Abstract
Purpose
We aimed at evaluating the role of 68Ga-PSMA-11 PET/CT-derived metabolic parameters for assessment of whole-body tumor burden and its capability to determine therapeutic response in patients with prostate cancer.
Methods
A total of 142 patients with biochemical recurrence of prostate cancer underwent PET/CT with [68Ga]Ga-PSMA-HBED-CC (68Ga-PSMA-11). Quantitative assessment of all 641 68Ga-PSMA-11-positive lesions in the field of view was performed to calculate PSMA-derived parameters, including whole-body PSMA tumor volume (PSMA-TV) and whole-body total lesion PSMA (TL-PSMA), as well as the established SUVmax and SUVmean values. All PET-derived parameters were tested for correlation with serum PSA levels and for association with Gleason scores.
In 23 patients who underwent 68Ga-PSMA-11 PET/CT before and after therapy with either external beam radiation, androgen deprivation, or docetaxel chemotherapy, SUVmax and TL-PSMA were compared to radiographic response assessment of CT images based on RECIST 1.1 criteria and to biochemical response determined by changes of serum PSA levels.
Results
PSMA-TV and TL-PSMA demonstrated a significant correlation with serum PSA levels (P < 0.0001) and TL-PSMA was significantly different for different Gleason scores. The agreement rate between TL-PSMA derived from PET and biochemical response was 87% (95% confidence interval, 0.66–0.97; Cohen’s κ = 0.78; P < 0.01) and, thus, higher than for SUVmax, which was 74% (95% CI, 0.52–0.90; κ = 0.55; P < 0.01). Furthermore, agreement with PSA was higher for TL-PSMA and SUVmax than for CT-based response evaluation. Discordant findings between PET and CT were most likely due to limitations of CT and RECIST in rating small lymph nodes as metastases, as well as bone involvement, which was sometimes not detectable in CT.
Conclusion
68Ga-PSMA-11 PET/CT-derived metabolic tumor parameters showed promising results for evaluation of treatment response. Especially, TL-PSMA demonstrated higher agreement rates with biochemical response compared to SUVmax. Larger, ideally prospective trials are needed to help to reveal the full potential of metabolic parameters derived from PET imaging with 68Ga-PSMA-11.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biochemical recurrence in prostate cancer (PC) is a major clinical problem, occurring in up to 30% of patients after radical prostatectomy [1] and in up to 60% of patients after primary external beam radiation therapy [2]. Initially, PC predominantly metastasizes to lymph nodes and bone; in the later stages, liver and lung can also be affected [3]. Exact detection of recurrence sites is of utmost importance to facilitate therapy planning. For this purpose, an imaging tool with high sensitivity and specificity is needed.
Recently, PET-compatible small-molecule inhibitors, targeting the prostate-specific membrane antigen (PSMA), a surface protein expressed 100- to 1000-fold higher in PC cells than in benign prostate tissue or normal tissue of most other organs, were proposed for PC imaging [4]. PET/CT with [68Ga]Ga-PSMA-HBED-CC (68Ga-PSMA-11) has proven to be an excellent tool for staging and restaging of PC [5, 6], offering superior diagnostic accuracy compared to conventional imaging modalities and choline PET [7]. Assessment of treatment response on PET/CT is primarily based on the appearance or disappearance of PET-positive lesions and on standardized uptake value (SUV) measurements [8]. However, for determination of overall response to therapy, individual lesion SUV measurements might be inadequate and the revised RECIST 1.1 criteria are of limited use in the assessment of bone lesions [9]. Furthermore, it is well known that serum PSA levels do not correlate with the size or number of tumor lesions [10], limiting the applicability of these metrics for response assessment. Therefore, especially for evaluation of therapy response, a parameter reflecting the whole-body tumor burden based on the size, number, and metabolic activity of tumor lesions, which is also easily applicable in clinical practice is urgently needed.
For 18F-FDG-PET/CT, such concepts already exist: For example, it has been demonstrated that metabolic tumor volume (MTV) and total lesion glycolysis (TLG) are able to predict poor response, as well as progression-free survival in patients with oesophageal adenocarcinoma who underwent induction chemotherapy [11]. In patients with diffuse large B-cell lymphoma, MTV was measured on 18F-FDG-PET/CT before and after two cycles of chemotherapy, being the only statistically significant predictor of progression-free survival [12].
