Abstract
The CRISPR/Cas9 system is a powerful genetic engineering technique that has been widely used in gene therapy, as well as in the development of novel antimicrobials and transgenic insects. However, several challenges, including the lack of effective host target genes and the off-target effects, limit the application of CRISPR/Cas9 in insects. To mitigate these difficulties, we established a highly efficient virus-inducible CRISPR/Cas9 system in transgenic silkworms. This system includes the baculovirus-inducible promoter 39K, which directs transcription of the gene encoding, the Cas9 protein, and the U6 promoter which targets the sgATAD3A site of the ATPase family AAA domain-containing protein 3 (ATAD3A) gene. The double-positive transgenic line sgATAD3A×39K-Cas9 (ATAD3A-KO) was obtained by hybridization; antiviral activity in this hybrid transgenic line is induced only after Bombyx mori nucleopolyhedrovirus (BmNPV) infection. The BmNPV-inducible system significantly reduced off-target effects and did not affect the economically important characteristics of the transgenic silkworms. Most importantly, this novel system efficiently and consistently edited target genes, inhibiting BmNPV replication after the transgenic silkworms were inoculated with occlusion bodies (OBs). The suppression of BmNPV by the virus-inducible system was comparable to that of the stably expressed CRISPR/Cas9 system. Therefore, we successfully established a highly efficient BmNPV-inducible ATAD3A-KO transgenic silkworm line, with improved gene targeting specificity and antiviral efficiency. Our study thereby provides insights into the treatment of infectious diseases and into the control of insect pests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The silkworm (Bombyx mori, B. mori) is an important model lepidopteron with high economic values. B. mori nucleopolyhedrovirus (BmNPV), a baculovirus family, is a major silkworm pathogen (Jiang and Xia 2014). Baculoviruses are a diverse group of viruses with double-stranded, circular, and super-spiral genomes, which vary in size from 80 to 180 kb, each encoding 90–180 genes (Kelly et al. 2007). Baculoviruses have a bi-directional life cycle: occluded derived virus (ODV) is responsible for the systemic infection of individual insects, and the budded virus (BV) plays an important role in secondary infection (Blissard and Rohrmann 1990; Kelly et al. 2007). Every year, BmNPV severely impacts the sericulture industry in China, causing major economic losses (Jiang and Xia 2014). Current strategies used to combat BmNPV include the cultivation of antiviral strains of B. mori via traditional breeding methods, and the creation of transgenic B. mori strains that either overexpress antiviral protein or lack (or underexpress) the genes required for BmNPV replication (Cheng et al. 2014; Jiang et al. 2012; Jiang and Xia 2014; Yang et al. 2008).
In 2004, Isobe et al. found that the inhibition of the late expression factor 1 (LEF-1) gene in BmNPV using RNA interference (RNAi) effectively increased viral resistance in silkworms. Since that time, numerous studies have focused on inhibiting the expression of key BmNPV genes transgenic silkworms using double-stranded RNAs (dsRNAs), small interfering RNAs (siRNAs), microRNA, and short hairpin RNAs (shRNAs) (Jiang et al. 2013; Subbaiah et al. 2013; Zhang et al. 2014a, b). Several proteins that effectively inhibit viral replication via RNAi in transgenic silkworms have already been identified, including a receptor factor (B. mori pattern recognition receptor family 2, BmPGRP2) and recognition receptor (B. mori receptor expression-enhancing protein, BmREEP, and a nuclear hormone receptor 96, BmNHR96) (Dong et al. 2015b, 2017a; Yang et al. 2017). Several additional proteins showed strong antiviral activity when extracted from silkworm larvae and the overexpressed, including Bmlispase-1, B. mori serine protease-2 (BmSP-2), B. mori Sprouty (BmSpry), BmAtlastin-n (in the dynamin superfamily), and B. mori NADH-oxidoreductase-like protein (BmNOX) (Cheng et al. 2014; Jiang et al. 2012; Liu et al. 2016; Yang et al. 2008). Overexpression of these proteins might also enhance viral resistance in silkworms (Cheng et al. 2014; Jiang et al. 2012; Liu et al. 2016; Yang et al. 2008).
However, RNAi also readily increases RNA accumulation, which may be toxic to host cells, and the use of CRISPR/Cas9 might lead to off-target effects on host development, limiting the application of these techniques sericulture (Dong et al. 2016; Zhang et al. 2014a). Therefore, there is an urgent need to establish a CRISPR/Cas9 system that not only inhibits BmNPV proliferation but also generates transgenic offspring without deleterious effects. The ATPase family AAA domain-containing protein 3 (ATAD3A) is a mitochondrial protein comprising two N-terminal coiled-coil domains and a conserved C-terminal ATPase domain (He et al. 2007; You et al. 2013). Previously, we demonstrated that BmNPV LEF-11 hijacks host BmATAD3A to promote virus multiplication, and that knocking down BmATAD3A inhibits BmNPV replication in silkworms (Dong et al. 2017b). Thus, the host receptor BmATAD3A is required for BmNPV replication and might be a useful target of antiviral research in transgenic silkworms.
We previously established a highly efficient clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein 9 (CRISPR/Cas9) system to disrupt BmNPV proliferation in vitro and in vivo (Dong et al. 2018, 2016). In the present study, we aimed to increase the possible targets of B. mori gene therapy and to test the use of a pathogen-dependent host factor as a target gene in insect infectious diseases research. To this end, we constructed a BmNPV-inducible ATAD3A-KO transgenic line, carrying a modified version of BmATAD3A to quickly inhibit viral infection. We did not observe any off-target effects in the BmNPV-inducible ATAD3A-KO transgenic line, nor were any developmental or economic characteristics of this strain significantly different from those of the stable expression system. Moreover, mortality and BmNPV gene expression analyses indicated that the inducible ATAD3A-KO transgenic line had the same antiviral abilities as the stable expression system. Thus, our novel BmNPV-inducible CRISPR/Cas9 system, which knocks out host BmATAD3A, effectively inhibits viral replication. Therefore, this system may be potentially useful for the treatment of infectious diseases in silkworm and for the control of insect pests.
