Abstract
Agar-degrading bacteria are crucial drivers for the carbon cycle in the marine environments due to their ability that use algae as a carbon source. Although numerous agar-degrading bacteria and agarases have been reported, little is known about expression levels of agar-degrading genes in wild strains. Here, the genome of an agar-hydrolyzing marine bacterium, Catenovulum maritimus Q1T, was sequenced and annotated with 11 agarase and 2 neoagarooligosaccharide hydrolase genes. Quantitative PCR revealed that all the annotated agar-degrading genes were expressed consistently that initially upregulated and then gradually downregulated under agarose induction. Moreover, the presence of glucose inhibited the agar-degrading ability, in terms of both gene expression and enzymatic activity. These facts indicated the agar-degrading ability of wild bacteria was mainly induced by agarose and repressed by the available carbon source. Additionally, a β-agarase, AgaQ1, belonging to the GH16 family, with high expression in strain Q1T, was cloned and characterized. Biochemical analysis showed that the recombinant AgaQ1 was substrate-specific, yielding neoagarotetraose and neoagarohexaose as the main products. It exhibited optimal activity at 40 °C, pH 8.0, and an agarose concentration of 1.6% (w/v). Besides, AgaQ1 showed a high-specific activity (757.7 U/mg) and stable enzymatic activity under different ion or agent treatments; thus, AgaQ1 has great potential in industrial applications.
Key points
• The genome of C. maritimus Q1T was sequenced and annotated with 11 agarases and 2 Nabh genes.
• The expression of agar-degrading genes in the strain C. maritimus Q1T was induced by agarose.
• Glucose was the carbon source utilized prior to agarose for bacterial growth.
• A β-agarase, AgaQ1, with high expression and activity was identified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
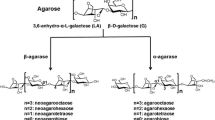
The oceans cover for more than 70% of the Earth’s surface and produce an abundance of algae, which are among the most potentially significant sources of sustainable biofuels (Wargacki et al. 2012; Menetrez 2012; Kawai and Murata 2016). Agar, as the main cell wall component of red algae (Rhodophyta), thus has a huge stock in the marine environment (Usov 1998). Structurally, agars constitute a family of galactans with a hybrid structure consisting of alternating 3-O-linked β-D-galactose (G) and 4-O-linked α-L-galactose (L). According to the nomenclature introduced by Knutsen et al. (1994), the main repeating moiety in agar is the agarobiose (G-LA), with the 3,6-anhydro-L-galactose monomer (LA) replacing the L monomer. The galactan backbones are often masked by ester sulfate groups, methyl groups, or pyruvic acid acetal groups, thereby increasing the number of possible agarobiose structures (Lahaye et al. 1989; Usov 1998; van de Velde et al. 2002). Given their tandem repetitive feature, agar hydrolysis is easily performed by chemical or biological treatments (Yun et al. 2017; Xu et al. 2018). The common products of agar hydrolysis are mixtures of oligosaccharides, the so-called agar-oligosaccharides, which are generally water-soluble, harmless, and easily absorbed by the body (Kim et al. 2017; Hong et al. 2019). Moreover, agar-oligosaccharides with a low degree of polymerization, such as neoagarobiose (NA2), neoagarotetraose (NA4), and neoagarohexaose (NA6), have also been reported to possess antioxidative activities, bacteriostatic ability, and moisture retention capacity when applied to human skin (Kang et al. 2014; Kim et al. 2017; Yun et al. 2017). Hence, agar-oligosaccharides have great potential for the applications in foods, medicines, nutraceuticals, cosmetics, and other fields (Wang et al. 2004; Yun et al. 2017; Park et al. 2020).
Although chemical and enzymatic methods are both used for agar degradation, the latter has more potential due to better controllability of products and the uncertain environmental effect of the former (Chi et al. 2012). Agarase, the first key enzyme in the agar-degrading pathway, is a glycoside hydrolase, mostly obtained from bacteria that can degrade agarose to agaro-oligosaccharides (AOS) or neoagaro-oligosaccharides (NAOS). Based on their cleavage pattern, agarases are classified as α-agarase (E.C. 3.2.1.158) if they hydrolyze the α-(1,3) glycosidic bonds of neoagarobiose repeating moieties to produces AOS (Potin et al. 1993), or β-agarase (E.C. 3.2.1.81) if they hydrolyze the β-(1,4) glycosidic bonds of agarobiose repeating moieties to produce NAOS (Kirimura et al. 1999). According to the carbohydrate-active enzymes (CAZY) database (http://www.cazy.org/), α-agarases are classified within the glycoside hydrolase (GH)-96 family, with 5 members now, while β-agarases are mainly classified into families: GH16, GH39, GH50, GH86, and GH118 families (Park et al. 2020). Therein, the GH16 family is the largest agarase group and contains hundreds of reported members with high enzyme activities (Fu and Kim 2010; Park et al. 2020).
Many agarases have been reported to digest agarose to NAOS or AOS, but the smallest product of hydrolysis has been NA2 or agarobiose, and none have been reported to digest agarose to monosaccharide, the direct carbon source for bacterial growth (Fu and Kim 2010; Chi et al. 2012). Hence, to further utilize NA2 or agarobiose, agar-degrading bacteria must also harbor NA2 or agarobiose hydrolase to support the final stage of agar degradation, even if no purified agarobiose hydrolase has yet been purified and identified (Kim et al. 2018). Neoagarooligosaccharide hydrolase (Nabh) was first discovered by Day and Yaphe in 1975, in the marine bacterium Pseudomonas atlantica, with the ability to cleave NA2 to LA and G (Day and Yaphe 1975). Subsequently, several Nabhs belonging to a novel family GH117 were successively reported, with different substrate preferences (Suzuki et al. 2002; Rebuffet et al. 2011; Jiang et al. 2020). Although there are many reports of the two key steps of agarose degradation (Chi et al. 2012; Park et al. 2020), an understanding of the expression level of both agarase and Nabh genes in wild bacteria is limited.
Numerous marine microorganisms have been reported to have agar-degrading ability, such as the genera Catenovulum (Yan et al. 2011), Aquimarina (Chen et al. 2012), Thalassomonas (Park et al. 2011), Colwellia (Xu et al. 2017a), and Tenacibaculum (Xu et al. 2017b). Among them, the genus Catenovulum was proposed by Yan et al. (2011) and contained three agar-hydrolyzing species C. agarivorans YM01T (Yan et al. 2011), C. maritimum Q1T (Li et al. 2015), and C. sediminis D2T (Shi et al. 2017). These three Catenovulum strains have been reported to show high enzymatic degradation and utilization of multiple polysaccharides, such as agarose, alginate, and starch (Shi et al. 2017). Particularly, strain C. maritimum Q1T can liquefy and grow on agarose as the sole carbon source, indicating that it can secrete highly active agarases, a trait which was further explored in this study.