To the best of our knowledge, the concept of whole-body tumor burden in patients with PC has so far only been assessed in one previous study by Schmuck et al. [13], in which up to a maximum of ten PSMA-positive lesions in the field of view (FOV) were included for determining the whole-body tumor load in a total of 101 patients.
Consequently, the aim of this study is to extend this previous work in a larger number of patients and by including all lesions in the FOV for calculation of metabolic parameters, as well as to compare PET findings with biochemical and RECIST 1.1 evaluations for patients who have been imaged multiple times for assessment of therapeutic response.
Materials and methods
Patients
A total of 142 patients who were referred due to biochemical recurrence of PC between March 2014 and October 2017 were retrospectively included in this study. The patients conformed to the following criteria:
-
Histopathologically confirmed PC
-
Completed primary therapy (prostatectomy with or without lymph node dissection or radiotherapy, optionally combined with androgen deprivation therapy)
-
Biochemical recurrence of PC
-
At least one PSMA-positive lesion on the 68Ga-PSMA-ligand PET/CT scan
The diagnosis of biochemically recurrent disease was carried out by the referring urologist and was based on the criteria outlined in the European Association of Urology (EAU) guidelines on PC [14, 15]. These define a biochemical recurrence of PC after radical prostatectomy by two consecutive PSA values of >0.2 ng/mL and rising, and after radiation therapy as rising PSA level > 2 ng/ml above the nadir PSA. The diagnosis of recurring disease was solely based on these criteria.
For each patient, a complete medical history including histology of confirmed PC (primary Gleason score), PSA values, past and present PC therapies, and demographic information was collected. Twenty-three patients underwent 68Ga-PSMA-ligand PET/CT before initiation and after completion of therapy. Patient characteristics are summarized in Tables 1 and 2.
68Ga-PSMA-HBED-CC (68Ga-PSMA-11) is an investigational radiopharmaceutical and is not yet US Food and Drug Administration- (FDA) or European Medicines Agency (EMA)-approved. It was therefore used under the conditions outlined in §13(2b) of the Arzneimittelgesetz (AMG; German Medicinal Products Act). Our protocol was approved by the Institutional Review Board of the University Hospital Erlangen, and written informed consent was obtained from all patients.
Radiosynthesis and formulation of 68Ga-PSMA-HBED-CC (68Ga-PSMA-11)
The fully automated good manufacturing practice (GMP)-compliant radiosynthesis of Glu-NH-CO-NH-Lys-(Ahx)-[68Ga(HBED-CC)] (short terms: 68Ga-PSMA-HBED-CC, also known as 68Ga-PSMA-11) was performed as described previously [16], with only slight modifications. 68Ga3+ was obtained from the 68Ge/68Ga radionuclide pharmacy-grade generator (1850 MBq, GalliaPharm™, Eckert & Ziegler AG, Berlin, Germany). The final formulation of 68Ga-PSMA-11 was done using isotonic phosphate-buffered saline (PBS) and sterile filtration of 14.5 mL. The radiochemical yield of 68Ga-PSMA-11 was 54 ± 5% (referred to the eluted 68Ga3+) and the radiochemical purity was >96% as determined by radio-HPLC [Chromolith Performance RP-18e, 100 × 4.6 mm, 5% acetonitrile (0.1% TFA; 0–0.5 min), 5–80% acetonitrile (0.1% TFA; 0.5–10 min), 2 mL/min, 280 nm]. The solution of 68Ga-PSMA-11 was applied to the patient via an intravenous bolus injection (mean of 134 ± 36 MBq).
Imaging procedure
68Ga-PSMA-11 PET/CT imaging was performed using a dedicated PET/CT system (Biograph mCT scanner, Siemens Medical Solutions).
