Abstract
Xylose is the second most abundant sugar derived from lignocellulose; it is considered less desirable than glucose for fermentation, and strategies that specifically increase xylose utilization in wild type or engineered cells are goals for biofuel production. Issues arise with xylose utilization because of carbohydrate catabolite repression, which is the preferential utilization of glucose relative to xylose in fermentations with both pure and mixed cultures. Taken together the low substrate utilization rates and solvent yields with xylose compared to glucose, many industrial fermentations ignore the xylolytic portion of the reaction in lieu of methods to maintain high glucose. This is shortsighted given the massive potential for xylose generation from a number of sustainable biomass feedstocks, based on utilization of the hemicellulose fraction(s) that enter pretreatment. A number of strategies have been developed in recent years to address xylose utilization and solvent production from xylose in systems with just xylose, or in systems with mixtures of glucose plus xylose, which are more typical of pretreated lignocellulose. The approaches vary in terms of complexity, stability, and ease of introduction to existing fermentation infrastructure (i.e., so-called drop-in fermentation strategies). Some approaches can be considered traditional engineering approaches (e.g., change the reaction conditions), while others are more subtle cellular approaches to eliminate the impacts of catabolite repression. Finally, genetic engineering has been used to increase xylose utilization, although this can be considered a relatively nascent approach compared to manipulations completed to date for glucose utilization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Solvents such as butanol, ethanol, and acetone are targets for both biofuels as well as biologically synthesized precursors in fine chemical production (Szwaja and Naber 2010; Cooney et al. 2009; Masum et al. 2014). Bio-butanol (technically n-butanol, but hereafter referred to as simply butanol) is particularly attractive as a supplement for gasoline-powered transportation infrastructure due to its combustion properties and relatively high energy content when compared to ethanol. One impediment to advancing bio-butanol as a global fuel is the lack of reasonable lignocellulosic feedstocks that can be fermented efficiently. Solvent production is generally divided into prokaryotic processes (e.g., Clostridia fermentations), and eukaryotic processes, which is mostly glucose and xylose fermenting yeasts. The following review only addresses advances in prokaryotic processes.
Butanol is generally considered an alternative to ethanol as a biologically derived liquid fuel, and the market is expected to expand through 2021 (de Maria 2016; Harvey and Meylemans 2011). The energy content of butanol is higher than ethanol; butanol releases 29.2 MJ L−1, and ethanol releases 19.6 MJ L−1 (Lee et al. 2008). As previously happened with methyl tert butyl ether (MTBE), the petrochemical industry has coalesced around butanol because it is more stable in storage and transport, and blends more readily with refined fuel. Biological processes such as acetone-butanol-ethanol (ABE) fermentation from lignocellulosic biomass are being researched to supplement or replace fossil fuels (Jiang et al. 2009; Atsumi et al. 2008; Connor and Liao 2009; Lee et al. 2012). Figure 1 is a conceptual model of the critical pathways in ABE fermentation, which indicates that multiple products can be generated during single fermentations.
ABE fermentation has been studied for almost a century, and the technology has been refined at both the cellular and engineering (bioreactor) scales. While the technology has always held promise, it does suffer from low solvent yields, limited substrate utilization, and low biomass turnover rate (Green 2011). Research addressing these limitations has focused on genetic modifications and/or reactor design modifications to increase solvent output, rather than specific yield (Harris et al. 2001; Qureshi and Maddox 1988; Roffler et al. 1987). One major issue is that solvent producers are seeking so-called drop-in technologies, which work with existing reactor infrastructure without too many modifications (Liang et al. 2002; Alkasrawi et al. 2003). While this is a very good goal, it is difficult in practice to adapt full-scale fermentations without changing the actual reactors involved.
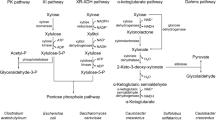
Hemicellulose monomers, the most critical of which is xylose, are desirable feedstocks for industrial ABE production since they do not compete directly with human food sources (Valentine et al. 2012). Xylose, a pentose sugar, comprises 30% of all plant-derived biomass (Kumar et al. 2009). The primary issue is that many microorganisms cannot ferment xylose effectively, or they lack the necessary machinery to transport and assimilate it into central metabolism (Gírio et al. 2010). This has made glucose the preferential sugar substrate, since it is readily fermented by most ABE producing strains (El Kanouni et al. 1998). Figure 2 indicates the general path from lignocellulosic biomass, to xylose-derived products.
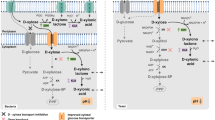
Members of the genus Clostridium rely either on xylose proton symporters or ATP-dependent xylose transport proteins to transport xylose across the cell membrane (Servinsky et al. 2010). Xylose is transformed prior to glycolysis, requiring ATP hydrolysis and regeneration of NAD+ and NADP+ cofactors (Jeffries 1983). Glucose is typically 100% fermented by the cells of interest, while xylose in ABE fermentation can be fermented at 50% or less (Xiao et al. 2012; Wu et al. 2016b; Mes-Hartree and Saddler 1982).
Solvent production with glucose versus xylose
Much attention has been placed on replacing glucose as a feedstock for current butanol fermentations overall substrate cost and competition with food sources (Yang et al. 2015; Xue et al. 2013; Zhang 2011). Recently, many have been investigating the use of hemicellulose as a feedstock due to natural abundance. Hemicellulose is a polymer which composes nearly 30–40% of the earth’s carbon (Kumar et al. 2009; Gong et al. 1981; Xiao et al. 2011), and xylose and arabinose are pentose monomers which compose hemicellulose. Although terrestrially abundant, pentose catabolism is limited or non-existent in many industrially relevant organisms, and although xylose flux is poorly understood in solventogenic organisms, many efforts are being made to elucidate the processes surrounding its uptake and metabolism for manipulation.