Materials and methods
Silkworm strains and viruses
The B. mori transgenic line IE1-Cas9 and the B. mori strain Dazao (control strain) were used in this study (Dong et al. 2018). Silkworm larvae were orally inoculated with wild-type (WT) BmNPV as previously described (Dong et al. 2018). Occlusion bodies (OBs) were harvested from the hemolymph of infected silkworm larvae as previously described (Dong et al. 2014). We counted OBs using a hemocytometer, and then stored the harvested OBs at 4 °C.
Vector construction
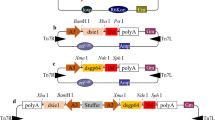
We constructed pBac [IE1-Cas9-Ser-PA-3×P3 EGFP afm] expression cassettes to express the Cas9 protein as described previously (Dong et al. 2018). In brief, we selected the BmATAD3A (GenBank accession number: XM_022262303.1) gene as the editing site; this gene is located at position nscaf1690 on B. mori chromosome 1. sgATAD3A primer dimers were synthesized using forward and reverse primers, then the primer dimers were ligated to the pSL1180-U6 vector after BbsI digestion, and the pSL1180-U6-sgATAD3A vector was obtained by sequencing this vector (Dong et al. 2016; Horn and Wimmer 2000). Finally, the U6-sgATAD3A was ligated to a pBac [3×P3 DsRed afm] vector to generate a red fluorescent protein transgenic vector for pBac [U6-sgATAD3A-3×P3 DsRed afm] using the BglII restriction endonuclease (Sarkar et al. 2006; Thomas et al. 2002). Meanwhile, the Hr3 enhancer and the 39K promoter of BmNPV were used to replace the IE1 promoter of the pSL1180-IE1-Cas9-Ser-PA vector, followed by the single digestion of pSL1180-Hr3-39k-Cas9-Ser-PA using AscI restriction endonuclease (Dong et al. 2018, 2016). The Hr3-39k-Cas9-Ser-PA fragment was ligated into the pBac [3×P3 EGFP afm] vector to obtain the green fluorescent protein transgenic vector pBac [Hr3-39k-Cas9-Ser-PA-3×P3 EGFP afm] (Dong et al. 2018, 2016; Kokoza et al. 2001; Thomas et al. 2002). All primers used in this study are given in Supplemental Table S1. All plasmids were confirmed with sequencing.
Microinjection and screening
Transgenic silkworm IE1-Cas9 constructs were prepared as previously described (Dong et al. 2018). In brief, the transgenic vectors pBac [Hr3-39K-Cas9-Ser-PA-3×P3 EGFP afm] and pBac [U6-sgATAD3A-3×P3 DsRed afm] were mixed with the helper plasmid pHA3PIG and injected into silkworm eggs as previously described (Dong et al. 2018; Tamura et al. 2000). The silkworm transgenic line uses the piggyBac transposon as a vector and the fluorescent protein (DsRed or EGFP) as a marker, under the control of an eye-specific promoter (Thomas et al. 2002). Therefore, G1-positive individuals were identified using green and red fluorescence microscopy. The double-positive individuals sgATAD3A×IE1-Cas9 and Hr3-39K-Cas9×sgATAD3A (sgATAD3A×39K-Cas9) were obtained after sgATAD3A was hybridized with IE1-CasS9 and with Hr3-39K-Cas9, respectively. The other three phenotypes of the sgATAD3A(−)×Cas9(−) line expressed neither the Cas9 protein nor the sgRNA target sequence; the sgATAD3A(−)×Cas9(+) line expressed the Cas9 protein only; and the sgATAD3A(+)×Cas9(−) line expressed the sgRNA target sequence only. These three lines were used as negative controls.
Sequencing
The complete genomic DNA of the silkworm transgenic lines sgATAD3A, sgATAD3A×IE1-Cas9, and sgATAD3A×39K-Cas9 were extracted using DNA extraction kits (Promega, Madison, Wisconsin, USA). The ATAD3A target site fragment was amplified with PCR using specific primers (Supplemental Table S1) and was ligated into a pEASY-T5 Zero cloning vector (TransGen Biotech, Haidian, Beijing, China) for monoclonal sequencing using M13 primers (Stahley and Stivers 2010) (Supplemental Table S1).
Off-target assays
To compare the off-target frequencies between the virus-inducible and the stable expression CRISPR/Cas9 systems in the silkworm genome, we predicted three possible off-target sites of sgATAD3A using CRISPR design tools (http://crispr.dbcls.jp/; (Naito et al. 2015)). We identified the three sites with the highest off-target frequencies. These sites were amplified with PCR, and the amplicons were ligated to pEASY-T5 Zero cloning vectors (Stahley and Stivers 2010). Vectors were sequenced with M13 primers (Supplemental Table S1) and aligned with the correct target gene sequences.
Mortality analyses
BmNPV OBs were purified and stored in our laboratory as previously described (Dong et al. 2018, 2014). Control silkworm larvae (strain Dazao) and transgenic silkworm larvae (39K-Cas9×sgATAD3A and sgATAD3A) were reared under standard conditions. After all larvae were grown to the fourth instar, the transgenic lines were identified using fluorescence microscopy, and then inoculated with identical doses of OBs. Larval mortality was calculated 10 days after OB infection. Three biological replicates were used per experimental group. All infected larvae were maintained individually under the same conditions.