A fully diagnostic, contrast-enhanced CT scan (120 kV, 170 mAs, 16 × 1.2-mm slice collimation, 0.5-s rotation time, pitch 1, reconstructed with filtered backprojection using B30f and B70f kernels, slice thickness 1.5 mm, and increment 1 mm) after injection of Imeron 350 at 1.5 ml/kg body weight was performed 1 h post-injection (p.i.) of 68Ga-PSMA-11 in a mean radioactivity of 134 ± 36 MBq. Immediately after CT scanning, a whole-body PET scan (skull base to mid-thighs) was acquired (3 min per bed, axial FOV of 21.8 cm per bed). The PET data were corrected for random and scattered coincidences, as well as for decay during scanning. PET data was corrected for attenuation by using the CT and reconstructed with an ordered subset expectation maximization (OSEM) algorithm with 2 iterations/12 subsets and point-spread-function modeling (Siemens TrueX). All corrections and reconstructions were carried out using the manufacturer’s software available on the PET/CT system.
Image analysis
All PET/CT datasets were analyzed with commercially available software (Syngo.via, Siemens Molecular Imaging, Hoffman Estates, IL, USA), allowing review of PET, CT, and fused imaging data. Visual evaluation was performed by two experienced nuclear medicine physicians and one radiologist. Diagnostic decisions were made by consensus.
PET image analysis
Excluding structures with known non-malignant uptake of PSMA (e.g. salivary glands and celiac ganglia), all remaining lesions with visually higher uptake than the respective normal region (gluteal muscle/lumbar vertebral body) were rated as PSMA-positive, suggesting local recurrence or metastases. For all suspected pathological lesions (n = 641), the mean and maximum SUV (SUVmean, SUVmax) and the MTV of each lesion was determined in VOIs with isocontours set at 45% of the maximum uptake within the respective focus. From these parameters, each lesion’s PSMA uptake was calculated by multiplying the respective MTV and mean SUV (Fig. 1). Following the suggestions of Schmuck et al. [13], this parameter is called total lesion PSMA or TL-PSMA and the MTV is called PSMA total volume or PSMA-TV. Furthermore, per-patient and per-lesion average values of the four mentioned parameters were calculated.
Assessment of whole-body tumor burden by 68Ga-PSMA-11 PET/CT in a 78-year-old patient (patient # 15 in Table 6), Gleason 8 tumor, serum PSA at examination 23 ng/ml with metastasized prostate cancer. The maximum intensity projection (a) shows several 68Ga-PSMA-11-positive metastases (indicated by circles). The left and right panels respectively demonstrate the segmentation of a lymph node metastasis (b, arrowhead) and a bone metastasis (c, arrow) to determine tumor volume and intensity of tracer uptake for calculation of PSMA-TV and TL-PSMA
Summation of the MTV over all lesions of a patient resulted in the whole-body PSMA-TV (wbPSMA-TV) summation of the PSMA uptake over all patient lesions in the whole-body TL-PSMA (wbTL-PSMA).
Response to treatment analysis
In 23 patients who underwent 68Ga-PSMA-11 PET/CT at baseline and for follow-up after therapy, radiographic response to treatment was assessed independently in PET and CT and compared to biochemical response (BR) based on PSA serum levels. Of the 23 patients, 13 patients underwent external beam radiation therapy, 9 patients androgen deprivation therapy, and 1 patient docetaxel chemotherapy. In detail, the response criteria were as follows:
-
Biochemical Response
-
Radiographic response in PET (RR-PET)
Per patient, five organ systems comprising lymph nodes, bone, liver, prostate/prostate bed, and other visceral metastatic sites were used for analysis. For each organ system, the two lesions with the highest SUVmax and with the highest TL-PSMA in the first PET examination were independently determined and selected as target lesions. However, only up to a maximum of five target lesions were selected for further evaluation in order to conform with the RECIST 1.1 criteria in this regard. SUVmax and TL-PSMA were determined as previously described and summed up for all target lesions.
According to slightly modified PET Response Criteria in Solid Tumors (PERCIST) 1.0 [20], post-treatment changes were interpreted as provided in Table 3.
In the following, the radiographic response as determined for SUVmax and TL-PSMA is referred to as RR-PET (SUVmax) and RR-PET (TL-PSMA), respectively.