Taking the agar-hydrolyzing marine bacterium C. maritimus Q1T, we clarified the dynamic expression characteristics of all annotated agar-degrading genes, and characterized an endo-type β-agarase, AgaQ1, with significant industrial potential.
Materials and methods
Strains
The strain C. maritimus Q1T, isolated from the surface of the marine alga, Porphyra yezoensis Ueda, was commonly cultured at 28 °C on marine agar 2216 (MA; BD Difco, NY, USA) or marine broth 2216 (MB; Difco) supplemented with 0.5% glucose (MBG) or 0.5% agarose (MBA) (Li et al. 2015). Escherichia coli DH5α and E. coli Rosetta™ (DE3), which were used as the hosts for cloning and expression, respectively, were cultured at 37 °C in Luria-Bertani (LB) medium.
Genome sequencing and analysis of the strain Q1T
The biomass of the strain Q1T for genome sequence was harvested after culturing in MBG for 2 days. Genomic DNA was extracted using a bacterial genomic DNA kit (Takara, Tokyo, Japan), according to the manufacturer’s instructions for Gram-stain negative bacteria. The quality and quantity of DNA samples were determined using a BioPhotometer (Eppendorf, Hamburg, Germany). Next, the DNA sample was randomly fragmented to produce DNA fragments of the desired length, followed by end repair and the adapter ligation. The qualified libraries were constructed and sequenced using Illumina MiSeq paired-end sequencing technology at Shanghai Personal Biotechnology (Shanghai, China). Raw sequencing data were assembled using Newbler version 2.8 (Margulies et al. 2005) and GapCloser version 1.12 (Luo et al. 2012). Protein-coding gene prediction and annotation were performed based on the SEED database of the RAST server (Aziz et al. 2008), the Swiss-Prot database (Bairoch and Apweiler 2000), and the NCBI Refseq database (Pruitt et al. 2005).
Sequence analysis of the agar-degrading enzyme genes
All agarase genes and Nabh genes of strain Q1T were identified based on the annotation of the Swiss-Prot database and CAZY database with the dbCAN2 meta server (Zhang et al. 2018), and further verified by the BLASTP tool against the NCBI database. Gene integrity was confirmed using ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/). The DNA sequences were translated into amino acid sequences using the Sequence Manipulation Suite (http://www.bioinformatics.org/sms/). The molecular weight, isoelectric point, signal peptide, and transmembrane domain of the proteins were estimated using the peptide mass tools on the ExPASy server of the Swiss Institute of Bioinformatics (Artimo et al. 2012). The circular map, based on the draft genome and showing the locations of all target genes, was generated by CGView Serve with the default parameters (Grant and Stothard 2008). The conserved domains in the analyzed proteins were annotated using Pfam 32.0 (El-Gebali et al. 2018) and reconfirmed with the NCBI structure tool. The amino acid sequences of other agarases were downloaded from the NCBI database and aligned using the CLUSTAL W algorithm in MEGA X (Kumar et al. 2018). A phylogenetic tree, based on amino acid sequences, was constructed using the neighbor-joining method, with 1000 bootstrap replicates in MEGA X (Kumar et al. 2018), and further edited using EvolView (Subramanian et al. 2019).
Determination of bacterial growth curve and the agarase activity of the strain Q1T
The growth curve of strain Q1T was drawn based on the OD600 values of biomass over time, using 100 mL of the MBA in a 250-mL conical flask. For the determination of agarase activity, the wild strain Q1T was cultured with three different media as the independent variable: MBG, MBA, and MBGA (MB with 0.5% glucose and 0.5% agarose). Samples were collected every 4 h from the time bacteria started to grow, and then preserved at − 20 °C until analysis of enzymatic activity. Agarase activity was quantified using a modified 3,5-dinitrosalicylic acid (DNS) method, as described below (please refer to the section “Enzyme activity assay”), modified to use 100 μL of the bacterial broth and 100 μL of the lysis buffer as reaction solutions.
Total RNA extraction, cDNA synthesis, and real-time quantitative PCR
For RNA extraction, the biomass of strain Q1T was collected at different growth times from the three different media as described above. Total RNA was extracted from the cells using a bacterial RNA extraction kit (Tiangen, Beijing, China), according to the manufacturer’s instructions. The quality, concentration, and integrity of extracted RNA were tested using a BioPhotometer (Eppendorf) and agarose gel electrophoresis. cDNA synthesis from the RNA templates was performed using a cDNA synthesis kit (Takara), with random primers. Real-time quantitative PCR (RT-qPCR) was performed using the StepOnePlus Real-Time PCR System (Applied Biosystems, CA, USA) with the SYBR Green PCR Master Mix (2Χ, Applied Biosystems), according to the manufacturer’s instructions. The reaction condition adopted was the default program of the instrument with the Fast model. The primer pairs for RT-qPCR were designed based on the MIQE guidelines (http://rdml.org/miqe), with the software Primer Premier 5 and listed in Table S1. The quantification technique used to analyze the data was that described by Schmittgen and Livak (2008). The geNorm tool was used to select the most stable genes for use as references. Data were normalized to the reference genes, 16S rRNA gene and recA, to increase the robustness of the procedure. All experiments were repeated three times with independent RNA samples.
Cloning of the agaQ1 gene
The whole sequence of agarase gene agaQ1 was amplified from the genomic DNA of strain Q1T with the primer sets: agaQ1F: 5’-GCGCGGATCCATGCTAAACAAAATCACGTTATG-3’ and agaQ1R: 5’-CGCGCTCGAGTTATTGGAACTTCCATTGCTG-3’, which contained the BamH 1 and Xhol 1 restriction enzyme sites (underlined), respectively. The amplified products were then purified and inserted into the expression vector pET-28a (+), using T4 DNA ligase (Takara). Subsequently, the recombinant plasmid, pET-28a (+)-agaQ1, was transformed into E. coli DH5α competent cells and further confirmed by Sanger sequencing with the universal primers, T7 and T7ter. Next, the plasmid pET-28a (+)-agaQ1 was extracted and purified from E. coli DH5α cells and transformed into E. coli Rosetta™ (DE3) competent cells for heterologous expression.