Quantitative real-time PCR (qPCR) DNA replication assay
All samples were collected at the corresponding times and stored at − 80 °C. Genomic DNA extraction from silkworm samples followed by qPCR was performed as previously described (Dong et al. 2017b, 2015c). A standard curve based on the cycle threshold (Ct) of serial dilution concentrations was generated. The glycoprotein (GP41) copy number at different time points of silkworm infection was calculated. The qPCR cycling program was as follows: 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s and 60 °C for 20 s. Each qPCR reaction volume contained 1 μM of each primer. Three biological replicates were analyzed per experimental group, and each qPCR was repeated three times.
Reverse transcription PCR (RT-PCR)
Total RNA was extracted from all experimental samples, and cDNA was synthesized as previously described (Dong et al. 2016). All RT-PCR analyses were conducted with a SYBR Select Master Mix Mixage reagent (Bio-Rad, Hercules, CA, USA), using primers specific to the following genes: BmATAD3A, immediate early 1 (IE-1), capsid protein encoding gene (VP39), gp64, and polyhedrin (POLY) (Supplemental Table S1). The B. mori gene sw22934 was used as the reference gene. All RT-PCRs used the following standard cycling conditions: 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s and 60 °C for 20 s. Each RT-PCR reaction volume contained 1 μM of each primer. Three biological replicates were analyzed per experimental group, and each RT-PCR was repeated three times.
Phenotypic analyses of transgenic individuals
After hybridization of the sgATAD3A and IE1-Cas9 (39K-Cas9) lines, all transgenic lines were raised under standard conditions until the fourth instar, and positive individuals were screened using fluorescence microscopy. Transgenic individuals from the sgATAD3A and sgATAD3A×IE1-Cas9 (39K-Cas9) lines were weighted and photographed on the first, third, and sixth day of the fifth instar (5L-1D, 5L-3D, and 5L-6D, respectively). Survival was calculated over the entire lifecycle, from larval hatching until moth death. Each transgenic line was represented by 30 larvae. All assays were performed three times.
Analysis of economic characteristics
Thirty cocoons from the transgenic lines sgATAD3A(−)×39K-Cas9(−), sgATAD3A(−)×39K-Cas9(+), sgATAD3A(+)×39K-Cas9(−), and sgATAD3A (+)×39K-Cas9(+) were randomly selected. For each cocoon, total volume and size were measured, and the shell rate was calculated. Each transgenic line was assessed based on the average of three independent replicates.
Statistical analysis
All experiments were performed three times. All data were expressed as the mean ± SD of three independent experiments. The statistical significance of differences between experimental groups was determined with Student’s t test in GraphPad Prism 6 (http://www.graphpad.com) (GraphPad Software, Inc., San Diego, CA, USA).
Results
CRISPR/Cas9-mediated editing of the B. mori genome in transgenic sgATAD3A×IE1-Cas9 larvae
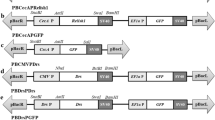
The baculovirus delayed early-expression gene 39K (pp31) encodes a phosphorylated DNA binding protein that is associated with the virogenic stroma in the nuclei of infected cells (Guarino et al. 1992). Previous studies have shown that, in BmNPV, the promoter of the delayed early gene 39K drives the transcription of foreign genes induced by baculovirus infection (Cao et al. 2016). Previously, we obtained the 39K promoter sequence from the BmNPV genome, and showed that this promoter was activated after BmNPV infection (Cao et al. 2016; Dong et al. 2016). To prevent the ATAD3A-KO transgenic line from influencing larval development, here we used the baculovirus-inducible promoter 39K to initiate Cas9 protein expression (Carson et al. 1988). We targeted the BmATAD3A gene at position 252 bp of its open reading frame. The 39K-Cas9-positive and sgATAD3A-postive transgenic lines fluoresced green and red, respectively, after transgenic injection with pBac [Hr3-39K-Cas9-Ser-PA-3×P3 EGFP afm] and pBac [U6-sgATAD3A-3×P3 DsRed afm] plasmids. The advantage of constructing two parental transgenic lines was that neither of these lines affected the development of B. mori unless the two transgenic lines hybridized. Four different transgenic silkworm phenotypes were obtained via the hybridization of the 39K-Cas9 and sgATAD3A transgenic lines: sgATAD3A(−)×39K-Cas9(−), sgATAD3A(−)×39K-Cas9(+), sgATAD3A(+)×39K-Cas9(−), and sgATAD3A(+)×39K-Cas9(+) (Fig. 1). Individuals positive for different transgenic phenotypes were identified using fluorescence microscopy. Only the sgATAD3A×39K-Cas9 transgenic line expressed both the sgATAD3A target sequence and the Cas9 protein; all other lines served as negative controls.
CRISPR/Cas9-mediated editing of the B. mori genome in sgATAD3A×IE1-Cas9 transgenic individuals. Transgenic vector construction of the pBac [IE1-Cas9-Ser-PA-3×P3 EGFP afm] and pBac [U6-sgATAD3A-3×P3 DsRed afm] injections using the helper plasmid. The G1 generations of the IE1-Cas9- and sgATAD3A-positive lines were detected using fluorescence microscopy. The G2 generation (sgATAD3A×IE1-Cas9 and sgATAD3A transgenic hybrid lines) was generated by G1 hybridization
Establishment of a virus-inducible transgenic CRISPR/Cas9 system
This virus-inducible CRISPR/Cas9 system did not induce Cas9 protein expression in the absence of viral infection, and had no significant effects on larval development. The basic principle underlying the activation of this system was the fact that the transcriptional activator IE1 binds the transcriptional activation element of the 39K promoter after BmNPV infection (Carson et al. 1988). This infection induces the 39K promoter to express the Cas9 protein, which cuts and edits the target gene by binding to the target RNA sequences (Fig. 2a) (Dong et al. 2016). When activated, this system inhibits viral proliferation by editing key host gene (Dong et al. 2016).