-
Radiographic response in X-ray CT (RR-CT)
Radiographic response to treatment was assessed according to RECIST 1.1 criteria [21]: The exact same lesions as in the PET response evaluation were selected as target lesions in CT. Since target lesions for RR-PET (SUVmax) and RR-PET (TL-PSMA) evaluation could be potentially different from each other, two potentially different sets of CT target lesions for each patient exist, one derived from PET SUVmax target lesions and another derived from PET TL-PSMA target lesions. Both sets of CT target lesions for each patient were independently processed and results are provided separately. Within each set of target lesions, all diameters were summed (longest for non-nodal lesions, short axis for nodal lesions). Bone metastases were assessed in terms of unequivocal progression. Post-treatment changes were interpreted as given in Table 3:
In the following, the radiographic response as determined for CT, based on target lesions defined by SUVmax and TL-PSMA is referred to as RR-CT (SUVmax) and RR-CT (TL-PSMA), respectively.
Statistical analysis
Descriptive statistics were computed for continuous and categorial variables, including mean, standard deviations (SD), and range for continuous variables and absolute and relative frequencies for categorial variables.
Correlation between PET-derived parameters and PSA levels were analyzed by using Spearman’s rank correlation.
A one-way analysis of variance (ANOVA) was used to evaluate whether SUVmax, SUVmean, wbPSMA-TV, and wbTL-PSMA were different for the patient groups with a Gleason score ≤ 7, Gleason = 8, and Gleason ≥ 9.
For the response-to-treatment analysis, classifications based on SUVmax, TL-PSMA, serum PSA-levels, and RECIST 1.1 criteria are listed individually.
Furthermore, the degree of agreement between radiographic and BR assessment was determined by calculation of percent agreement and Cohen’s κ [22], including 95 % confidence intervals (95% CIs) for the agreement and level of significance for κ. In this context, interpretation of κ was based on a classification provided by Landis and Koch [23]: 0.0, poor; 0.0–0.20, slight; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, substantial; 0.81–1.00, almost perfect agreement.
For all analyses, a P value < 0.05 was considered statistically significant. Statistical analyses were performed using Matlab version R2012b (The Math Works Inc., Natick, MA, USA).
Results
Descriptive statistics
In 142 patients, a total of 641 lesions were detected. A total of 361 lymph node metastases were found in 88 (62%) patients, 239 bone metastases in 53 (37%) patients, 34 local recurrences in 34 (24%) patients, and 7 other metastases in 5 (4%) patients. On average, 4.5 ± 8.2 lesions (range 1–82) were found per patient.
Only one 68Ga-PSMA-11-positive lesion in the FOV was found for 53 (37%) patients, while 89 (63%) had more than 1 lesion.
The number of patients at a respective Gleason score was: 7 patients with Gleason score = 6, 39 patients with Gleason score = 7, 36 patients with Gleason score = 8, 57 patients with Gleason score = 9, and 3 patients with Gleason score = 10.
Mean, SD, and range of the four PET parameters SUVmean, SUVmax, PSMA-TV, and TL-PSMA are summarized in Table 4 on per-patient and per-lesions bases.
Correlation of serum PSA levels with PET parameters
SUVmean, SUVmax, wbPSMA-TV, and wb TL-PSMA correlated with serum PSA levels in a significant way.
For those patients with more than one lesion (n = 89), only SUVmax, wbPSMA-TV, and wbTL-PSMA correlated with serum PSA levels at a statistically significant level, while in patients with only one lesion (n = 53), all four parameters correlated significantly with PSA.
When analyzing for mean value differences between the Gleason groups, only the parameter wbTL-PSMA was significantly different (one-way ANOVA P < 0.05; Fig. 2).
An overview of the correlation between the four PET parameters and the serum PSA levels is provided in Table 5, for all patients, as well as for the respective subgroups, including correlation parameters and significance levels.
Additionally, from the plot of wbPSMA-TV versus PSA serum level (Fig. 3), the correlation can be seen.
Evaluation of response to therapy by using biochemical, PET, and CT criteria
A total of 23 patients with 223 68Ga-PSMA-11-positive lesions which received baseline and follow-up 68Ga-PSMA-11-PET/CT were included in the therapeutic response evaluation, consisting of 13 patients who underwent radiation therapy, 9 patients who underwent androgen deprivation therapy, and 1 patient who underwent chemotherapy (see Fig. 4 for 68Ga-PSMA-11-PET/CT imaging in a patient who underwent baseline and follow-up PET/CT after radiation therapy of a PSMA-positive lymph node metastasis).