Expression and purification of the recombinant enzyme AgaQ1
E. coli Rosetta cells harboring the recombinant plasmid pET-28a (+)-agaQ1 were cultured at 37 °C in LB broth supplemented with 50 μg/mL kanamycin. When the cell density reached 0.6–0.8 (OD600), 0.1 mM (final concentration) isopropyl β-D-1-thiogalactopyranoside (IPTG) was added to induce the expression of AgaQ1. After further overnight incubation at 16 °C, the crude fermentation broth containing bacterial cells was collected to verify agarase activity. Concurrently, bacterial biomass was collected by centrifugation at 10,000×g for 10 min at 4 °C, and then washed twice with ice-cold 50 mM Tris-HCl buffer. The collected bacteria were subsequently re-suspended in 1/20 volume Tris-HCl buffer and disrupted by sonication (300 W, 6 s bursts, and 10 s pulses for 20 min) in an ice-water bath. Crude inclusion bodies of AgaQ1 were collected after centrifugation at 12,000×g for 30 min. The purification and renaturation of inclusion bodies were performed according to the methods described by Han et al. (2013). Purity and molecular mass of AgaQ1 were examined by SDS-PAGE, and the amino acid sequence was confirmed by LC-MS/MS, supplemented with the Mascot search engine version 2.3.1, which was carried out by Beijing Protein Innovation (Beijing, China) using the Prominence nano LC system (Shimadzu, Tokyo, Japan) interfacing with the MicrOTOF-QII system (Bruker Daltonics, MA, USA). The concentration of purified and renatured protein was measured using the BCA Protein Assay Kit (Beyotime, Shanghai, China), with bovine serum albumin (BSA) as the standard.
Enzyme activity assay
The agar-degrading activity of the wild strain Q1T and recombinant AgaQ1 was visualized by staining with Lugol’s iodine solution and quantified by detecting the reducing sugar using the DNS method (Miller 1959), with some adjustment. In brief, the reaction system was first prepared with 50 μL of the enzyme solution and 150 μL of lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 5 mM Imidazole, pH 8.0) containing 1.0% (w/v) melted agarose. After incubation at 40 °C for 30 min, the reaction was stopped by adding 100 μL DNS reagent (1% 3,5-dinitrosalicylic acid, 30% potassium sodium tartrate, and 1.5% NaOH), and then immersed in boiling water for 5 min. Then, 700 μL of distilled water was added and mixed, to chill the reaction system and dilute the reaction solution to a volume of 1 mL. The absorbance of the reaction solution was measured at 520 nm using a VersaMax Microplate Reader (Molecular Devices, CA, USA). The alkalified (pH > 11) and boiled enzyme solution was used as the negative control. d-galactose was used as the standard for calculating agarase-specific activity. Enzyme activity (U) was defined as the amount of enzyme that produced 1 μmol of reducing sugars per minute.
Biochemical characterization of AgaQ1
Seven polysaccharides, including agarose, cellulose, gelatin, carrageenan, alginate, starch, and agar powder, dissolved in lysis buffer at a concentration of 1.0% (w/v), were individually assayed to determine the degradable substrates of AgaQ1. The temperature tests were performed with lysis buffer containing 1.0% (w/v) agarose at a temperature range of 30–80 °C. A pH range of 5.5–10.0 was tested, using the following buffers (Sangon Biotech, Shanghai, China): MES (pH 5.5 and 6.0), PIPES (pH 7.0), HEPES (pH 8.0), and CAPSO (pH 9.0 and 10.0), at a concentration of 20 mM. The optimal substrate concentration was confirmed at agarose concentrations ranging from 0.1 to 2.0% (w/v), while the effects of various metal ions, chelators, and reducing reagents on the activity of AgaQ1 were assayed using lysis buffer supplemented with 1.0% agarose and various ions or agents (10 mM). The agarase activity, under the different conditions listed above, was determined using the modified DNS method. Relative activity was defined as the percentage of the maximum agarolytic activity, except for the effect of various ions and reagents, whose relative activity was in contrast to the condition without any ions or reagents.
Identification of products of hydrolysis and the minimum hydrolytic substrate
To determine the hydrolytic products of purified AgaQ1, the hydrolysis reaction was carried out at 40 °C using 50 μL of the purified agarase solution and 150 μL of the lysis buffer containing 1.0% (w/v) agarose with a reaction time varying from 5 min to 72 h. The hydrolytic products were detected using thin layer chromatography (TLC) method, according to the process described by Cui et al. (2014), using Silica Gel 60 TLC plates (Merck, NJ, USA). Three agar-oligosaccharides, NA2, NA4, and NA6, obtained from Seebio Biotech (Shanghai, China) were used as standard markers. Identification of the minimum hydrolysis substrate was performed using purified 0.2% (w/v) NA4 or NA6 in the lysis buffer as the only substrate. The reaction time and detection method were the same as those used for the identification of products of agarose hydrolysis.
Accession numbers
All sequences used in this study were deposited in NCBI database with the following accession numbers: agaQ1-MH005810, agaQ2-MH005811, agaQ3-MH005812, agaQ4-MH005813, agaQ5-MH005814, agaQ7-MH005816, agaQ10-MH005818, agaQ11-MH005819, agaQ12-MH005820, agaQ13-MH005821, agaQ14-MH005822, recA-MK770644, 16S rRNA gene-NR_146038.1. The genome sequences of strain Catenovulum maritimus Q1T were deposited in NCBI database with the accession number NZ_LAZL00000000.1. The strain Catenovulum maritimus Q1T is available in the China Center of Industrial Culture Collection (CICC) and the German Collection of Microorganisms and Cell Cultures (DSMZ) with accessions numbers CICC 10836T and DSM 28813T, respectively.
Results
Genome analysis of strain Q1T
In the genomic sequence of strain Q1T, approximately 6.7 million raw reads were obtained and assembled into 62 contigs, with a total genome length of 4.5 Mbp, a mean coverage of 346 ×, and a N50 value of 155,862 bp. The average G+C content of the genome was 37.9 mol%. Automated annotation identified 3751 open reading frames (ORFs), 57 tRNA genes, and 2 CRISPR sequences. Moreover, there were 2482 protein-coding genes annotated with the Swiss-Prot database and 56 carbohydrate-active enzymes with the CAZY database, including 11 agarase genes and 2 Nabh genes, which were located at different positions in the genome without any contiguity (Fig. 1). Based on the phylogenetic analysis, most of the annotated agarases were clustered into the various GH families of β-agarase: AgaQ1 and AgaQ3 belong to GH16, AgaQ4, AgaQ5, AgaQ7, AgaQ11, AgaQ13, and AgaQ14 belong to GH50, AgaQ10 belongs to GH86, and AgaQ2 belongs to GH118 (Fig. 2, Table 1). In addition, an α-agarase, AgaQ12, belonging to GH96 was also annotated with a longer amino acid sequence than any of the β-agarases (Fig. 2, Table 1). The two annotated Nabhs, Nabh1 and Nabh2, were classified into the family GH117 based on their high similarities to other reported Nabhs (Table 1). These multiple agar-degrading enzymes have also explained the high agar-degrading activity of the wild strain Q1T on agar plates. Detailed information on the 11 annotated agarase genes and 2 Nabh genes, and their GenBank accession numbers are listed in Table 1.