The favorability of hexose over pentose sugars is apparent from the rate of substrate co-fermentation within Clostridia (Chen et al. 2013; Grimmler et al. 2010; Jeffries 1983). Xylose can be metabolized in bacterial cells following entry catalyzed by proton motive force (symport) (Jeffries 1983; Walmsley et al. 1998; Ma 1958). Following entry into the cell, xylose is metabolized via the pentose phosphate pathway (PPP) or the phosphoketolase pathway in organisms such as C. acetobutylicum, accounting for the slower rate of utilization attributed to the requirement of additional metabolic steps prior to entry into central and/or solventogenic downstream pathways.
In the PPP, xylose is phosphorylated to xylulose-5-phosphate, and from this position, xylulose-5-phosphate is converted to fructose-6-phosphate or glyceraldehyde-3-phosphate, allowing for entry into glycolysis (Ma 1958). Recently, evidence has shown that under high xylose concentrations (20 g L−1), the phosphoketolase pathway in C. acetobutylicum is upregulated, indicating that some solventogenic Clostridia possess the ability to metabolize xylose in parallel pathways under high substrate stress (Liu et al. 2012). This allows for xylose to be converted to glyceraldeyhyde-3-phosphate or acetyl-phosphate, which can be shunted to acetyl-CoA, thus bypassing glycolysis if the phosphoketolase pathway is activated.
Reports suggest that it is this difference in substrate uptake that may be responsible for the preferential utilization of glucose by solventogenic microbial genera. It makes sense from an energy balance perspective; xylose uptake is active and consumes ATP, while glucose uptake is passive and does not necessarily require ATP.
Carbohydrate catabolite repression (CCR) assures that glucose will be preferentially utilized and that xylose will not be taken up by the cells until glucose falls below a threshold concentration. CCR is a signal cascade in cells that can utilize both glucose and xylose, in which metabolites of the glucose uptake and utilization pathway downregulate genes involved in xylose utilization. This mechanism decreases the value of high xylose content lignocellulosic biomass. If cells are going to preferentially utilize glucose, and the solvent yield from xylose is generally low, then most industrial fermentations are going to ignore the xylose fermentation component altogether and focus only on glucose. Again, this is a very limiting approach given the mass of xylose that can be developed from many lignocellulosic sources. A number of processes can help overcome this xylose limitation; they are introduced below.
Acetone, butanol, ethanol (ABE) fermentation with xylose as the primary substrate
The methods tested for increasing xylose utilization for solventogenic fermentations have generally fallen within three broad areas: (a) developing mixed microbial cultures that will increase overall xylose utilization kinetics, and therefore the solvent generation kinetics, (b) amending continuous or fed-batch reactors with electron transfer molecules that disrupt overall metabolism and change substrate utilization rate and extent, and (c) genetic engineering of specific xylose uptake and utilization genes for increased solvent production. These methods can also be combined to maximize xylose utilization and solvent yield. Each of these is discussed below.
Co-cultures and mixed cultures
Pure cultures are generally more desirable with which to work during industrial fermentations, mainly because of purity of the products generated and the absence of cells that may metabolize the end products. In addition, the fermentation conditions are simpler when only a single genus or species is present; multiple cells may have diverse nutritional needs. This has worked very well for glucose fermentation, but less so for xylose fermentation.
Developing mixed cultures or co-cultures with two specific microbial genera has also been a long-standing method of generating desirable end products. Co- and mixed cultures are sometimes more stable than pure cultures, which means that they can be grown in continuous culture as opposed to fed batch mode, which slows production. Co- and mixed cultures also respond differently to metabolites; there may be less repression due to one member of the community quickly utilizing glucose which allows the xylose fermenter to directly ferment xylose even in reactors with both substrates present.
A recent co-culture was developed using a yeast, Saccharomyces cerevisiae, and Clostridium acetobutylicum, which fermented mixed glucose plus xylose and generated high solvent yields when both cells were present (Qi et al. 2015). Initially, it was thought that pre-treating the substrate mixture with S. cerevisiae would be the most effective method of increasing overall solvent yield, with the yeast quickly eliminating glucose from the medium and allowing C. acetobutylicum to ferment the xylose unaffected by CCR. Solvent yield in this case was 0.03 g g−1 higher than the non-pre-treated substrate mixture. However, when cells were incubated together rather than in series, the overall productivity rates (g L−1 h−1 solvent generated) doubled, with simultaneous glucose and xylose utilization. While successful, this method does require specialized culture conditions that are favorable to both the yeast and the Bacterium. It is unknown if the dual-favorable conditions decrease overall productivity, given that the fermentation conditions are not ideal for either cell involved.
A co-culture developed using C. beijerinckii and the Fe3+ reducer Geobacter metallireducens increased xylose utilization and subsequent hydrogen and solvent production (Zhang et al. 2013a, Zhang et al. 2013b). Glucose utilization was the same amongst all treatments with this co-culture, and both the glucose utilization rate and solvent yields were similar in the co-culture and the pure culture C. beijerinckii. However, xylose utilization increased in the co-culture, especially when inoculated with an electron shuttling molecule (anthraquinone 2,6-disulfonate, AQDS) that serves as an electron acceptor for G. metallireducens. The suggested mechanism was that acetate utilization by the respiratory G. metallireducens altered substrate uptake kinetics, possibly by limiting catabolite repression or changing the overall energy balance of the combined reactions. Geobacter are not butanol oxidizers, so this system was suggested as a potential butanol generating industrial co-culture.