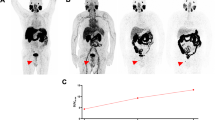
68Ga-PSMA-11 PET/CT-derived metabolic parameters for assessment of therapeutic response in a 71-year-old patient (patient # 10 in Table 6), Gleason 7 tumor, and serum PSA at first examination of 1.7 ng/ml. Maximum intensity projection (a). Baseline (b) and follow- up PET/CT after radiation therapy (c) demonstrate a 68Ga-PSMA-11-positive metastasis in the pelvis (arrow). Serum PSA level at second examination decreased by 30% (biochemical PR), SUVmax decreased by 38% (RR-PET PR), TL-PSMA decreased by 48% (RR-PET PR), while radiographic response assessment based on CT indicated stable disease (SD)
Based on the BR, 2 patients were diagnosed with CR, 9 with PR, 1 with SD, and 11 with PD. With assessment of response based on SUVmax from PET, 2 patients were diagnosed with CR, 6 with PR, 0 with SD, and 15 with PD. Based on the measurement of TL-PSMA, 2 patients were diagnosed with CR, 7 with PR, 0 with SD, and 14 with PD. For the response assessment based on CT target lesions as defined by the SUVmax criterion, 1 patient was diagnosed with CR, 4 with PR, 8 with SD, and 10 with PD. For CT target lesions defined by the TL-PSMA criterion, one patient was diagnosed with CR, seven with PR, six with SD, and nine with PD. A detailed overview of each patient’s response evaluation is provided in Table 6.
The agreement between BR and RR-PET (SUVmax) was 74% and between BR and RR-PET (TL-PSMA) 87%. The agreement of BR and RR-CT (SUVmax) was 39% and for BR and RR-CT (TL-PSMA) 56%. The agreement between RR-PET (SUVmax) and RR-CT (SUVmax) was 56%. The agreement of RR-PET (TL-PSMA) and RR-CT (TL-PSMA) was 61%. An overview of the agreement is provided in Table 7, along with a 95% CI for the agreement, Cohen’s Kappa values, and level of significance for Cohen’s Kappa.
Discussion
In the current work, we were able to significantly extend the previous study of Schmuck et al. [13] by increasing the population to 142 patients and the analyzed lesion number to 641 lesions. Additionally, we analyzed baseline and follow-up PET/CT data of 23 patients under therapy and compared response evaluation based on PSA serum levels, PET-derived SUVmax and TL-PSMA, and CT-derived PERCIST.
Correlation of PSA and PET-derived metabolic parameters
We could demonstrate that SUVmean, SUVmax, whole-body PSMA-TV, and whole-body TL-PSMA correlated significantly with serum PSA levels in our patient population.
In the comparable study of Schmuck et al. [13], the authors found a significant correlation of whole-body PSMA-TV and whole-body TL-PSMA with serum PSA levels, which is analogous to our results. Nevertheless, some differences between their results and ours are present. For example, their results suggested no correlation of SUVmax and SUVmean values with serum PSA levels. The differences might be explainable due to the higher patient number (101 vs. 142) and higher number of 68Ga-PSMA-11-positive lesions (304 vs. 641) which were evaluated in our study.
Furthermore, deviating results for correlation of SUVmax and SUVmean with PSA could be explained by differences of the number of patient lesions of the study populations. When analyzing the sub-groups of patients which have more than one or only one 68Ga-PSMA-11-positive lesion, we found that the correlation of SUVmax and SUVmean with PSA was higher for the latter group. Most likely, in patients with only one lesion, SUVmean and SUVmax are surrogate parameters which express the entire tumor load of a patient as sufficient as PSA, PSMA-TV, and TL-PSMA. However, since we do not have detailed knowledge on the Schmuck et al. patient population in this regard, this is speculative.
Interestingly, we found that TL-PSMA was the only parameter which was significantly different for primary Gleason score, while other PET-derived parameters like SUVmax, SUVmean, and PSMA-TV were not. An explanation for this might be that in contrast to PSMA-TV, which only takes into account the MTV, TL-PSMA also considers the degree of PSMA-expression of tumor cells which is regularly elevated at higher Gleason scores [24, 25].