The locations of all annotated agarase and Nabh genes in the circular genome of strain Catenovulum maritimus Q1T. The rings from inner to outer: ring 1, GC skew; ring 2, G+C content; ring 3, the locations of annotated agarase and Nabh genes; ring 4, 5, all predicted ORFs of strain Catenovulum maritimus Q1T
Phylogeny and protein structures of all annotated agarases (in bold) in strain Catenovulum maritimus Q1T and representatives of some related GH families. The tree was constructed based on amino acid sequences using MEGA X with neighbor-joining method. The protein domains were annotated with Pfam 32.0 with e value of 1.0 as the threshold. Bootstrap values of the tree (expressed as percentages of 1000 replications) over 70% are shown as black dots at branching nodes. The background colors indicated different GH families
Expression profiles of all agar-degrading genes in the strain Q1T
Before examining the expression profiles of agar-degrading genes in strain Q1T, a growth curve was first drawn that divided the growth phase of strain Q1T into three parts: lag phase (0–8 h), logarithmic phase (8–20 h), and stationary phase (20–36 h), with a generation time of approximately 2.1 h (Fig. S1). Three times (16 h, 20 h, and 26 h) at specific points in the growth curve were chosen to harvest bacteria for RT-qPCR analysis. To calculate the relative expression of all genes under this study, the culture condition of MBG was designed as the experimental control. As is shown in Fig. 3a, when agarose alone was used as the inducer, the expression levels of all annotated agarase and Nabh genes were initially high, decreasing steadily from the 16 to the 26 h. However, when both agarose and glucose were used as inducers, the expression initially increased from the 16 to the 20 h, and then decreased from the 20 to the 26 h, which suggested that the presence of glucose delayed the expression of all agar-degrading genes. These facts indicate that both agarase and Nabhs were produced inductively during the bacterial logarithmic phase to degrade agarose as a carbon source for bacterial growth, but were inhibited by the presence of glucose.
Gene expression and phenotypic results of the wild strain Catenovulum maritimus Q1T under agarose induction. a The expression profiles of all annotated agarase and Nabh genes of strain Q1T under different treatments, Aga, AG represent the induction of agarose and agarose plus glucose, respectively; 16 h, 20 h, 26 h represent three culture times when collected samples. b Transparent zone caused by agarose degradation of fermentation broth. CK, negative control with 100 μL of the MBA; F1 and F2, 100 μL of the MBA with inoculation of strain Q1T. c Relative agarase activity of the wild strain Q1T under three treatments: MBG, MB supplemented with 0.5 % glucose; MBGA, MB supplemented with 0.5% glucose and 0.5% agarose; MBA, MB supplemented with 0.5% agarose. Samples were collected every 4 h from the time bacteria started to grow
Agar-degrading activity of strain Q1T
The agar-degrading ability of strain Q1T was visualized by the transparent zones (Fig. 3b) and quantified by detecting the amount of reducing sugar (Fig. 3c). Although the ability to degrade agar showed a steadily decreasing trend under agarose induction, agarase activity remained higher than in cultures containing glucose (Fig. 3c). This consistent phenomenon with the gene expression levels under agarose inductive conditions indicated that agarase secretion from bacteria was induced by its substrate, agarose, and inhibited by glucose (Fig. 3a, 3c). However, under both agarose and glucose induction, the enzyme activities continued to increase during the stationary phase, rather than following the gene expression profiles by increasing initially, then decreasing (Fig. 3a, 3c). These conflicting results might be caused by the difference in durability between gene expression and protein activity. Gene expression profiles are more sensitive to environmental change, whereas protein activity can be sustained for longer, especially if secreted to extracellular conditions.
Analysis of the AgaQ1 gene and amino acid sequence
The gene agaQ1 consists of an ORF of 1,314 nucleotides, encoding a protein of 437 amino acid residues. The predicted protein AgaQ1 has a molecular mass of 48.6 kDa, and its calculated isoelectric point is 5.94, higher than all other close neighbors (Table 2). SignalP 4.1 analysis indicated that AgaQ1 contained a signal peptide which was composed of the first 20 amino acid residues, and no transmembrane helices were predicted. Conserved domain analysis with the full protein sequence suggested that AgaQ1 not only contained a family GH16 catalytic module (Ala20–Lys287) but also contained a Ricin module (Ser310–Phe436), related to carbohydrate combination, near the C terminus (Fig. 2). A three-dimensional structure simulation of the catalytic module of AgaQ1 shows that 1 α-helix and 14 β-strands are present and form a β-jelly roll motif (Fig. 4a), which is a conserved secondary structure for β-agarases in the GH16 family (Allouch et al. 2003). Moreover, catalytic residues of AgaQ1 were annotated with three amino acids, Glu147, Asp149, and Glu152, located in a β-strand of the catalytic module, similar to its relative neighbors (Fig. 4a, 4b). A BLASTP search, based on the NCBI database, revealed that the deduced amino acid sequence of AgaQ1 shared the highest sequence identity (92%) with the β-agarase YM01-3, which was derived from strain C. agarivorans YM01T with the GenBank accession no. AGU13985 (Cui et al. 2014), followed by the β-agarase AguD from an uncultured bacterium (GenBank accession no. AAP49316) (76% similarity) (Voget et al. 2003). Phylogenetic analysis based on the full protein sequences showed that AgaQ1 was clustered with other β-agarases in the family GH16, and was distanced from other families, which was also confirmed by the conserved protein domains (Fig. 2). These results revealed that AgaQ1 obtained from the strain C. maritimus Q1T is a β-agarase located in the family GH16.
Structure and sequence analysis of AgaQ1. a 3D structure prediction of the catalytic domain in AgaQ1 using the SWISS-MODEL server. The structure was simulated based on a known agarase with the SMTL ID, 3wz1.1 (57.4% identity). The different colors represent N-terminal (blue), C-terminal (green), alpha helix (cyan), beta strand (red), loop (light red), and catalytic residues (yellow). b Comparison of the amino acid sequences of the catalytic domain in AgaQ1 (20–276 aa) with five close and known agarases belonging to the family GH16. The conserved residues are indicated with sequence logo (above) and blue color. AGU13985.1: β-agarase from Catenovulum agarivorans YM01; ADD60418.1: β-agarase from Pseudoalteromonas sp. AG4; BAK08910.1: β-agarase from Microbulbifer thermotolerans JAMB A94; BAC99022.1: β-agarase from Microbulbifer elongatus JAMB-A7. The predicted catalytic residues of the catalytic domain in AgaQ1 are represented as red triangle symbols
Expression and purification of recombinant AgaQ1
Preliminary enzyme activity of recombinant strain E. coli showed that the crude fermentation broth formed a transparent zone on agarose plates (Fig. 5a), indicating that the recombinant protein, AgaQ1, together with a His6 tag, was successfully expressed. Furthermore, the enzymatic hydrolysates of the crude fermentation broth, with agarose as the substrate showed that NA2 and NA4 were the major products (Fig. 5b). Although the supernatant of the cell lysate showed high agar-degrading activity, most of the target proteins were located in the precipitates of the cell lysate, as inclusion bodies (Fig. 5c). After purification and renaturation, the renatured protein eluent was obtained and showed a single band with a molecular weight of approximately 50 kDa on SDS-PAGE analysis (Fig. 5c). Further analysis by LC-MS/MS indicated that the amino acid sequence of the purified protein was in accordance with the deduced sequence of AgaQ1 (Fig. S2). Together, this indicated that purified AgaQ1 with agarase activity had been obtained.