Most recently, a genetically engineered co-culture of Escherichia coli was developed that limited the impacts of CCR on the cells and increased the overall rate and extent of xylose fermentation to generate butanol (Saini et al. 2017). An E. coli strain was isolated that was not sensitive to the impacts of CCR and could therefore utilize xylose and glucose without specific preference. However, xylose-derived butanol yields remained low. The co-culture distributed the necessary reactions between two different strains, which increased overall butanol yield. The primary mechanism segregated butyrate synthesis and butyrate conversion between the glycolytic strain and the xylolytic strain, with the glycolytic strain converting the majority of the butyrate to butanol. In addition, eliminating the glucose-6-phosphate dehydrogenase pathway prevented glucose metabolism from directly impacting xylose metabolism, by limiting glucose interaction with the pentose phosphate pathway. This allowed simultaneous glucose and xylose fermentation, without glucose catabolism interfering with the xylose fermentation pathway. However, as with many genetically modified or even discovered mutant strains, these reactions were first predicated on having obtained the CCR-insensitive strain (Saini et al. 2017).
Batch and continuous fermentation amendments or enhancements
Technologies or strategies that merely amend existing pure, co-, and mixed cultures are sometimes considered ideal from a practical perspective. These are the so-called drop in strategies that require little or no change to existing infrastructure and also do not require the presence of sensitive, costly, or fastidious microbial cultures. They are often considered a replacement for genetic modification, but amendments to actual culture conditions can be used with any cells, whether the cells are wild-type, mutant, or genetically modified strains.
The simplest manipulation is pH, which impacts the onset and longevity of solvent production during ABE fermentations. Acids generated lower the pH, and each cell has a pH threshold at which acidogenesis slows down and solventogenesis increases. Several data suggest that xylose utilization increases, primarily in pure cultures, when pH is controlled and is maintained slightly higher than would be expected if the fermentation was allowed to go to completion, with the pH dropping to 4.0–4.5 (Jiang et al. 2014; Procentese et al. 2015). However, the impacts of pH seem to be most profound xylose is alone as the fermentable substrate. Jiang et al. (2014) report that xylose utilization and solvent production productivity and yield doubled, when the pH was maintained at 5.0 as opposed to dropping below 4.0 over the course of a 100-h fed-batch reaction with C. acetobutylicum (Jiang et al. 2014). Cell yield was significantly higher in the controlled pH reactor, which may have been partially responsible for the increases reported. The results were less pronounced when glucose and xylose were simultaneously fed to the reactor, and systems with higher glucose/xylose ratios were not influenced by pH; the yields were similar irrespective of pH control (Jiang et al. 2014).
Qiu et al. (2016) demonstrated that a municipal sludge derived mixed culture generated hydrogen and solvents with xylose as the sole substrate at a pH range from 4.0 to 10.0 as the initial pH, but without further pH control over the course of a fed-batch reaction (Qiu et al. 2016). Phylogenetic data using 16S rRNA gene indicated that the dominant populations were expected genera including Clostridium, Bacteroidetes, and uncultured phylotypes most closely related to known ABE fermenters (Qiu et al. 2016). Respiratory populations were low or absent, which was expected with the lack of a terminal electron acceptor. Xylose was completely consumed in all incubations run between pH 6.0 and 9.0 as the starting pH, but utilization dropped off sharply at pH 5.0 and pH 10.0, as did all other metabolites. Hydrogen yield was relatively high in this culture in comparison to solvents, which argues that mixed cultures are less efficient at solvent production and that pH has little impact on mixed culture solvent yield.
Nutrient supplementation is another simple approach to increase xylose fermentation and solvent yield, and data suggest that zinc impacts xylose-derived ABE synthesis. Clostridium acetobutylicum was incubated in several volumetric scales of batch reactor with xylose as the sole fermentable substrate or in mixtures with glucose, and zinc added at 1.0 mg L−1 (which was operationally defined as high relative to the basal medium). High zinc concentrations increased xylose utilization and solvent production, and the data indicated that perhaps zinc impacted xylose transport, but at this point, the exact mechanism is unknown (Wu et al. 2016a, Wu et al. 2016b). The data indicate a modest synergistic effect of co-added calcium at higher than basal level concentrations and that the combined nutrients influenced carbon flux within the cells from predominately acidogenesis to predominately solventogenesis. They attributed this to an uncharacterized “redox balance” (Wu et al. 2016a, Wu et al. 2016b), which leads to the last drop in manipulation that can be performed.
Fermentation in general is predicated on redox balance; the absence of a terminal electron acceptor necessitates a greater balance between inputs, metabolites, and outputs (products) during fermentation. Anything that unbalances the fermentation process increases or decreases the rate at which substrates are fermented, and the productivity and yield of solvents generated.
Redox active molecules and electron shuttling compounds (e.g., neutral red or anthraquinone-2,6-disulfonate (AQDS)) are reported to alter fermentative metabolism, although the exact mechanisms involved are not yet known. Thus far, all data suggest that redox mediators increase solvent production rates, and overall solvent titers; however, most data were generated with glucose as the sole fermentable substrate (Girbal et al. 1995; Peguin et al. 1994a, Peguin et al. 1994b; Peguin and Soucaille 1996; Vasconcelos et al. 1994). The first reports demonstrated that electron mediators such as neutral red and methyl viologen altered substrate oxidation, solvent production, and more recently hydrogen production (Park et al. 1999; Peguin and Soucaille 1995; Rao and Mutharasan 1987; Ye et al. 2012). Solvent production was generally influenced by the redox mediator methyl viologen, in pH-controlled fed-batch reactors with different Clostridial cultures; however, the single most affected variable was butanol production, and subsequent studies focused on this strategy for increasing butanol output (Peguin and Soucaille 1996). Most recently, a series of fed-batch reactors were manipulated using a potentiostat to poise the aqueous medium at operationally defined “near oxic” conditions, and data indicated that solvent yield increased relative to controls (Shin et al. 2002). It is unsurprising that fermentative redox manipulations went in this direction, given the increase in electrode manipulations for microbial reactions over the past several years. Although no exact mechanisms were developed for any of these systems, the best theory was that NAD+/NADH ratios were altered and that in turn increased production of solvents by redirecting carbon and electrons to these pathways (Meyer and Papoutsakis 1989; Singh et al. 2009). Again, in all cases referenced above, glucose was the sole substrate and experiments did not address improving glucose utilization due to extracellular electron transfer. More recent data have considered substrate utilization, and substrates other than glucose.