Establishment of a virus-inducible transgenic CRISPR/Cas9 system. a Schematic of the virus-inducible CRISPR/Cas9 system. b RT-PCR analysis of Cas9 gene transcription after infection with different concentrations of BmNPV. Total RNA from each 39K-Cas9 larvae was isolated 96 h p.i. with 1 × 101, 1 × 102, 1 × 103, 1 × 104, and 1 × 105 OBs/larva. c RT-PCR analysis of Cas9 gene transcription after infection with BmNPV at a density of 1 × 105 OBs/larva at 0, 12, 24, 48, 72, and 96 h p.i. Cas9 gene expression are shown as the mean of three independent replicates. NS, not significant. (***P < 0.001)
We determined the sensitivity of the virus-inducible CRISPR/Cas9 system to BmNPV infection using RT-PCR. We measured the expression of the Cas9 gene in larvae inoculated with OBs at different times post infection and infected. We found that the expression levels of the Cas9 gene 96-h post-infection (h p.i.) were 2-, 2.4-, 4.3-, 17.5-, and 22.8-fold higher when inoculated with 1 × 101, 1 × 102, 1 × 103, 1 × 104, and 1 × 105 OBs/larva, respectively (Fig. 2b). When inoculated with 1 × 105 OBs/larva, Cas9 gene expression increased 1.8-, 1-, 73.6-, 157.5-, and 191.1-fold after 12, 24, 48, 72, and 96 h p.i., respectively (Fig. 2c). These results indicated that the virus-inducible CRISPR/Cas9 system was activated rapidly, even at low concentration of BmNPV.
Gene editing efficiency of the inducible CRISPR/Cas9 system
To systematically compare the gene editing efficiency of the virus-inducible system to that of the stable system, we first determined the level of Cas9 gene expression in both systems. We found that Cas9 was upregulated by the inducible system at 48 h p.i., and was more strongly expressed in the inducible system than the stable system by 72 h p.i. (Fig. 3a). In contrast, Cas9 gene expression in the stable expression system remained stable for most of the infection duration, decreasing slightly during the later stages of infection.
Gene editing efficiency of the inducible CRISPR/Cas9 system. a RT-PCR analysis of Cas9 gene transcription in the virus-inducible system (sgATAD3A×39K-Cas9) and the stable expression system (sgATAD3A×IE1-Cas9) after infection with BmNPV at a density of 1 × 106 OBs/larva at 0, 12, 24, 48, 72, 96, and 120 h p.i. Cas9 gene expression levels are shown as the mean of three independent replicates. b–c The gene editing efficiency of the virus-inducible CRISPR/Cas9 system in transgenic larvae uninfected and infected with BmNPV. The wild-type (WT) BmATAD3A gene sequence is shown above in black; the target sgATAD3A sequence is shown in green. Also shown is a representative chromatogram demonstrating the WT sequence targeted by sgATAD3A-mediated genomic editing. The shaded area indicates sequence the insertion or deletion sequence. The GenBank accession number of the BmATAD3A sequence is XM_022262303.1. d Off-target analysis of the virus-inducible CRISPR/Cas9 system in transgenic silkworms. The most common off-target sites are shown in green, with the corresponding chromosome location indicated. PAM representative protospacer adjacent motif sequence
Sequencing showed that the target sites of sgATAD3A×IE1-Cas9 edited the BmATAD3A gene, with an editing efficiency up to 100% (Supplemental Fig. S1). Sequencing of the sgATAD3A×39K-Cas9 line identified no obvious mutations, deletions, or insertions in the absence of BmNPV infection (Fig. 3b). In contrast, the sgATAD3A×IE1-Cas9 line caused genetic modifications, specifically a large deletion and insertion at the target site, without BmNPV infection (Fig. 3b). Only the transgenic line sgATAD3A×39K-Cas9 edited the B. mori genome after BmNPV infection. The most frequent deletions and insertions were 698 bp and 466 bp along, respectively. Most of the inserted sequences involved target site attachments, whereas the deleted sequences were key domain sequences of BmATAD3A (Fig. 3c). No gene editing was observed in any of the transgenic lines used as negative controls.
To prevent off-target effects, we excluded the three top off-target sites during sgRNA design. We used T cloning and sequencing to determine whether these off-target sites were non-specific editing sites in the transgenic sgATAD3A×39K-Cas9 line. No off-target effects were detected in the three non-specific editing sites of sgATAD3A (Fig. 3d). Thus, the virus-inducible CRISPR/Cas9 lines only edited the target site after BmNPV infection, and had no significant effects on non-specific loci in silkworms.
Economic characteristics of virus-inducible ATAD3A-KO transgenic silkworms
To determine the impact of the virus-inducible CRISPR/Cas9 system on silkworm development, we analyzed the development of silkworm larvae and measured changes in larval weight at different developmental stages. We found no significant differences in larval weight or developmental progression between the virus-inducible line sgATAD3A×39K-Cas9 and the normal line (sgATAD3A) at 5L-1D, 5L-3D, and 5L-6D (Fig. 4a). However, development and growth in the ATAD3A-KO transgenic line sgATAD3A×IE1-Cas9 were significantly delayed relative to the control line (sgATAD3A; Supplemental Fig. S2A). In addition, larval weight was ten times greater in the normal line (sgATAD3A), as compared to the ATAD3A-KO transgenic line sgATAD3A×IE1-Cas9 at 5L-1D, 5L-3D, and 5L-6D (Supplemental Fig. S2A).