The enzyme activity and purity of the recombinant agarase AgaQ1. a Transparent zones of agarose degradation by agarases in crude fermentation broth of recombinant E. coli with (below) or without (above) Lugol’s iodine solution. b TLC images of agarose degradation from the crude recombinant agarase AgaQ1 before purification. S1, marker NA2; S2, marker NA4; S3, marker NA6; AgaQ1, degrading products of AgaQ1 from the crude fermentation of recombinant E. coil. c, SDS-PAGE image of purified recombinant agarase AgaQ1. M, molecular mass marker; CK, total cellular extract from E. coli Rosetta™ (DE3) before induction; lane 1, total cellular extract after induction; lane 2, total cellular soluble extract after induction; lane 3, total cellular insoluble extract after induction; lane 4, the inclusion body dissolved in urea solution; lane 5, purified AgaQ1 in elution solution of affinity chromatography
Biochemical characterization and enzyme activity of AgaQ1
Reducing sugars were produced when agar powder or agarose was used as the enzyme substrate, but no reducing sugars were detected when using other polysaccharides. This result revealed that AgaQ1 is a substrate-specific agarase that only degrades agar or agarose. The optimal reaction temperature of AgaQ1 was 40 °C (Fig. 6a), and the optimal pH was 8 (Fig. 6b). When the reaction temperature exceeded 60 °C or the pH was above 10, AgaQ1 lost more than 70% of its maximum activity, whereas under conditions of 40–50 °C and pH 6.0–9.0, AgaQ1 retained more than 80% of its maximum activity, indicating a wider range of effective reaction conditions. The optimal concentration of substrate (agarose) was 1.6% (w/v) (Fig. 6c). The agarase activity of AgaQ1 was inhibited by Cu2+, but significantly increased by Fe2+, Co2+, and dithiothreitol (DTT) (Fig. 6d). Notably, the presence of 10 mM DTT increased enzymatic activity by up to 340%, indicating that breaking the disulfide bonds may considerably increase the activity of AgaQ1. Under the optimal conditions of 40 °C, pH 8.0, and 10 mM DTT, the specific activity of the agarase AgaQ1 on agarose was 757.7 U/mg, which is higher than most other reported agarases (Table 2, Fu and Kim 2010).
Biochemical characteristics of the purified AgaQ1. a The effect of temperature on the agarolytic activity with the temperature range of 30–80 °C. b The effect of pH on the agarolytic activity with the pH range of 5.5–10.0. c The effect of agarose concentration on the agarolytic activity with the concentration range of 0.1–2.0%. d The effect of different chemical reagents on the agarolytic activity. Asterisks represent significant difference using Student’s t test (**p < 0.01, ***p < 0 .001). Means ± standard deviations (SD) of triplicate were presented in this study
Identification of hydrolytic products and minimum hydrolysis substrate of AgaQ1
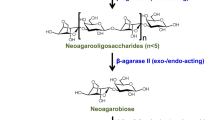
Purified AgaQ1 hydrolyzed agarose to various polymerization degrees at the initial reaction stage (Fig. 7a). After 1 h of incubation, NA4 and NA6 were determined as the major products of the reaction system, and neither NA2 nor galactose was detected (Fig. 7a). The same result was obtained from the other hydrolysis test, which was performed by adding enzyme solution twice (0 h and 24 h) to confirm the stability of hydrolytic products (Fig. S3). These facts suggest that AgaQ1 is an endo-type β-agarase, and the final hydrolyzed products are NA4 and NA6. Additionally, AgaQ1 was also found to hydrolyze purified NA6 to NA4 and NA2 (Fig. 7b), but when NA4 or NA2 was used as the substrate, no other oligosaccharide was generated. Thus, NA6 was the minimum hydrolysis substrate of AgaQ1.
Discussion
In this study, we analyzed the draft genome of a wild agar-degrading bacterium, C. maritimus Q1T, and found 11 annotated agarase genes and 2 Nabh genes located in different GH families (Fig. 2). Agarases belonging to different GH families have been reported to display different digestion profiles towards agarose; NA4 is usually the main product of agarases in the GH16 family, NA2 for those in the GH50 family, and NA6, NA8, or larger NAOS polymers for those in the GH86 family (Fu and Kim 2010). In addition, many reported agarases and Nabhs can use different polymers of NAOS as substrates to produce a lower degree of polymerization (Suzuki et al. 2002; Han et al. 2016). These facts indicate that agarases and Nabhs from different GH families, present in strain Q1T, acted jointly to digest agarose to monosaccharides to provided carbon sources for bacterial growth. Furthermore, a signal peptide was annotated to all agarases found in strain Q1T, but none were annotated to Nabhs (Fig. 2, Table 1), suggesting that the Nabhs act within the cell, whereas the agarases act extracellularly. However, due to the presence of transmembrane helices in agarases AgaQ2, AgaQ3, AgaQ10, and AgaQ12 (Table 1), it is suggested that these four agarases work in the cytoplasm, which further supports the notion of combined and systematic action for agar-degrading enzymes.
In addition, we detected the expression profiles of all annotated agar-degrading genes in strain Q1T and found similar patterns in the expression of all annotated agarase and Nabh genes, even under different treatments (Fig. 3c), which suggested that their expression was consistent and mainly controlled by agarose induction rather than the growth phase. This result was also consistent with the observation that the presence of agarose substrate caused higher degrading activities than constitutive activities without agarose induction. This further implies that most agar-degrading activities of strain Q1T were induced by the substrate agar or agarose, rather than constitutive expression or growth conditions, and supports the conclusion that agar-degrading enzymes in strain Q1T indeed act synergistically. Additionally, the available carbon source, glucose, provoked an obvious inhibition of agarase secretion, which is consistent with previous studies for Streptomyces coelicolor (Romero-Rodríguez et al. 2016; Kwakman and Postma 1994), indicating that glucose was utilized prior to agarose for bacterial growth.