While early studies did not consider impacts on xylose utilization, they were the foundation upon which the current field of unbalanced fermentation has been built, for the purpose of increasing xylose utilization and solvent recovery. The term unbalanced fermentation refers to any strategies or technologies that introduce an extracellular electron acceptor that is not used in respiratory processes to generate energy as adenosine triphosphate (ATP). In other words, a so-called electron sink is added to fermentative cultures, which redirects some fraction of electron flow. The general result is a disruption of standard fermentation, which expresses itself in different ways.
Exogenous molecules are not even necessary to increase butanol production from sugars, just merely a change in redox potential. Recent reports suggest that fermentations conducted in the presence of electrodes that maintain “oxidizing” conditions (EH between +0.2 V and + 0.4 V) increased butanol production (Wietzke and Bahl 2012). Enzyme data suggest that the cells (Clostridium acetobutylicum) expressed a redox sensing protein (Rex), which is still being characterized (Wietzke and Bahl 2012). Xylose utilization was not part of that specific study, but the researchers suggested that this change would impact all fermentable substrates.
Hydrogen is the functional electron sink for fermentation. Excess reducing equivalents are expended during rapid fermentation as molecular hydrogen, which is released extracellularly to maintain an intracellular redox balance (Xin et al. 2014; Ye et al. 2011, Ye et al. 2012). Fermentative cells, however, will transfer electrons to a number of molecules which includes redox active quinones (e.g., AQDS), ferric iron, and manganese dioxide (Popovic and Finneran 2018; Popovic et al. 2017; Ye et al. 2011, Ye et al. 2012; Zhang et al. 2013b). These electron sinks alter carbon and electron flow during fermentation, which simultaneously increases xylose utilization and solvent production. In some cases, the overall biomass yield increases, but these are not being used as respiratory terminal electron acceptors (Popovic et al. 2017 #3525).
Fed batch reactors of C. beijerinckii amended with either AQDS or riboflavin were incubated with and without ferric iron (which was added as the common environmental mineral phase ferrihydrite). Data demonstrated that extracellular electron transfer disrupted metabolism similarly to prior molecules, but in this case, the ultimate electron sink was the environmentally relevant mineral iron, which has implications for natural systems as well as engineered systems. The process by which redox active molecules accept and then donate electrons to an alternate acceptor is referred to as electron shuttling (Popovic et al. 2017), and has been used in environmental remediation for several decades. It is only now gaining momentum in engineered reactors.
The changes to xylose utilization rate and extent, as well as the butanol production yields, in any system amended with both electron shuttling molecules and ferric iron were substantial. It was especially notable because these cultures were not incubated with acetate, which is added to increase butanol yield in traditional fermentations. Past data indicated that electron shuttles alone increased butanol yield, but the continuous recycling of the oxidized form of the molecule (by electron transfer to ferric iron) increased xylose utilization as well. The redox mediators did not impact glucose; it was always utilized 100% irrespective of the test conditions. However, extracellular electron transfer did increase butanol production with glucose as the primary substrate (Popovic et al. 2017; Zhao et al. 2017). These data demonstrate a strategy for targeting xylose uptake and utilization in solvent generating wild-type cells, which could be retrofitted to existing fermentation systems without the need to radically alter engineering infrastructure (and therefore move in the direction of a “drop in” technology).
An electron flow conceptual model was developed to describe the fate of reducing equivalents and carbon in ABE fermentations that were subjected to unbalanced fermentations, and how carbon and electron flow amongst the various pathways responsible resulted in increased butanol production. Data suggest that increased ATP yield and NAD+/NADH ratios are critical to both (Popovic et al. 2017; Zhao et al. 2017), but more work is required to elucidate the exact mechanisms by which redox active quinones and/or ferric iron increase xylose utilization and increased butanol production.
Genetic engineering of known xylose fermenters
Genetic engineering can include insertion or deletion of specific genes that impact xylose utilization and solvent production, or genetic tools to develop mutants that selectively utilize xylose to generate solvents. The majority of recent genetic advances have focused on two metabolic pathways: xylose uptake and phosphorylation, or altering the carbon catabolite repression system (Basu et al. 2017; Boonsombuti et al. 2014; Bruder et al. 2015; Liu et al. 2018; Ratnaparkhe et al. 2016; Zhang et al. 2017). Table 1 summarizes the reported genetic modifications and/or mutant strains that have been developed in support of xylose fermentation for solvent production.
Lignocellulosic pretreatment for xylose recovery and butanol production
Feedstock pretreatment is a critical aspect of solvent production. While past pretreatment approaches have selected against xylose enrichment, a number of recent pretreatment advances specifically select for xylose. Pretreatment is an entirely different subject, but it is worth mentioning here that several different lignocellulosic materials can be treated to produce high masses of xylose and that xylose will be available for ABE fermentation.
Materials that have been reported to date include birch Kraft black liquor, enzyme-treated corn fiber hydrolysate, acid-treated corn fiber, sea weed extract, and wood pulp hydrolysate (acid treated) (Kudahettige-Nilsson et al. 2015). Data from studies done with these varying materials suggest that ABE yield differs depending on the starting biomass feedstock, but all materials generated solvents, with butanol as the solvent recovered in the highest yield for all cases reported (Kudahettige-Nilsson et al. 2015). In summary, the data suggest that butanol is the optimal solvent target for lignocellulosic biomass that is high in xylose content.