Economically important characteristics of virus-inducible ATAD3A-KO transgenic silkworms. a Phenotypes and body weights of transgenic silkworm larvae (ATAD3A-KO line). Transgenic lines sgATAD3A×39K-Cas9 and sgATAD3A were raised the fifth instar (5L) under the same conditions. Thirty larvae from each group were evaluated, with three independent replicates. Larval weight was measured at 5L-1D, 5L-3D, and 5L-6D.. b Lifespan of the transgenic lines sgATAD3A×39K-Cas9 and sgATAD3A, from incubation to moth. Each line was represented by 30 larvae. Three independent replicates were analyzed. c Cocoon shell rate of the transgenic hybrid lines. Each value represents the average of 30 repeated measurements
Survival rates between the virus-inducible and normal lines were not significantly different, and the life cycle of both lines was about 32–38 days (Fig. 4b). The life cycle of the transgenic line sgATAD3A×IE1-Cas9 was 20 days longer than that of the normal line (sgATAD3A); the ATAD3A-KO completed metamorphosis in 55–60 days (Supplemental Fig. S2B). This delay was caused by the knock out of the BmATAD3A gene during metamorphosis. Each transgenic line was represented by 30 larvae, and three different larvae were assessed per line. We identified no significant differences in economically important factors between the transgenic hybrid line and the control line. In the transgenic hybrid pupae, whole cocoon weight was 0.7–1.3 g, cocoon shell weight was 0.13–0.21 g, and the cocoon shell rate was 13–22%. There were no significant differences in any of these measures between the virus-inducible line sgATAD3A(+)×39K-Cas9(+) and any of the normal lines (sgATAD3A(−)×39K-Cas9(+), sgATAD3A(+)×39K-Cas9(−), and sgATAD3A(−)×39K-Cas9(−)) (Fig. 4c and Supplemental Fig. S3). Thus, the BmNPV-inducible Cas9 system effectively edited the target gene without any significant effects on larval development or economically important characters.
Antiviral activity of the virus-inducible CRISPR/Cas9 system
The fourth instar larvae of the transgenic lines generated by hybridizing the BmNPV-inducible line sgATAD3A×39K-Cas9 with normal line sgATAD3A were infected with BmNPV at a density of 1 × 106 OBs/larva. The survival rate of the sgATAD3A×39K-Cas9 line was 85% up until 10 days p.i., whereas the sgATAD3A line exhibited large-scale mortality within 5–8 days after OB inoculation (Fig. 5a). Total DNA was isolated from the sgATAD3A×39K-Cas9 and sgATAD3A lines, and quantified with qPCR. qPCR analysis showed that the copies of BmNPV DNA gradually increased in the normal line sgATAD3A after OB inoculation, whereas in the virus-inducible line BmNPV copies increased significantly between 0 and 48 h p.i., then gradually decreased, and finally disappeared entirely 48 to 120 h after OB infection (Fig. 5b). The abundance of viral DNA in the sgATAD3A×39K-Cas9 line was about 1000-fold lower than that of the normal line (Fig. 5b).
Antiviral activity of the virus-inducible CRISPR/Cas9 system. a Survival rate of the transgenic lines sgATAD3A×39K-Cas9 and sgATAD3A after the inoculation of the fourth instar larvae with 1 × 106 OBs/larva. Each transgenic line was screened in triplicate, with each replicate including 30 larvae. Mortality rate was determined 10 days after inoculation. b GP41 expression, as quantified with qPCR, representing BmNPV DNA replication in the transgenic lines sgATAD3A×39K-Cas9 and sgATAD3A at 0, 12, 24, 48, 72, 96, and 120 h post inoculation of fourth instar larvae with BmNPV at a density 1 × 106 OBs/larva. c Relative expression levels of BmNPV genes ie-1, gp64, vp39, and poly in transgenic lines sgATAD3A×39K-Cas9 and sgATAD3A, as quantified with qPCR. The expression level of each gene at each time point is the mean of three independent replicates. NS, not significant. ***means are significantly different (P < 0.001)
Total RNA was extracted from the sgATAD3A×39K-Cas9 and sgATAD3A lines, and the expression levels of several BmNPV genes (immediate early gene ie-1, early gene gp64, late gene vp39, and very late gene poly) were analyzed by RT-PCR. BmNPV ie-1, gp64, vp39, and poly were expressed at very low levels in the sgATAD3A×39K-Cas9 line after inoculation with 1 × 106 OBs/larva (Fig. 5c). In contrast, the expression of these genes gradually increased in normal line sgATAD3A.
Discussion
Genomic editing based on the CRISPR/Cas9 system is a new and effective genetic editing tool that has been widely used for targeting, inactivating, and deleting genes for genome-wide screenings and even for infectious disease gene therapy (Dong et al. 2015a; Ebina et al. 2013; Kennedy et al. 2015; Ma et al. 2015). However, the application of the CRISPR system to insect pathogens is still relatively basic and limited (Tsubota and Sezutsu 2017; Zhang et al. 2016). Here, we constructed a virus-inducible CRISPR/Cas9 system in transgenic silkworms that not only effectively inhibited viral proliferation but also reduced off-target effects and host toxicity. In addition, this virus-inducible CRISPR/Cas9 system could effectively edit both BmNPV genes and host factors. Our modified inducible CRISPR/Cas9 system may potentially be useful for determining insect gene function, for designing biological controls, and for the modification of malaria vectors.