Monitoring of response to treatment
Currently, assessment of therapeutic response in PC is based on changes of serum PSA levels, on the evaluation of X-ray CT imaging, using the RECIST 1.1 criteria, and other clinical outcome parameters [26]. Little is known about the role of hybrid imaging and especially the potential of PET/CT in this regard. A few reports on the use of 18F-choline and 11C-choline PET/CT are available [27,28,29]. Also, information on the role of PSMA PET/CT in monitoring response to treatment and on enhanced metabolic PET parameters such as TL-PSMA and PSMA-TV is scarce and only a few reports are available. In the 68Ga-PSMA-ligand PET/CT study of Seitz et al. [30], a comparison between response monitoring based on PET and on RECIST 1.1 CT evaluation was performed. In 23 patients undergoing docetaxel chemotherapy, they found that response assessment with PET showed a higher concordance to changes of PSA serum levels, compared to the RECIST 1.1 evaluation. These differences were not statistically significant, which the authors attribute to the too limited number of patients in the study.
In the study of Schmuck et al. [13], response-to-treatment assessment with PET was compared to changes in PSA level. Specifically, the correlation between changes in PSA level and PET-derived parameters SUVmax, SUVmean, PSMA-TV, and TL-PSMA were calculated. The authors found that changes in PSMA-TV and TL-PSMA correlated significantly with changes of PSA, while no such correlation was found for SUVmax and SUVmean. The authors thus conclude that PSMA-TV and TL-PSMA might facilitate therapy monitoring.
Congruently, our results also suggest a potential superiority of parameters derived from 68Ga-PSMA-11 PET over X-ray CT in assessing response of PCA to treatment. Agreement between SUVmax/TL-PSMA and BR evaluation was substantially higher compared to the agreement between CT assessment based on RECIST 1.1 and BR, although this difference did not reach statistical significance. Notably, similar to the results of Schmuck et al., the agreement to BR assessment was higher for TL-PSMA than for SUVmax values, although the difference was not statistically significant as well.
An explanation for the potential superiority of TL-PSMA could be that while SUVmax represents the highest metabolic activity of a single voxel within the tumor, TL-PSMA also takes into account the MTV of metastatic lesions.
Limitations
Our study suffers from several limitations. First of all, due to the low number of only 23 patients who underwent baseline and follow-up PET/CT for evaluation of therapeutic response, the results have to be interpreted with caution. Possible biases stemming from patient referrals and treatments cannot be excluded due to the retrospective nature of this analysis. Furthermore, this analysis was conducted as a single-center study and histological confirmation as a gold standard of each PSMA-positive lesion would have been preferable, but was not feasible.
While PERCIST 1.0 criteria are already established for evaluation of therapeutic response in FDG-PET studies [31], there are as yet no established standardized response criteria for PSMA-PET. As a surrogate, we adapted PERCIST 1.0 criteria for assessment of treatment response, which could potentially overcome several limitations of solely morphological-based criteria. For example, lesions, e.g. bone metastases without soft tissue mass > 10 mm, which are rated unmeasurable by RECIST 1.1 criteria [21] could now be included in the evaluation. Consequently, MTV could be addressed directly, instead of lesion diameter sums [32]. Thus, e.g. TL-PSMA, combining metabolic tumor activity and MTV, might be an ideal parameter for therapeutic response evaluation. Nevertheless, although other groups used the same modified PERCIST criteria for PSMA-PET [33] as we did, the response thresholds have to be validated in larger studies.
Lastly, radiographic response assessment was compared to BR as measured by PSA serum levels, which is also recommended for assessment of many therapies in advanced PC by the current guidelines of the EAU [34]. However, Ceci et al. [17] and the EAU recommendations for therapy monitoring in patients with advanced castration-resistant prostate cancer (CRPC) pointed out that PSA alone might not be sufficiently reliable for assessment of treatment response in all cases.
To determine the validity of radiographic response assessment, long-term follow-up and survival in treated patients should be evaluated in larger prospective multicenter trials.