Conclusions and prospects
Xylose utilization in solvent production has increased during the past decade, and advances in research guarantee that it will continue to increase in the years ahead. All recent data suggest that the underlying questions are being investigated to develop xylose-derived solvents, primarily butanol, into a competitive market on the world biofuel stage. Lignocellulosic biomass pre-treatment technologies have become more cost effective, and the number of available feedstocks has increased. Xylose has been historically considered a low value product during pre-treatment, but that was the result of having few downstream reactions predicated on xylose use. The review above suggests that the number of available technologies for xylose fermentation to generate solvents is already modest to large, and that with each new advance, the field comes closer to having xylose be as preferable a substrate as glucose, although there is still a large gap between xylose use and glucose use.
While this has the greatest number of technological implications for biofuel production, it does raise questions as to how xylose fermentation in natural environments or alternative engineered environments (e.g., wastewater treatment) influences carbon and electron flow. The Firmicutes are often dominant members of the microbial community in all environments and therefore exert a large level of influence on carbon flow. The reactions above (i.e., biofuels) are viewed through the aperture of “products.” However, if these reactions also occur in natural or non-biofuel systems, then fermentative Clostridia and other solvent generating genera can be viewed as influencing the remainder of the reactions, by producing molecules that serve as electron donors for respiratory cells. It is reasonable to expect that in higher Fe(III) environments, xylose fermentation will increase, and butanol will become a more prevalent product. That will select for different downstream metabolic processes than standard fermentations.
In terms of engineered biofuel production, one very active research area is the development of mixed microbial cultures rather than relying strictly on pure cultures. Mixed microbial cultures (be it binary cultures or those containing multiple different genera) are considered more stable than pure cultures. However, there is less chance of genetic manipulations, and product yields are lower. So, it is a tradeoff between stability and longevity of the culture (and the general ease of manipulating it in a reactor), versus the purity and yield of products. A number of recent studies have focused on how mixed cultures can be developed, that catalyze similar reactions, and reach similar product yields (Li et al. 2017; Panitz et al. 2014; Popovic et al. 2017; Raganati et al. 2014; Ratnaparkhe et al. 2016; Roth and Tippkotter 2016; Sandoval-Espinola et al. 2015; Van Hecke et al. 2016; Zhang et al. 2017).
One additional thought is the intersection between the redox manipulations described above, and the burgeoning field of microbial bioelectrochemistry. Bioelectrochemistry is the use of electrodes to manipulate electron donors, electron acceptors, or general redox conditions within microbial incubations. It is not unreasonable to postulate that electrodes could be used in place of redox active molecules. If successful, it is also reasonable to conclude that electrodes could be very simply added to any industrial fermentation to manipulate reactor conditions, which favor both xylose utilization and butanol generation.
References
Alkasrawi M, Eriksson T, Börjesson J, Wingren A, Galbe M, Tjerneld F, Zacchi G (2003) The effect of Tween-20 on simultaneous saccharification and fermentation of softwood to ethanol. Enzym Microb Technol 33:71–78
Atsumi S, Cann AF, Connor MR, Shen CR, Smith KM, Brynildsen MP, Liao JC (2008) Metabolic engineering of Escherichia coli for 1-butanol production. Metab Eng 10:305–311
Basu A, Xin FX, Lim TK, Lin QS, Yang KL, He JZ (2017) Quantitative proteome profiles help reveal efficient xylose utilization mechanisms in solventogenic Clostridium sp strain BOH3. Biotechnol Bioeng 114(9):1959–1969. https://doi.org/10.1002/bit.26332
Boonsombuti A, Komolpis K, Luengnaruemitchai A, Wongkasemjit S (2014) Enhancement of ABE fermentation through regulation of ammonium acetate and D-xylose uptake from acid-pretreated corncobs. Ann Microbiol 64(2):431–439. https://doi.org/10.1007/s13213-013-0673-2
Bruder M, Moo-Young M, Chung DA, Chou CP (2015) Elimination of carbon catabolite repression in Clostridium acetobutylicum—a journey toward simultaneous use of xylose and glucose. Appl Microbiol Biotechnol 99(18):7579–7588. https://doi.org/10.1007/s00253-015-6611-4
Chen Y, Zhou T, Liu D, Li A, Xu S, Liu Q, Ying H (2013) Production of butanol from glucose and xylose with immobilized cells of Clostridium acetobutylicum. Biotechnol Bioprocess Eng 18:234–241
Connor MR, Liao JC (2009) Microbial production of advanced transportation fuels in non-natural hosts. Curr Opin Biotechnol 20:307–315
Cooney C, Wallner T, McConnell S, Gillen JC, Abell C, Miers SA, Naber JD (2009) Effects of blending gasoline with ethanol and butanol on engine efficiency and emissions using a direct-injection, spark ignition engine. In: ASME 2009 Internal Combustion Engine Division Spring Technical Conference ASME 157-165
de Maria P (ed) (2016) Industrial biorenewables: a practical viewpoint, 1st edn. Hoboken, John Wiley & Sons, Inc.