Previously, we used the CRISPR/Cas9 system to establish the first transgenic cell line that completely inhibited BmNPV DNA replication in insect cells (Dong et al. 2016). This system has been successfully used to create antiviral transgenic individuals by connecting the expression frames of sgRNA and Cas9 or via the hybridization of sgRNA lines and Cas9 lines (Chen et al. 2017; Dong et al. 2018). To expand the range of applications of transgenic antiviral compounds and to improve transgenic antiviral ability, we targeted the gene of a BmNPV replication-dependent host factor for the genetic modification of transgenic antiviral lines. Although the ATAD3A-KO lines effectively inhibited viral replication, the development of these silkworms was adversely affected (Supplemental Fig. S2). Here, we succeeded in preventing this negative host impact by establishing a virus-inducible system (Fig. 4a, b). We identified no significant differences in cocoon shell rate between the virus-inducible transgenic and the normal silkworms, suggesting that this transgenic line might be practical for silkworm breeding (Fig. 4c). Our study provides a novel approach to the study of silkworm antivirals compounds, and, combined with previous studies, will lead to silkworm lines that more effectively inhibit viral replication.
The most important aspect of our virus-inducible transgenic CRISPR/Cas9 system was that the system was not activated in the absence of viral infection. The basic principle of this system was that the Cas9 protein was not expressed under normal conditions, so sgRNA was activated alone. The virus-inducible transgenic CRISPR/Cas9 system was rapidly activated, resulting in reduced BmNPV virulence and/or a shorter course of infection. The system then edited target host-dependent factors or key genes to inhibit viral replication (Fig. 2a). This gene editing transgenic system had several advantages compared to stable expression system: First, the system did not cause cytotoxic effects due to long-term Cas9 protein expression. Second, this system did not affect host development due to Cas9 protein and target gene expression (Fig. 4a). Third, this system did not induce off-target effects due to the long-term expression of Cas9 (Fig. 3c). Fourth, the short-term activation of the Cas9 system was more efficient a than stable expression system.
We plan to apply this inducible system in future studies function of insect genes, pest control, and malaria transmission by activating the system at regular intervals (McLean and Jacobs-Lorena 2016; Ricroch 2017; Taning et al. 2017; Tsubota and Sezutsu 2017). This type of use relies on the modification and optimization of the BmNPV-inducible promoter 39K and its regulatory elements (Cao et al. 2016; Mistretta and Guarino 2005; Regev et al. 2006). Our inducible CRISPR/Cas9 system provides a framework for the study of insect infectious diseases. We intend to apply the system to the study of major pathogens (such as baculoviruses, retroviruses, bacteria, fungi, and microsporidia) by screening the inducible promoters and target genes of hosts and disease vectors (Pelosse et al. 2017). More importantly, this virus-inducible system provides new insights into the use of the CRISPR system for gene therapy, agricultural production, and animal model construction.
In conclusion, we developed a highly efficient virus-inducible CRISPR/Cas9 system that increased the antiviral ability of transgenic silkworms, while minimizing host toxicity and off-target effects. This virus-inducible CRISPR/Cas9 system provides a novel approach for the breeding of disease-resistant insect lines. We plan to optimize this inducible CRISPR/Cas9 system for application to viral, fungal, and bacterial diseases of insects, as well as to those caused by microsporidia. Here, we have also established a new insect pathogen control system that may effectively increase the disease resistance of beneficial insects and reduce the spread of harmful insects.
References
Blissard GW, Rohrmann GF (1990) Baculovirus diversity and molecular biology. Annu Rev Entomol 35:127–155. https://doi.org/10.1146/annurev.en.35.010190.001015
Cao MY, Kuang XX, Li HQ, Lei XJ, Xiao WF, Dong ZQ, Zhang J, Hu N, Chen TT, Lu C, Pan MH (2016) Screening and optimization of an efficient B. mori nucleopolyhedrovirus inducible promoter. J Biotechnol 231:72–80. https://doi.org/10.1016/j.jbiotec.2016.05.037
Carson DD, Guarino LA, Summers MD (1988) Functional mapping of an AcNPV immediately early gene which augments expression of the IE-1 trans-activated 39K gene. Virology 162(2):444–451
Chen S, Hou C, Bi H, Wang Y, Xu J, Li M, James AA, Huang Y, Tan A (2017) Transgenic clustered regularly interspaced short palindromic repeat/Cas9-mediated viral gene targeting for antiviral therapy of B. mori nucleopolyhedrovirus. J Virol 91(8):e02465–e02416. https://doi.org/10.1128/JVI.02465-16