Conclusion
SUVmean and SUVmax, as well as advanced PET-derived parameters which express the total MTV, such as whole-body PSMA-TV and TL-PSMA, demonstrated a significant correlation with serum PSA levels. TL-PSMA was the only PET-derived parameter which was significantly different between patient groups with different Gleason scores. Additionally, response-to-therapy assessment using TL-PSMA showed the highest agreement to monitoring based on PSA levels, superior to SUVmax-based evaluation and response assessment based on CT data and RECIST 1.1 criteria.
Altogether, this suggests a possible role of 68Ga-PSMA-11 PET/CT for therapeutic response evaluation in patients with PC.
References
Freedland SJ, Presti JC, Amling CL, Kane CJ, Aronson WJ, Dorey F, et al. Time trends in biochemical recurrence after radical prostatectomy: results of the SEARCH database. Urology. 2003;61:736–41.
Khuntia D, Reddy CA, Mahadevan A, Klein EA, Kupelian PA. Recurrence-free survival rates after external-beam radiotherapy for patients with clinical T1–T3 prostate carcinoma in the prostate-specific antigen era. Cancer. 2004;100:1283–92.
Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–83.
Afshar-Oromieh A, Babich JW, Kratochwil C, Giesel FL, Eisenhut M, Kopka K, et al. The rise of PSMA ligands for diagnosis and therapy of prostate cancer. J Nucl Med. 2016;57:79S–89S.
Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–74.
Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic efficacy of 68 gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195:1436–43.
Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13:226.
Ben-Haim S, Ell P. 18F-FDG PET and PET/CT in the evaluation of cancer treatment response. J Nucl Med. 2009;50:88–99.
Bäuerle T, Semmler W. Imaging response to systemic therapy for bone metastases. Eur Radiol. 2009;19:2495–507.
Sandblom G, Ladjevardi S, Garmo H, Varenhorst E. The impact of prostate-specific antigen level at diagnosis on the relative survival of 28,531 men with localized carcinoma of the prostate. Cancer. 2008;112:813–9.
van Rossum PS, Fried DV, Zhang L, Hofstetter WL, Ho L, Meijer GJ, et al. The value of 18F-FDG PET before and after induction chemotherapy for the early prediction of a poor pathologic response to subsequent preoperative chemoradiotherapy in oesophageal adenocarcinoma. Eur J Nucl Med Mol Imaging. 2017;44:71–80.
Mikhaeel NG, Smith D, Dunn JT, Phillips M, Møller H, Fields PA, et al. Combination of baseline metabolic tumour volume and early response on PET/CT improves progression-free survival prediction in DLBCL. Eur J Nucl Med Mol Imaging. 2016;43:1209–19.
Schmuck S, von Klot CA, Henkenberens C, Sohns JM, Christiansen H, Wester H-J, et al. Initial experience with volumetric 68Ga-PSMA I&T PET/CT for assessment of whole-body tumor burden as a quantitative imaging biomarker in patients with prostate cancer. J Nucl Med. 2017;58:1962–8.
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent—update 2013. Eur Urol. 2014;65:124–37.
Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–42.
Eder M, Neels O, Müller M, Bauder-Wüst U, Remde Y, Schäfer M, et al. Novel preclinical and radiopharmaceutical aspects of [68Ga] Ga-PSMA-HBED-CC: a new PET tracer for imaging of prostate cancer. Pharmaceuticals. 2014;7:779–96.
Ceci F, Castellucci P, Graziani T, Schiavina R, Renzi R, Borghesi M, et al. 11C-choline PET/CT in castration-resistant prostate cancer patients treated with docetaxel. Eur J Nucl Med Mol Imaging. 2016;43:84–91.
Picchio M, Berardi G, Fodor A, Busnardo E, Crivellaro C, Giovacchini G, et al. 11C-choline PET/CT as a guide to radiation treatment planning of lymph-node relapses in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2014;41:1270–9.
Jereczek-Fossa BA, Beltramo G, Fariselli L, Fodor C, Santoro L, Vavassori A, et al. Robotic image-guided stereotactic radiotherapy, for isolated recurrent primary, lymph node or metastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82:889–97.