El Kanouni A, Zerdani I, Zaafa S, Znassni M, Loutfi M, Boudouma M (1998) The improvement of glucose/xylose fermentation by Clostridium acetobutylicum using calcium carbonate. World J Microbiol Biotechnol 14:431–435
Fu HX, Yang ST, Wang MQ, Wang JF, Tang IC (2017a) Butyric acid production from lignocellulosic biomass hydrolysates by engineered Clostridium tyrobutyricum overexpressing xylose catabolism genes for glucose and xylose co-utilization. Bioresour Technol 234:389–396. https://doi.org/10.1016/j.biortech.2017.03.073
Fu HX, Yu L, Lin M, Wang JF, Xiu ZL, Yang ST (2017b) Metabolic engineering of Clostridium tyrobutyricum for enhanced butyric acid production from glucose and xylose. Metab Eng 40:50–58. https://doi.org/10.1016/j.ymben.2016.12.014
Girbal L, Croux C, Vasconcelos I, Soucaille P (1995) Regulation of metabolic shifts in Clostridium acetobutylicum ATCC-824. FEMS Microbiol Rev 17(3):287–297. https://doi.org/10.1111/j.1574-6976.1995.tb00212.x
Gírio FM, Fonseca C, Carvalheiro F, Duarte LC, Marques S, Bogel-Łukasik R (2010) Hemicelluloses for fuel ethanol: A review. Bioresource Technol 101(13):4775–4800
Gong CS, Chen LF, Flickinger MC, Tsao GT (1981) Conversion of hemicellulose carbohydrates. In Bioenergy (pp. 93-118). Springer Berlin Heidelberg
Green EM (2011) Fermentative production of butanol—the industrial perspective. Curr Opin Biotechnol 22:337–343
Grimmler C, Held C, Liebl W, Ehrenreich A (2010) Transcriptional analysis of catabolite repression in Clostridium acetobutylicum growing on mixtures of D-glucose and D-xylose. J Biotechnol 150:315–323
Harris LM, Blank L, Desai RP, Welker NE, Papoutsakis ET (2001) Fermentation characterization and flux analysis of recombinant strains of Clostridium acetobutylicum with an inactivated solR gene. J Ind Microbiol Biotechnol 27(5):322–328
Harvey BG, Meylemans HA (2011) The role of butanol in the development of sustainable fuel technologies. J Chem Technol Biotechnol 86(1):2–9
Jeffries TW (1983) Utilization of xylose by bacteria, yeasts, and fungi. Springer, Berlin, Heidelberg, pp 1–32
Jiang Y, Xu C, Dong F, Yang Y, Jiang W, Yang S (2009) Disruption of the acetoacetate decarboxylase gene in solvent-producing Clostridium acetobutylicum increases the butanol ratio. Metabolic Engineering 11(4-5):284–291
Jiang W, Wen ZQ, Wu MB, Li H, Yang J, Lin JP, Lin YJ, Yang LR, Cen PL (2014) The effect of pH control on acetone-butanol-ethanol fermentation by Clostridium acetobutylicum ATCC 824 with xylose and D-glucose and D-xylose mixture. Chin J Chem Eng 22(8):937–942. https://doi.org/10.1016/j.cjche.2014.06.003
Kudahettige-Nilsson RL, Helmerius J, Nilsson RT, Sojblom M, Hodge DB, Rova U (2015) Biobutanol production by Clostridium acetobutylicum using xylose recovered from birch Kraft black liquor. Bioresour Technol 176:71–79. https://doi.org/10.1016/j.biortech.2014.11.012
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind Eng Chem Res 48(8):3713–3729
Lee SY, Park JH, Jang SH, Nielsen LK, Kim J, Jung KS (2008) Fermentative butanol production by Clostridia. Biotechnol Bioeng 101(2):209–228
Lee J, Jang Y, Choi SJ, Im JA, Song H, Cho JH, Seung DY, Papoutsakis ET, Bennett GN, Lee SY (2012) Metabolic Engineering of Clostridium acetobutylicum ATCC 824 for Isopropanol-Butanol-Ethanol Fermentation. Appl Environ Microbiol 78(5):1416–1423
Li HL, Xiong L, Chen XF, Wang C, Qi GX, Huang C, Luo MT, Chen XD (2017) Enhanced enzymatic hydrolysis and acetone-butanol-ethanol fermentation of sugarcane bagasse by combined diluted acid with oxidate ammonolysis pretreatment. Bioresour Technol 228:257–263. https://doi.org/10.1016/j.biortech.2016.12.119
Liang TM, Cheng SS, Wu KL (2002) Behavioral study on hydrogen fermentation reactor installed with silicone rubber membrane. Int J Hydrog Energy 27:1157–1165
Liu L, Zhang L, Tang W, Gu Y, Hua Q, Yang S, Yang C (2012) Phosphoketolase pathway for xylose catabolism in Clostridium acetobutylicum revealed by 13C metabolic flux analysis. J Bacteriol 194:5413–5422
Liu J, Lin QL, Chai XY, Luo YC, Guo T (2018) Enhanced phenolic compounds tolerance response of Clostridium beijerinckii NCIMB 8052 by inactivation of Cbei_3304. Microbial Cell Factories 17 doi: 10.1186/s12934-018-0884-0
Ma C (1958) Metabolism of pentoses by Clostridia II: The fermentation of C14-labeled pentoses by Clostridium perfringens, Clostridium beijerinckii, and Clostridium butylicum. J Bacteriol 75:335–338
Masum BM, Kalam MA, Masjuki HH, Palash SM, Fattah IR (2014) Performance and emission analysis of a multi cylinder gasoline engine operating at different alcohol–gasoline blends. Royal Soc Chem Adv 4:27898–27904
Mes-Hartree M, Saddler J (1982) Butanol production of Clostridium acetobutylicum grown on sugars found in hemicellulose hydrolysates. Biotechnol Lett 4(4):247–252
Meyer CL, Papoutsakis ET (1989) Increased levels of ATP and NADH are associated with increased solvent production in continuous cultures of Clostridium acetobutylicum. Appl Microbiol Biotechnol 30:450–459