Cheng Y, Wang XY, Du C, Gao J, Xu JP (2014) Expression analysis of several antiviral related genes to BmNPV in different resistant strains of silkworm, B mori. J Insect Sci 14:76. https://doi.org/10.1093/jis/14.1.76
Dong ZQ, Zhang J, Chen XM, He Q, Cao MY, Wang L, Li HQ, Xiao WF, Pan CX, Lu C, Pan MH (2014) B. mori nucleopolyhedrovirus ORF79 is a per os infectivity factor associated with the PIF complex. Virus Res 184:62–70. https://doi.org/10.1016/j.virusres.2014.02.009
Dong C, Qu L, Wang H, Wei L, Dong Y, Xiong S (2015a) Targeting hepatitis B virus cccDNA by CRISPR/Cas9 nuclease efficiently inhibits viral replication. Antivir Res 118:110–117. https://doi.org/10.1016/j.antiviral.2015.03.015
Dong XL, Liu TH, Wang W, Pan CX, Wu YF, Du GY, Chen P, Lu C, Pan MH (2015b) BmREEPa is a novel gene that facilitates BmNPV entry into silkworm cells. PLoS One 10(12):e0144575. https://doi.org/10.1371/journal.pone.0144575
Dong ZQ, Hu N, Zhang J, Chen TT, Cao MY, Li HQ, Lei XJ, Chen P, Lu C, Pan MH (2015c) Oligomerization of baculovirus LEF-11 is involved in viral DNA replication. PLoS One 10(12):e0144930. https://doi.org/10.1371/journal.pone.0144930
Dong ZQ, Chen TT, Zhang J, Hu N, Cao MY, Dong FF, Jiang YM, Chen P, Lu C, Pan MH (2016) Establishment of a highly efficient virus-inducible CRISPR/Cas9 system in insect cells. Antivir Res 130:50–57. https://doi.org/10.1016/j.antiviral.2016.03.009
Dong XL, Wu YF, Liu TH, Wang W, Pan CX, Adur M, Zhang MJ, Pan MH, Lu C (2017a) B. mori protein BmREEPa and BmPtchd could form a complex with BmNPV envelope protein GP64. Biochem Biophys Res Commun 490(4):1254–1259. https://doi.org/10.1016/j.bbrc.2017.07.004
Dong ZQ, Hu N, Dong FF, Chen TT, Jiang YM, Chen P, Lu C, Pan MH (2017b) Baculovirus LEF-11 hijack host ATPase ATAD3A to promote virus multiplication in B. mori cells. Sci Rep 7:46187. https://doi.org/10.1038/srep46187
Dong Z, Dong F, Yu X, Huang L, Jiang Y, Hu Z, Chen P, Lu C, Pan M (2018) Excision of nucleopolyhedrovirus form transgenic silkworm using the CRISPR/Cas9 system. Front Microbiol 9:209. https://doi.org/10.3389/fmicb.2018.00209
Ebina H, Misawa N, Kanemura Y, Koyanagi Y (2013) Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus. Sci Rep 3:2510. https://doi.org/10.1038/srep02510
Guarino LA, Dong W, Xu B, Broussard DR, Davis RW, Jarvis DL (1992) Baculovirus phosphoprotein pp31 is associated with virogenic stroma. J Virol 66(12):7113–7120
He J, Mao CC, Reyes A, Sembongi H, Di Re M, Granycome C, Clippingdale AB, Fearnley IM, Harbour M, Robinson AJ, Reichelt S, Spelbrink JN, Walker JE, Holt IJ (2007) The AAA+ protein ATAD3 has displacement loop binding properties and is involved in mitochondrial nucleoid organization. J Cell Biol 176(2):141–146. https://doi.org/10.1083/jcb.200609158
Horn C, Wimmer EA (2000) A versatile vector set for animal transgenesis. Dev Genes Evol 210(12):630–637
Jiang L, Xia Q (2014) The progress and future of enhancing antiviral capacity by transgenic technology in the silkworm B. mori. Insect Biochem Mol Biol 48:1–7. https://doi.org/10.1016/j.ibmb.2014.02.003
Jiang L, Wang G, Cheng T, Yang Q, Jin S, Lu G, Wu F, Xiao Y, Xu H, Xia Q (2012) Resistance to B. mori nucleopolyhedrovirus via overexpression of an endogenous antiviral gene in transgenic silkworms. Arch Virol 157(7):1323–1328. https://doi.org/10.1007/s00705-012-1309-8
Jiang L, Zhao P, Cheng T, Sun Q, Peng Z, Dang Y, Wu X, Wang G, Jin S, Lin P, Xia Q (2013) A transgenic animal with antiviral properties that might inhibit multiple stages of infection. Antivir Res 98(2):171–173. https://doi.org/10.1016/j.antiviral.2013.02.015
Kelly BJ, King LA, Possee RD (2007) Introduction to baculovirus molecular biology. Methods Mol Biol 388:25–54. https://doi.org/10.1007/978-1-59745-457-5_2
Kennedy EM, Kornepati AV, Cullen BR (2015) Targeting hepatitis B virus cccDNA using CRISPR/Cas9. Antivir Res 123:188–192. https://doi.org/10.1016/j.antiviral.2015.10.004
Kokoza V, Ahmed A, Wimmer EA, Raikhel AS (2001) Efficient transformation of the yellow fever mosquito Aedes aegypti using the piggyBac transposable element vector pBac[3xP3-EGFP afm]. Insect Biochem Mol Biol 31(12):1137–1143
Liu TH, Dong XL, Pan CX, Du GY, Wu YF, Yang JG, Chen P, Lu C, Pan MH (2016) A newly discovered member of the Atlastin family, BmAtlastin-n, has an antiviral effect against BmNPV in B. mori. Sci Rep 6:28946. https://doi.org/10.1038/srep28946
Ma H, Dang Y, Wu Y, Jia G, Anaya E, Zhang J, Abraham S, Choi JG, Shi G, Qi L, Manjunath N, Wu H (2015) A CRISPR-based screen identifies genes essential for West-Nile-virus-induced cell death. Cell Rep 12(4):673–683. https://doi.org/10.1016/j.celrep.2015.06.049
McLean KJ, Jacobs-Lorena M (2016) Genetic control of malaria mosquitoes. Trends Parasitol 32(3):174–176. https://doi.org/10.1016/j.pt.2016.01.002
Mistretta TA, Guarino LA (2005) Transcriptional activity of baculovirus very late factor 1. J Virol 79(3):1958–1960. https://doi.org/10.1128/JVI.79.3.1958-1960.2005
Naito Y, Hino K, Bono H, Ui-Tei K (2015) CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 31(7):1120–1123. https://doi.org/10.1093/bioinformatics/btu743