All annotated agarase genes in strain Q1T were expressed under agarose induction, although their expression profiles were not identical (Fig. 3a). However, it remains unclear whether the annotated agarase genes are really associated with the ability of bacteria to degrade agar. In order to confirm this and to develop more efficient agarase sources for future industrial application, we cloned and characterized a β-agarase, AgaQ1, owing to its high expression level after agarose induction in strain Q1T. AgaQ1 is the first reported agarase obtained from the strain C. maritimus Q1T. It belongs to the family GH16 and shares the highest similarity with the β-agarase YM01-3. Interestingly, β-agarase YM01-3 has the highest reported agarase activity (Cui et al. 2014), which implies that AgaQ1, as its closest relative, might also possess a relative higher agarose-degrading activity. The gelling temperature of agar is reported to be around 38 °C and the natural seawater has a weakly alkaline pH (Fu and Kim 2010); however, the optimal conditions for YM01-3 are a temperature of 60 °C and pH of 6.0 (Cui et al. 2014). These facts suggest that AgaQ1 with an optimal temperature of 40 °C and pH of 8.0 would be a better industrial agarase for large-scale applications due to its alkali-tolerance and lower optimal temperatures, with the potential to decrease energy costs and improve the machining efficiency (Chen et al. 2019a). Although hundreds of agarases with certain enzymatic activity have been reported, most of them have a relative low activity, even under optimal conditions, indicating that only a few agarases have the potential for industrial application (Table 2; Cui et al. 2014; Fu and Kim 2010). However, the β-agarase AgaQ1 not only gave a higher activity value (757.7 U/mg) than most reported ones, but also showed stable activity after treatment with various ions and agents. These strengths further suggested the great potential of AgaQ1 in industrial applications, especially in agar degradation and in the preparation of agar-oligosaccharides.
Purified AgaQ1 degraded agarose to NA4 and NA6 as the major products, which is consistent with most reported agarases in the GH16 family (Fu and Kim 2010; Park et al. 2020), but slightly different from the major products (NA2 and NA4) of AgaQ1 in crude fermentation broth (Fig. 5b, 7a). Additional experiments confirmed that purified AgaQ1 can degrade NA6 to NA4 and NA2, which indicated the ability to produce NA2 of purified AgaQ1 was present but weak. When recombinant AgaQ1 was in the crude fermentation broth before purification, some of them were directly from soluble expression of the agaQ1 gene and with higher enzymatic activity. Under these conditions, it is possible that AgaQ1 directly hydrolyzed agarose to NA2 and NA4 (Fig. 5b). However, in view of the 340% DTT effect on the enzymatic activity and the lower activity value than that of YM01-3, AgaQ1 lost most of its activity in comparison with the native enzyme after purification and renaturation. Singh et al. (2015) noted that bioactive proteins are hard to recover their full activities from the inclusion bodies, due to the incomplete refolding of the protein structures. These facts would explain why purified AgaQ1 lost its ability to degrade agarose to NA2 directly.
In conclusion, this study not only improved our understanding of agar-degrading genes and activities under agarose induction but also expanded the available agarase resources, with great potential for future use in industry. This study provides an initial exploration of agarase function based on gene expression. With such promising applications, further research into the mechanisms of high-activity agarases is urgently needed.
References
Allouch J, Jam M, Helbert W, Barbeyron T, Kloareg B, Henrissat B, Czjzek M (2003) The three-dimensional structures of two β-agarases. J Biol Chem 278:47171–47180. https://doi.org/10.1074/jbc.M308313200
Artimo P, Jonnalagedda M, Arnold K, Baratin D, Csardi G, de Castro E, Duvaud S, Flegel V, Fortier A, Gasteiger E, Grosdidier A, Hernandez C, Ioannidis V, Kuznetsov D, Liechti R, Moretti S, Mostaguir K, Redaschi N, Rossier G, Xenarios I, Stockinger H (2012) ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res 40:W597–W603. https://doi.org/10.1093/nar/gks400
Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O (2008) The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9:75. https://doi.org/10.1186/1471-2164-9-75
Bairoch A, Apweiler R (2000) The SWISS-PROT protein sequence database and its supplement TrEMBL in 2000. Nucleic Acids Res 28:45–48. https://doi.org/10.1093/nar/28.1.45
Chen WM, Sheu FS, Sheu SY (2012) Aquimarina salinaria sp. nov., a novel algicidal bacterium isolated from a saltpan. Arch Microbiol 194:103–112. https://doi.org/10.1007/s00203-011-0730-9
Chen X, Lin H, Jin M, Zeng R, Lin M (2019a) Characterization of a novel alkaline β-agarase and its hydrolysates of agar. Food Chem 295:311–319. https://doi.org/10.1016/j.foodchem.2019.05.132
Chen YP, Wu HT, Wang GH, Wu DY, Hwang IE, Chien MC, Pang HY, Kuo JT, Liaw LL (2019b) Inspecting the genome sequence and agarases of Microbulbifer pacificus LD25 from a saltwater hot spring. J Biosci Bioeng 127:403–410. https://doi.org/10.1016/j.jbiosc.2018.10.001
Chi WJ, Chang YK, Hong SK (2012) Agar degradation by microorganisms and agar-degrading enzymes. Appl Microbiol Biotechnol 94:917–930. https://doi.org/10.1007/s00253-012-4023-2
Cui F, Dong S, Shi X, Zhao X, Zhang XH (2014) Overexpression and characterization of a novel thermostable β-Agarase YM01-3, from marine bacterium Catenovulum agarivorans YM01T. Mar Drugs 12:2731–2747. https://doi.org/10.3390/md12052731
Cui X, Jiang Y, Chang L, Meng L, Yu J, Wang C, Jiang X (2018) Heterologous expression of an agarase gene in Bacillus subtilis, and characterization of the agarase. Int J Biol Macromol 120:657–664. https://doi.org/10.1016/j.ijbiomac.2018.07.118
Day DF, Yaphe W (1975) Enzymatic hydrolysis of agar: purification and characterization of neoagarobiose hydrolase and p-nitrophenyl α-galactoside hydrolases. Can J Microbiol 21:1512–1518. https://doi.org/10.1139/m75-223