Panitz JC, Zverlov VV, Pham VTT, Stuzl S, Schieder D, Schwarz WH (2014) Isolation of a solventogenic Clostridium sp strain: fermentation of glycerol to n-butanol, analysis of the bcs operon region and its potential regulatory elements. Syst Appl Microbiol 37(1):1–9. https://doi.org/10.1016/j.syapm.2013.10.004
Park DH, Laivenieks M, Guettler MV, Jain MK, Zeikus JG (1999) Microbial utilization of electrically reduced neutral red as the sole electron donor for growth and metabolite production. Appl Environ Microbiol 65:2912–2917
Peguin S, Delorme P, Goma G, Soucaille P (1994a) Enhanced alcohol yields in batch cultures of Clostridium acetobutylicum using a 3-electrode poteniometric system with methyl viologen as electron carrier. Biotechnol Lett 16(3):269–274. https://doi.org/10.1007/bf00134624
Peguin S, Goma G, Delorme P, Soucaille P (1994b) Metabolic flexibility of Clostridium acetobutylicum in response to methyl viologen addition. Appl Microbiol Biotechnol 42(4):611–616
Peguin S, Soucaille P (1995) Modulation of carbon and electron flow in Clostridium acetobutylicum by iron limitation and methyl viologen addition. Appl Environ Microbiol 61:403–405
Peguin S, Soucaille P (1996) Modulation of metabolism of Clostridium acetobutylicum grown in chemostat culture in a three-electrode potentiostatic system with methyl viologen as electron carrier. Biotechnol Bioeng 51(3):342–348
Popovic J, Finneran KT (2018) Electron shuttling to ferrihydrite selects for fermentative rather than Fe3+-reducing biomass in xylose-fed batch reactors derived from three different inoculum sources. Biotechnol Bioeng 115(3):577–585. https://doi.org/10.1002/bit.26494
Popovic J, Ye XF, Haluska A, Finneran KT (2017) Ferric iron and extracellular electron shuttling increase xylose utilization and butanol production during fermentation with multiple solventogenic bacteria. Appl Microbiol Biotechnol 101(21):8053–8061. https://doi.org/10.1007/s00253-017-8533-9
Procentese A, Raganati F, Olivieri G, Russo ME, Salatino P, Marzocchella A (2015) Continuous xylose fermentation by Clostridium acetobutylicum—assessment of solventogenic kinetics. Bioresour Technol 192:142–148. https://doi.org/10.1016/j.biortech.2015.05.041
Qi GX, Xiong L, Huang C, Chen XF, Lin XQ, Chen XD (2015) Solvents production from a mixture of glucose and xylose by mixed fermentation of Clostridium acetobutylicum and Saccharomyces cerevisiae. Appl Biochem Biotechnol 177(4):996–1002. https://doi.org/10.1007/s12010-015-1790-0
Qiu CS, Zheng YZ, Zheng JF, Liu Y, Xie CY, Sun LP (2016) Mesophilic and thermophilic biohydrogen production from xylose at various initial pH and substrate concentrations with microflora community analysis. Energy Fuel 30(2):1013–1019. https://doi.org/10.1021/acs.energyfuels.5b02143
Qureshi N, Maddox IS (1988) Reactor design for the ABE fermentation using cells of Clostridium acetobutylicum immobilized by adsorption onto bonechar. Bioprocess Eng 3(2):69–72
Raganati F, Procentese A, Olivieri G, Salatino P, Marzocchella A (2014) Biobutanol production from hexose and pentose sugars. In: Bardone E, Bravi M, Keshavarz T (eds) Ibic2014: 4th International Conference on Industrial Biotechnology. Chemical engineering transactions, vol 38, pp 193–198
Rao G, Mutharasan R (1987) Altered electron flow in continuous cultures of Clostridium acetobutylicum induced by viologen dyes. Appl Environ Microbiol 53:1232–1235
Ratnaparkhe S, Ratnaparkhe MB, Jaiswal AK, Kumar A (2016) Strain Engineering for Improved Bio-Fuel Production. Curr Metabolom 4(1):38–48
Roffler S, Blanch HW, Wilke CR (1987) Extractive Fermentation of Acetone and Butanol: Process Design and Economic Evaluation. Biotechnol Prog 3(3):131–140
Roth J, Tippkotter N (2016) Evaluation of lignocellulosic material for butnaol prodcution using enzymatic hydrolysate medium. Cellul Chem Technol 50(3–4):405–410
Saini M, Lin LJ, Chiang CJ, Chao YP (2017) Synthetic consortium of Escherichia coli for n-butanol production by fermentation of the glucose-xylose mixture. J Agric Food Chem 65(46):10040–10047. https://doi.org/10.1021/acs.jafc.7b04275
Sandoval-Espinola WJ, Chinn M, Bruno-Barcena JM (2015) Inoculum optimization of Clostridium beijerinckii for reproducible growth. FEMS Microbiol Lett 362(19) doi: 10.1093/femsle/fnv164
Servinsky MD, Kiel JT, Dupuy NF, Sund CJ (2010) Transcriptional analysis of differential carbohydrate utilization by Clostridium acetobutylicum. Microbiol 156(11):3478–3491
Shin H, Zeikus J, Jain M (2002) Electrically enhanced ethanol fermentation by Clostridium thermocellum and Saccharomyces cerevisiae. Appl Microbiol Biotechnol 58(4):476–481
Singh A, Lynch MD, Gill RT (2009) Genes restoring redox balance in fermentation-deficient E. coli NZN111. Metabol Eng 11(6):347–354
Szwaja S, Naber JD (2010) Combustion of n-butanol in a spark-ignition IC engine. Fuel 89(7):1573–1582