Pelosse M, Crocker H, Gorda B, Lemaire P, Rauch J, Berger I (2017) MultiBac: from protein complex structures to synthetic viral nanosystems. BMC Biol 15(1):99. https://doi.org/10.1186/s12915-017-0447-6
Regev A, Rivkin H, Gurevitz M, Chejanovsky N (2006) New measures of insecticidal efficacy and safety obtained with the 39K promoter of a recombinant baculovirus. FEBS Lett 580(30):6777–6782. https://doi.org/10.1016/j.febslet.2006.11.037
Ricroch AE (2017) What will be the benefits of biotech wheat for European agriculture? Methods Mol Biol 1679:25–35. https://doi.org/10.1007/978-1-4939-7337-8_2
Sarkar A, Atapattu A, Belikoff EJ, Heinrich JC, Li X, Horn C, Wimmer EA, Scott MJ (2006) Insulated piggyBac vectors for insect transgenesis. BMC Biotechnol 6:27. https://doi.org/10.1186/1472-6750-6-27
Stahley MR, Stivers JT (2010) Mechanism and specificity of DNA strand exchange catalyzed by vaccinia DNA topoisomerase type I. Biochemistry 49(13):2786–2795. https://doi.org/10.1021/bi902204v
Subbaiah EV, Royer C, Kanginakudru S, Satyavathi VV, Babu AS, Sivaprasad V, Chavancy G, Darocha M, Jalabert A, Mauchamp B, Basha I, Couble P, Nagaraju J (2013) Engineering silkworms for resistance to baculovirus through multigene RNA interference. Genetics 193(1):63–75. https://doi.org/10.1534/genetics.112.144402
Tamura T, Thibert C, Royer C, Kanda T, Abraham E, Kamba M, Komoto N, Thomas JL, Mauchamp B, Chavancy G, Shirk P, Fraser M, Prudhomme JC, Couble P (2000) Germline transformation of the silkworm B. mori L. using a piggyBac transposon-derived vector. Nat Biotechnol 18(1):81–84. https://doi.org/10.1038/71978
Taning CN, Van Eynde B, Yu N, Ma S, Smagghe G (2017) CRISPR/Cas9 in insects: applications, best practices and biosafety concerns. J Insect Physiol 98:245–257. https://doi.org/10.1016/j.jinsphys.2017.01.007
Thomas JL, Da Rocha M, Besse A, Mauchamp B, Chavancy G (2002) 3×P3-EGFP marker facilitates screening for transgenic silkworm B. mori L. from the embryonic stage onwards. Insect Biochem Mol Biol 32(3):247–253
Tsubota T, Sezutsu H (2017) Genome editing of silkworms. Methods Mol Biol 1630:205–218. https://doi.org/10.1007/978-1-4939-7128-2_17
Yang H, Fan W, Wei H, Zhang J, Zhou Z, Li J, Lin J, Ding N, Zhong B (2008) Transgenic breeding of anti-B. mori L. nuclear polyhedrosis virus silkworm B. mori. Acta Biochim Biophys Sin Shanghai 40(10):873–876
Yang JG, Liu TH, Dong XL, Wu YF, Zhang Q, Zhou L, Chen P, Lu C, Pan MH (2017) In vivo RNA interference of BmNHR96 enhances the resistance of transgenic silkworm to BmNPV. Biochem Biophys Res Commun 493(1):332–339. https://doi.org/10.1016/j.bbrc.2017.09.022
You WC, Chiou SH, Huang CY, Chiang SF, Yang CL, Sudhakar JN, Lin TY, Chiang IP, Shen CC, Cheng WY, Lin JC, Shieh SH, Chow KC (2013) Mitochondrial protein ATPase family, AAA domain containing 3A correlates with radioresistance in glioblastoma. Neuro-Oncology 15(10):1342–1352. https://doi.org/10.1093/neuonc/not077
Zhang J, He Q, Zhang CD, Chen XY, Chen XM, Dong ZQ, Li N, Kuang XX, Cao MY, Lu C, Pan MH (2014a) Inhibition of BmNPV replication in silkworm cells using inducible and regulated artificial microRNA precursors targeting the essential viral gene lef-11. Antivir Res 104:143–152. https://doi.org/10.1016/j.antiviral.2014.01.017
Zhang P, Wang J, Lu Y, Hu Y, Xue R, Cao G, Gong C (2014b) Resistance of transgenic silkworm to BmNPV could be improved by silencing ie-1 and lef-1 genes. Gene Ther 21(1):81–88. https://doi.org/10.1038/gt.2013.60
Zhang B, Chen XF, Huang X, Yang X (2016) Research advances on animal genetics in China in 2015. Yi Chuan 38(6):467–507. https://doi.org/10.16288/j.yczz.16-205
Funding
This study was funded by The National Natural Science Foundation of China (Grant Nos. 31472153 and 31572466) and the China Agriculture Research System (CARS-18).
Author information
Authors and Affiliations
Contributions
Z.D., F.D., and L.H. performed vector cloning, sequencing, cell culturing, and PCR. Z.D., F.D., and Z.H. conducted transgenic injections. M.C., Z.H., Q.Q., and J.L. participated in mortality analyses and DNA replication assays. Z.D., M.P., and C.L. conceived the experimental design and participated in data analysis. Z.D., M.P., P.C., and C.L. were involved in the preparation of the manuscript. The final manuscript was reviewed and approved by all authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any experiments with human participants or animals (except invertebrates, which are exempt from ethical concerns) performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 395 kb)
Rights and permissions
About this article
Cite this article
Dong, Z., Huang, L., Dong, F. et al. Establishment of a baculovirus-inducible CRISPR/Cas9 system for antiviral research in transgenic silkworms. Appl Microbiol Biotechnol 102, 9255–9265 (2018). https://doi.org/10.1007/s00253-018-9295-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9295-8