Dong J, Tamaru Y, Araki T (2007) Molecular cloning, expression, and characterization of a β-Agarase gene, agaD, from a marine bacterium, Vibrio sp. strain PO-303. Biosci Biotechnol Biochem 71:38–46. https://doi.org/10.1271/bbb.60304
El-Gebali S, Mistry J, Bateman A, Eddy SR, Luciani A, Potter SC, Qureshi M, Richardson LJ, Salazar GA, Smart A, Sonnhammer ELL, Hirsh L, Paladin L, Piovesan D, Tosatto SCE, Finn RD (2018) The Pfam protein families database in 2019. Nucleic Acids Res 47:D427–D432. https://doi.org/10.1093/nar/gky995
Fu XT, Kim SM (2010) Agarase: Review of major sources, categories, purification method, enzyme characteristics and applications. Mar Drugs 8:200–218. https://doi.org/10.3390/md8010200
Grant JR, Stothard P (2008) The CGView Server: a comparative genomics tool for circular genomes. Nucleic Acids Res 36:W181–W184. https://doi.org/10.1093/nar/gkn179
Han WJ, Gu JY, Liu HH, Li FC, Wu ZH, Li YZ (2013) An extra peptide within the catalytic module of a β-agarase affects the agarose degradation pattern. J Biol Chem 288:9519–9531. https://doi.org/10.1074/jbc.M112.412247
Han W, Cheng Y, Wang D, Wang S, Liu H, Gu J, Wu Z, Li F (2016) Biochemical characteristics and substrate degradation pattern of a novel exo-type β-agarase from the polysaccharide-degrading marine bacterium Flammeovirga sp. strain MY04. Appl Environ Microbiol 82:4944–4954. https://doi.org/10.1128/AEM.00393-16
Hong SJ, Lee JH, Kim EJ, Lee YH, Jung HM, Hong SK (2019) Safety evaluation of β-agarase preparations from Streptomyces coelicolor A3(2). Regul Toxicol Pharmacol 101:142–155. https://doi.org/10.1016/j.yrtph.2018.11.005
Jam M, Flament D, Allouch J, Potin P, Thion L, Kloareg B, Czjzek M, Helbert W, Michel G, Barbeyron T (2005) The endo-β-agarases AgaA and AgaB from the marine bacterium Zobellia galactanivorans: two paralogue enzymes with different molecular organizations and catalytic behaviours. Biochem J 385:703–713. https://doi.org/10.1042/BJ20041044
Jiang C, Liu Z, Sun J, Mao X (2020) Characterization of a novel α-neoagarobiose hydrolase capable of preparation of medium- and long-chain agarooligosaccharides. Front Bioeng Biotechnol 7:470. https://doi.org/10.3389/fbioe.2019.00470
Kang OL, Ghani M, Hassan O, Rahmati S, Ramli N (2014) Novel agaro-oligosaccharide production through enzymatic hydrolysis: physicochemical properties and antioxidant activities. Food Hydrocoll 42:304–308. https://doi.org/10.1016/j.foodhyd.2014.04.031
Kawai S, Murata K (2016) Biofuel production based on carbohydrates from both brown and red macroalgae: recent developments in key biotechnologies. Int J Mol Sci 17:145. https://doi.org/10.3390/ijms17020145
Kim JH, Yun EJ, Yu S, Kim KH, Kang NJ (2017) Different levels of skin whitening activity among 3,6-anhydro-l-galactose, agarooligosaccharides, and neoagarooligosaccharides. Mar Drugs 15:321. https://doi.org/10.3390/md15100321
Kim SW, Kim YW, Hong CH, Lyo IW, Lim HD, Kim GJ, Shin HJ (2018) Recombinant agarase increases the production of reducing sugars from HCl-treated Gracilaria verrucosa, a red algae. Algal Res 31:517–524. https://doi.org/10.1016/j.algal.2017.01.008
Kirimura K, Masuda N, Iwasaki Y, Nakagawa H, Kobayashi R, Usami S (1999) Purification and characterization of a novel β-agarase from an alkalophilic bacterium, Alteromonas sp. E-1. J Biosci Bioeng 87:436–441. https://doi.org/10.1016/S1389-1723(99)80091-7
Knutsen SH, Myslabodski DE, Larsen B, Usov AI (1994) A modified system of nomenclature for red algal galactans. Botanica Marina 37:163–170. https://doi.org/10.1515/botm.1994.37.2.163
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Kwakman JH, Postma PW (1994) Glucose kinase has a regulatory role in carbon catabolite repression in Streptomyces coelicolor. J Bacteriol 176:2694–2698. https://doi.org/10.1128/jb.176.9.2694-2698.1994
Lahaye M, Yaphe W, Viet MTP, Rochas C (1989) 13C-NMR spectroscopic investigation of methylated and charged agarose oligosaccharides and polysaccharides. Carbohydrate Research 190:249–265. https://doi.org/10.1016/0008-6215(89)84129-1
Li DQ, Zhou YX, Liu T, Chen GJ, Du ZJ (2015) Catenovulum maritimus sp. nov., a novel agarolytic gammaproteobacterium isolated from the marine alga Porphyra yezoensis Ueda (AST58-103), and emended description of the genus Catenovulum. Antonie Van Leeuwenhoek 108:427–434. https://doi.org/10.1007/s10482-015-0495-2
Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J, He G, Chen Y, Pan Q, Liu Y, Tang J, Wu G, Zhang H, Shi Y, Liu Y, Yu C, Wang B, Lu Y, Han C, Cheung DW, Yiu S-M, Peng S, Xiaoqian Z, Liu G, Liao X, Li Y, Yang H, Wang J, Lam TW, Wang J (2012) SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience 1:2047–217X–1–18. https://doi.org/10.1186/2047-217X-1-18
Mai Z, Su H, Zhang S (2016) Isolation and characterization of a glycosyl hydrolase family 16 β-Agarase from a mangrove soil metagenomic library. Int J Mol Sci 17. https://doi.org/10.3390/ijms17081360
Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen Z, Dewell SB, Du L, Fierro JM, Gomes XV, Godwin BC, He W, Helgesen S, Ho CH, Irzyk GP, Jando SC, Alenquer MLI, Jarvie TP, Jirage KB, Kim J-B, Knight JR, Lanza JR, Leamon JH, Lefkowitz SM, Lei M, Li J, Lohman KL, Lu H, Makhijani VB, McDade KE, McKenna MP, Myers EW, Nickerson E, Nobile JR, Plant R, Puc BP, Ronan MT, Roth GT, Sarkis GJ, Simons JF, Simpson JW, Srinivasan M, Tartaro KR, Tomasz A, Vogt KA, Volkmer GA, Wang SH, Wang Y, Weiner MP, Yu P, Begley RF, Rothberg JM (2005) Genome sequencing in microfabricated high-density picolitre reactors. Nature 437:376–380. https://doi.org/10.1038/nature03959
Menetrez MY (2012) An overview of algae biofuel production and potential environmental impact. Environ Sci Technol 46:7073–7085. https://doi.org/10.1021/es300917r
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Oh C, Nikapitiya C, Lee Y, Whang I, Kim SJ, Kang DH, Lee J (2010) Cloning, purification and biochemical characterization of beta agarase from the marine bacterium Pseudoalteromonas sp. AG4. J Ind Microbiol Biotechnol 37:483–494. https://doi.org/10.1007/s10295-010-0694-9
Ohta Y, Hatada Y, Nogi Y, Miyazaki M, Li Z, Akita M, Hidaka Y, Goda S, Ito S, Horikoshi K (2004) Enzymatic properties and nucleotide and amino acid sequences of a thermostable β-agarase from a novel species of deep-sea Microbulbifer. Appl Microbiol Biotechnol 64:505–514. https://doi.org/10.1007/s00253-004-1573-y
