was the lowest SUVmax value found in neoplastic lesions amongst the studied patients. For manual quantification, we used the value of 41% of the SUVmax in each lesion [20]. There are no studies determining which value is better when using 68Ga-PSMA-PET. Determining an ideal value would be necessary for standardization, since by increasing the threshold we reduce the measured volume.
One limitation of this study was the lack of histological confirmation of lesions found in 68Ga-PSMA-PET. In addition, this study did not evaluate the ideal TL-PSMA value to separate groups of patients with high or low disease volume. Other studies are needed to validate TL-PSMA as a prognostic factor in prostate cancer patients and as a parameter to evaluate therapeutic response.
Conclusion
TL-PSMA strongly and independently correlates with PSA levels of prostate cancer patients with BR and is the method of choice for whole-body tumor burden quantifications, which can be easily done on a semi-automatic software. Also, TL-PSMA could be used as an imaging biomarker to separate patients into groups with high or low tumor burden, instead of considering only the number of lesions. Further studies should be performed so that this parameter can be regarded as a prognostic factor or used for therapeutic response evaluation and theranostic applications.
References
Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–42.
Tilki D, Kim S, Il HuB, Dall’Era MA, Evans CP. Ultrasensitive prostate specific antigen and its role after radical prostatectomy: a systematic review. J Urol. 2015;193:1525–31.
Einspieler I, Rauscher I, Düwel C, Krönke M, Rischpler C, Habl G, et al. Detection efficacy of hybrid 68 Ga-PSMA Ligand PET/CT in prostate cancer patients with biochemical recurrence after primary radiation therapy defined by phoenix criteria. J Nucl Med. 2017;58:1081–7.
Lin C, Itti E, Haioun C, Petegnief Y, Luciani A, Dupuis J, et al. Early 18F-FDG PET for prediction of prognosis in patients with diffuse large B-cell lymphoma: SUV-based assessment versus visual analysis. J Nucl Med. 2007;48:1626–32.
Carkaci S, Sherman CT, Ozkan E, Adrada BE, Wei W, Rohren EM, et al. 18F-FDG PET/CT predicts survival in patients with inflammatory breast cancer undergoing neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2013;40:1809–16.
Marinelli B, Espinet-Col C, Ulaner GA, McArthur HL, Gonen M, Jochelson M, et al. Prognostic value of FDG PET/CT-based metabolic tumor volumes in metastatic triple negative breast cancer patients. Am J Nucl Med Mol Imaging. 2016;6:120–7.
Kaira K, Higuchi T, Naruse I, Arisaka Y, Tokue A, Altan B, et al. Metabolic activity by 18F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur J Nucl Med Mol Imaging. 2018;45:56–66.
Etchebehere EC, Araujo JC, Milton DR, Erwin WD, Wendt RE, Swanston NM, et al. Skeletal tumor burden on baseline 18F-fluoride PET/CT predicts bone marrow failure after 223Ra therapy. Clin Nucl Med. 2016;41:268–73.
Rohren EM, Etchebehere EC, Araujo JC, Hobbs BP, Swanston NM, Everding M, et al. Determination of skeletal tumor burden on 18F-fluoride PET/CT. J Nucl Med. 2015;56:1507–12.
Schmidkonz C, Cordes M, Schmidt D, Bäuerle T, Goetz TI, Beck M, et al. 68 Ga-PSMA-11 PET/CT-derived metabolic parameters for determination of whole-body tumor burden and treatment response in prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:1862–72.
Schmuck S, von Klot CA, Henkenberens C, Sohns JM, Christiansen H, Wester H-J, et al. Initial experience with volumetric 68 Ga-PSMA I&T PET/CT for assessment of whole-body tumor burden as a quantitative imaging biomarker in patients with prostate cancer. J Nucl Med. 2017;58:1962–8.
Hirata K, Kobayashi K, Wong K-P, Manabe O, Surmak A, Tamaki N, et al A Semi-Automated Technique Determining the Liver Standardized Uptake Value Reference for Tumor Delineation in FDG PET-CT. Chen K, editor. PLoS One. 2014;9:e105682.
von Eyben FE, Picchio M, von Eyben R, Rhee H, Bauman G. 68 Ga-labeled prostate-specific membrane antigen ligand positron emission tomography/computed tomography for prostate cancer: a systematic review and meta-analysis. Eur Urol Focus Eur Assoc Urol. 2018;4:686–93.
Etchebehere EC, Araujo JC, Fox PS, Swanston NM, Macapinlac HA, Rohren EM. Prognostic factors in patients treated with 223Ra: the role of skeletal tumor burden on baseline 18F-fluoride PET/CT in predicting overall survival. J Nucl Med. 2015;56:1177–84.
Zacho HD, Nielsen JB, Afshar-Oromieh A, Haberkorn U, DeSouza N, De Paepe K, et al. Prospective comparison of 68 Ga-PSMA PET/CT, 18F-sodium fluoride PET/CT and diffusion weighted-MRI at for the detection of bone metastases in biochemically recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:1884–97.
Dyrberg E, Hendel HW, Huynh THV, Klausen TW, Løgager VB, Madsen C, et al. 68 Ga-PSMA-PET/CT in comparison with 18F-fluoride-PET/CT and whole-body MRI for the detection of bone metastases in patients with prostate cancer: a prospective diagnostic accuracy study. Eur Radiol. 2019;29:1221–30.
Kyriakopoulos CE, Chen Y-H, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36:1080–7.
Hofman MS, Hicks RJ, Maurer T, Eiber M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. RadioGraphics. 2018;38:200–17.
Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2017;44:678–88.
Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54.
Acknowledgements
We thank the Real Nuclear team for their support during this project.
Funding
None. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The local Institutional Review Board approved this study. Since this was a retrospective study with secondary data, the Local Ethics Committee did not request individual informed consent. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brito, A.E.T., Mourato, F.A., de Oliveira, R.P.M. et al. Evaluation of whole-body tumor burden with 68Ga-PSMA PET/CT in the biochemical recurrence of prostate cancer. Ann Nucl Med 33, 344–350 (2019). https://doi.org/10.1007/s12149-019-01342-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-019-01342-z