Valentine J, Clifton-Brown J, Hastings A, Robson P, Allison G, Smith P (2012) Food vs. fuel: the use of land for lignocellulosic ‘next generation’energy crops that minimize competition with primary food production. Glob Change Biol Bioenergy 4:1–19
Van Hecke W, Vandezande P, Dubreuil M, Uyttebroek M, Beckers H, De Wever H (2016) Biobutanol production from C5/C6 carbohydrates integrated with pervaporation: experimental results and conceptual plant design. J Ind Microbiol Biotechnol 43(1):25–36. https://doi.org/10.1007/s10295-015-1717-3
Vasconcelos I, Girbal L, Soucaille P (1994) Regulation of carbon and electron flow in Clostridium acetobutylicum grown in chemostat culture at neutral pH on mixtures of glucose and glycerol. J Bacteriol 176(5):1443–1450
Walmsley AR, Barrett MP, Bringaud F, Gould GW (1998) Sugar transporters from bacteria, parasites and mammals: structure–activity relationships. Trends Biochem Sci 23:476–481
Wietzke M, Bahl H (2012) The redox-sensing protein rex, a transcriptional regulator of solventogenesis in Clostridium acetobutylicum. Appl Microbiol Biotechnol 96:749–761
Wu YD, Xue C, Chen LJ, Bai FW (2016a) Impact of zinc supplementation on the improved fructose/xylose utilization and butanol production during acetone-butanol-ethanol fermentation. J Biosci Bioeng 121(1):66–72. https://doi.org/10.1016/j.jbiosc.2015.05.003
Wu YD, Xue C, Chen LJ, Yuan WJ, Bai FW (2016b) Synergistic effect of calcium and zinc on glucose/xylose utilization and butanol tolerance of Clostridium acetobutylicum. FEMS Microbiol Lett 363(5) doi: 10.1093/femsle/fnw023
Xiao H, Gu Y, Ning Y, Yang Y, Mitchell WJ, Jiang W, Yang S (2011) Confirmation and elimination of xylose metabolism bottlenecks in glucose phosphoenolpyruvate-dependent phosphotransferase system-deficient Clostridium acetobutylicum for simultaneous utilization of glucose, xylose, and arabinose. Appl Environ Microbiol 77(22):7886–7895
Xiao H, Li Z, Jiang Y, Yang Y, Jiang W, Gu Y, Yang S (2012) Metabolic engineering of d-xylose pathway in Clostridium beijerinckii to optimize solvent production from xylose mother liquid. Metab Eng 14(5):569–578
Xin FX, Wu YR, He JZ (2014) Simultaneous fermentation of glucose and xylose to butanol by Clostridium sp strain BOH3. Appl Environ Microbiol 80(15):4771–4778. https://doi.org/10.1128/aem.00337-14
Xue C, Zhao XQ, Liu CG, Chen LJ, Bai FW (2013) Prospective and development of butanol as an advanced biofuel. Biotechnol Adv 31:1575–1584
Yang M, Kuittinen S, Zhang J, Vepsäläinen J, Keinänen M, Pappinen A (2015) Co-fermentation of hemicellulose and starch from barley straw and grain for efficient pentoses utilization in acetone–butanol–ethanol production. Bioresour Technol 179:128–135
Ye X, Zhang X, Morgenroth E, Finneran KT (2011) Anthrahydroquinone-2,6,-disulfonate (AH2QDS) increases hydrogen molar yield and xylose utilization in growing cultures of Clostridium beijerinckii. Appl Microbiol Biotechnol 92:855–864
Ye XF, Zhang XY, Morgenroth E, Finneran KT (2012) Anthrahydroquinone-2,6-disulfonate increases the rate of hydrogen production during Clostridium beijerinckii fermentation with glucose, xylose, and cellobiose. Int J Hydrog Energy 37(16):11701–11709. https://doi.org/10.1016/j.ijhydene.2012.05.018
Zhang F, Rodriguez S, Keasling JD (2011) Metabolic engineering of microbial pathways for advanced biofuels production. Curr Opin Biotechnol 22:775–783
Zhang JZ, Yu L, Xu MM, Yang ST, Yan QJ, Lin M, Tang IC (2017) Metabolic engineering of Clostridium tyrobutyricum for n-butanol production from sugarcane juice. Appl Microbiol Biotechnol 101(10):4327–4337. https://doi.org/10.1007/s00253-017-8200-1
Zhang X, Ye X, Finneran KT, Zilles JL, Morgenroth E (2013a) Interactions between Clostridium beijerinckii and Geobacter metallireducens in co-culture fermentation with anthrahydroquinone-2, 6-disulfonate (AH2QDS) for enhanced biohydrogen production from xylose. Biotechnol Bioeng 110(1):164–172. https://doi.org/10.1002/bit.24627
Zhang X, Ye X, Guo B, Finneran KT, Zilles JL, Morgenroth E (2013b) Lignocellulosic hydrolysates and extracellular electron shuttles for H-2 production using co-culture fermentation with Clostridium beijerinckii and Geobacter metallireducens. Bioresour Technol 147:89–95. https://doi.org/10.1016/j.biortech.2013.07.106
Zhao XH, Kasbi M, Chen JK, Peres S, Jolicoeur M (2017) A dynamic metabolic flux analysis of ABE (acetone-butanol-ethanol) fermentation by Clostridium acetobutylicum ATCC 824, with riboflavin as a by-product. Biotechnol Bioeng 114(12):2907–2919. https://doi.org/10.1002/bit.26393
Acknowledgements
We thank Anne Haluska, Xiaofeng Ye, and Xinyu Zhang for data and support that were presented in this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict(s) of interest. This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Finneran, K.T., Popovic, J. Solvent production from xylose. Appl Microbiol Biotechnol 102, 8707–8715 (2018). https://doi.org/10.1007/s00253-018-9254-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9254-4