Park S, Choi WC, Oh TK, Yoon JH (2011) Thalassomonas agariperforans sp. nov., an agarolytic bacterium isolated from marine sand. Int J Syst Evol Microbiol 61:2573–2576. https://doi.org/10.1099/ijs.0.027821-0
Park SH, Lee CR, Hong SK (2020) Implications of agar and agarase in industrial applications of sustainable marine biomass. Appl Microbiol Biotechnol 104:2815–2832. https://doi.org/10.1007/s00253-020-10412-6
Potin P, Richard C, Rochas C, Kloareg B (1993) Purification and characterization of the α-agarase from Alteromonas agarlyticus (Cataldi) comb. nov., strain GJ1B. FEBS J 214:599–607. https://doi.org/10.1111/j.1432-1033.1993.tb17959.x
Pruitt KD, Tatusova T, Maglott DR (2005) NCBI Reference Sequence (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res 33:D501–D504. https://doi.org/10.1093/nar/gki025
Rebuffet E, Groisillier A, Thompson A, Jeudy A, Barbeyron T, Czjzek M, Michel G (2011) Discovery and structural characterization of a novel glycosidase family of marine origin. Environ Microbiol 13:1253–1270. https://doi.org/10.1111/j.1462-2920.2011.02426.x
Romero-Rodríguez A, Ruiz-Villafán B, Tierrafría VH, Rodríguez-Sanoja R, Sánchez S (2016) Carbon catabolite regulation of secondary metabolite formation and morphological differentiation in Streptomyces coelicolor. Appl Biochem Biotechnol 180:1152–1166. https://doi.org/10.1007/s12010-016-2158-9)
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108. https://doi.org/10.1038/nprot.2008.73
Shi YL, Lu XZ, Yu WG (2008) A new β-agarase from marine bacterium Janthinobacterium sp. SY12. World J Microbiol Biotechnol 24:2659–2664. https://doi.org/10.1007/s11274-008-9792-5
Shi MJ, Du WJ, Wang C, Chen GJ, Du ZJ (2017) Catenovulum sediminis sp. nov., isolated from coastal sediment. Int J Syst Evol Microbiol 67:3894–3898
Singh A, Upadhyay V, Upadhyay AK, Singh SM, Panda AK (2015) Protein recovery from inclusion bodies of Escherichia coli using mild solubilization process. Microb Cell Fact 14:41. https://doi.org/10.1186/s12934-015-0222-8
Subramanian B, Gao S, Lercher MJ, Hu S, Chen WH (2019) Evolview v3: a webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res 47:W270–W275. https://doi.org/10.1093/nar/gkz357
Suzuki H, Sawai Y, Suzuki T, Kawai K (2002) Purification and characterization of an extracellular α-neoagarooligosaccharide hydrolase from Bacillus sp. MK03. J Biosci Bioeng 93:456–463. https://doi.org/10.1016/S1389-1723(02)80092-5
Tawara M, Sakatoku A, Tiodjio RE, Tanaka D, Nakamura S (2015) Cloning and characterization of a novel agarase from a newly isolated Bacterium simiduia sp. strain TM-2 able to degrade various seaweeds. Appl Biochem Biotechnol 177:610–623. https://doi.org/10.1007/s12010-015-1765-1
Usov AI (1998) Structural analysis of red seaweed galactans of agar and carrageenan groups. Food Hydrocoll 12:301–308. https://doi.org/10.1016/S0268-005X(98)00018-6
Van De Velde F, Knutsen SH, Usov AI, Rollema HS, Cerezo AS (2002) 1H and 13C high resolution NMR spectroscopy of carrageenans: application in research and industry. Trends Food Sci Technol 13:73–92. https://doi.org/10.1016/S0924-2244(02)00066-3
Voget S, Leggewie C, Uesbeck A, Raasch C, Jaeger KE, Streit WR (2003) Prospecting for novel biocatalysts in a soil metagenome. Appl Environ Microbiol 69:6235–6242. https://doi.org/10.1128/aem.69.10.6235-6242.2003
Wang J, Jiang X, Mou H, Guan H (2004) Anti-oxidation of agar oligosaccharides produced by agarase from a marine bacterium. J Appl Phycol 16:333–340. https://doi.org/10.1023/B:JAPH.0000047944.40463.e6
Wargacki AJ, Leonard E, Win MN, Regitsky DD, Santos CNS, Kim PB, Cooper SR, Raisner RM, Herman A, Sivitz AB, Lakshmanaswamy A, Kashiyama Y, Baker D, Yoshikuni Y (2012) An engineered microbial platform for direct biofuel production from brown macroalgae. Science 335:308–313. https://doi.org/10.1126/science.1214547
Xu ZX, Zhang HX, Han JR, Dunlap CA, Rooney AP, Mu DS, Du ZJ (2017a) Colwellia agarivorans sp. nov., an agar-digesting marine bacterium isolated from coastal seawater. Int J Syst Evol Microbiol 67:1969–1974. https://doi.org/10.1099/ijsem.0.001897
Xu Z, Yu P, Mu D, Liu Y, Du Z (2017b) Tenacibaculum agarivorans sp. nov., an agar-degrading bacterium isolated from marine alga Porphyra yezoensis Ueda. Int J Syst Evol Microbiol 67:5139–5143. https://doi.org/10.1099/ijsem.0.002432
Xu XQ, Su BM, Xie JS, Li RK, Yang J, Lin J, Ye XY (2018) Preparation of bioactive neoagaroligosaccharides through hydrolysis of Gracilaria lemaneiformis agar: a comparative study. Food Chem 240:330–337. https://doi.org/10.1016/j.foodchem.2017.07.036
Yan S, Yu M, Wang Y, Shen C, Zhang XH (2011) Catenovulum agarivorans gen. nov., sp. nov., a peritrichously flagellated, chain-forming, agar-hydrolysing gammaproteobacterium from seawater. Int J Syst Evol Microbiol 61:2866–2873. https://doi.org/10.1099/ijs.0.027565-0
Yun EJ, Yu S, Kim KH (2017) Current knowledge on agarolytic enzymes and the industrial potential of agar-derived sugars. Appl Microbiol Biotechnol 101:5581–5589. https://doi.org/10.1007/s00253-017-8383-5
Zhang H, Yohe T, Huang L, Entwistle S, Wu P, Yang Z, Busk PK, Xu Y, Yin Y (2018) dbCAN2: a meta server for automated carbohydrate-active enzyme annotation. Nucleic Acids Res 46:W95–W101. https://doi.org/10.1093/nar/gky418
Funding
This work was financially supported by the Weihai Ocean Economic Innovation and Development Demonstration Project.
Author information
Authors and Affiliations
Contributions
Z.X., D.M., and Z.D. designed the research. Z.X. performed most experiments. Q.L. constructed the recombined strain. Z.X and P.Y. analyzed data. Z.X. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals that were performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary materials
ESM 1
(PDF 325 kb)
Rights and permissions
About this article
Cite this article
Xu, ZX., Yu, P., Liang, QY. et al. Inducible expression of agar-degrading genes in a marine bacterium Catenovulum maritimus Q1T and characterization of a β-agarase. Appl Microbiol Biotechnol 104, 10541–10553 (2020). https://doi.org/10.1007/s00253-020-10969-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10969-2