Joo Hyun O, Lodge MA, Wahl RL. Practical PERCIST: a simplified guide to PET response criteria in solid tumors 1.0. Radiology. 2016;280:576.
Eisenhauer E, Therasse P, Bogaerts J, Schwartz L, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74.
Kasperzyk JL, Finn SP, Flavin RJ, Fiorentino M, Lis RT, Hendrickson WK, et al. Prostate-specific membrane antigen protein expression in tumor tissue and risk of lethal prostate cancer. Cancer Epidemiology and Prevention Biomarkers. 2013; https://doi.org/10.1158/1055-9965.EPI-13-0668.
Marchal C, Redondo M, Padilla M, Caballero J, Rodrigo I, Garcia J, et al. Expression of prostate specific membrane antigen (PSMA) in prostatic adenocarcinoma and prostatic intraepithelial neoplasia. Histol Histopathol. 2004;19:715–8.
Thalgott M, Rack B, Eiber M, Souvatzoglou M, Heck MM, Kronester C, et al. Categorical versus continuous circulating tumor cell enumeration as early surrogate marker for therapy response and prognosis during docetaxel therapy in metastatic prostate cancer patients. BMC Cancer. 2015;15:458.
De Giorgi U, Caroli P, Burgio SL, Menna C, Conteduca V, Bianchi E, et al. Early outcome prediction on 18F-fluorocholine PET/CT in metastatic castration-resistant prostate cancer patients treated with abiraterone. Oncotarget. 2014;5:12448.
De Giorgi U, Caroli P, Scarpi E, Conteduca V, Burgio SL, Menna C, et al. 18F-Fluorocholine PET/CT for early response assessment in patients with metastatic castration-resistant prostate cancer treated with enzalutamide. Eur J Nucl Med Mol Imaging. 2015;42:1276–83.
Schwarzenböck SM, Eiber M, Kundt G, Retz M, Sakretz M, Kurth J, et al. Prospective evaluation of [11C] choline PET/CT in therapy response assessment of standardized docetaxel first-line chemotherapy in patients with advanced castration refractory prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:2105–13.
Seitz AK, Rauscher I, Haller B, Krönke M, Luther S, Heck MM, et al. Preliminary results on response assessment using 68 Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur J Nucl Med Mol Imaging. 2017:1–11.
Min SJ, Jang HJ, Kim JH. Comparison of the RECIST and PERCIST criteria in solid tumors: a pooled analysis and review. Oncotarget. 2016;7:27848.
Eiber M, Herrmann K, Calais J, Hadaschik B, Giesel FL, Hartenbach M, et al. PROstate cancer molecular imaging standardized evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med. 2018;59:469–78.
Seitz AK, Rauscher I, Haller B, Krönke M, Luther S, Heck MM, et al. Preliminary results on response assessment using 68Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur J Nucl Med Mol Imaging. 2017:1–11.
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–79.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflicts of interest
Schmidkonz C, Cordes M, Beck M, Goetz Th I, Schmidt D, Prante O, Kuwert T, Ritt P: The Clinic of Nuclear Medicine in Erlangen has research cooperation with Siemens in the field of SPECT/CT, but not related to the data contained in this manuscript.
Kuwert T: has received honoraria for lectures on behalf of Siemens Molecular Imaging.
Goebell P: has received honoraria for lectures and participation in expert meetings from Pfizer, Janssen-Cilag, Novartis, GSK, BMS, and Bayer.
Wullich B: has received honoraria for lectures and participation in expert meetings from Janssen-Cilag and Astellas.
Uder M: has received honoraria for lectures and grants from Siemens, Bayer, Bracco, and Medtronic.
Bäuerle T: has received Honoria for lectures and grants from Bayer, Bracco, and Boeringer Ingelheim.
Beck M: has received honoraria for lectures from Bayer.
Rights and permissions
About this article
Cite this article
Schmidkonz, C., Cordes, M., Schmidt, D. et al. 68Ga-PSMA-11 PET/CT-derived metabolic parameters for determination of whole-body tumor burden and treatment response in prostate cancer. Eur J Nucl Med Mol Imaging 45, 1862–1872 (2018). https://doi.org/10.1007/s00259-018-4042-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4042-z