Abstract
Clostridium acetobutylicum TISTR 1462 and Clostridium beijerinckii TISTR 1461 were chosen to optimize acetone–butanol–ethanol (ABE) fermentation by using glucose as a carbon source. The enhancement in its productivity by adding various concentrations of ammonium acetate was studied. Then, the variation of glucose/xylose ratios in the pre-grown medium was investigated. The results showed that both increased ammonium acetate in the production medium and D–xylose in the pre-grown medium could produce more ABE. With these conditions, using corncob hydrolysate as a substrate, 20.58 g/L ABE was produced from C. beijerinckii TISTR 1461 with 0.44 g/L/h and 0.45 of ABE productivity and yield, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
The fluctuation of oil prices and worldwide environmental regulatory concerns have led researchers to seek alternative energy resources that are less dependent on fossil fuels. In recent years, ethanol production has been increasing because it can be blended with gasoline to reduce gasoline consumption, while at the same time helping to reduce greenhouse gas emissions (Talebnia et al. 2010). Among various alternative fuels, butanol has been recognized as one of the most promising and can be produced via fermentation or petrochemical processing in the same way as ethanol. However, butanol has additional advantages due to its low Reid vapor pressure (RVP) and higher energy (Ladisch 1991). In addition, it can be blended with gasoline at a higher concentration than ethanol, and less energy is required to separate butanol from water than to separate ethanol from water. Moreover, without necessitating any modifications of vehicles, butanol can compete with ethanol because it is not corrosive like ethanol (Lee et al. 2008).
The solventogenic products—acetone, butanol, and ethanol (ABE)—produced from ABE fermentation have a conventional ratio of 6:3:1, respectively (Jones and Woods 1986). Clostridium acetobutylicum and C. beijerinckii are among the prominent solventogenic species capable of ABE formation via fermentation, with C. acetobutylicum being the species that is most often used. In some cases, the productivity of the fermentation can be enhanced by adding ammonium acetate (Gu et al. 2009), or strain degeneration can be prevented by adding acetate or butyrate to C. beijerinckii NCIMB 8052 (Lee et al. 2008; Chen and Blaschek 1999b), C. beijerinckii BA101 (Chen and Blaschek 1999a), or C. acetobutylicum strain 77 (a mutant from C. acetobutylicum ATCC 824) (Matta-el-ammouri et al. 1987). It has been reported that butyrate and acetate pathways play important roles in the energy metabolism of various strains of solventogenic products; therefore, solventogenesis is triggered, which appears to be essential for butanol production. So far, however, there have been no studies of the two strains provided by the Thailand Institute of Scientific and Technological Research (TISTR), C. acetobutylicum TISTR 1462 and C. beijerinckii TISTR 1461, in terms of how they might enhance ABE production. In this study, we chose these two strains as the representatives for C. acetobutylicum and C. beijerinckii (Keis et al. 2001).
One of the limitations of ABE fermentation is the cost of substrates, to the extent that substrate costs have been identified as a major factor affecting the economic viability of industrial ABE fermentation. In the past, production facilities have even been shut down due to the high price of molasses (Jones and Woods 1986). Therefore, the possibility of using abundant and renewable sources of lignocellulosic residues, such as corncob waste, as sources of monomeric sugars for conversion into biobutanol holds great promise. Indeed, it makes this waste more attractive for producing a variety of valuable products, including biofuels. In Thailand, corncobs are considered agricultural waste, with over 35 million kg generated each year. Corncobs consist mainly of cellulose (39 %), hemicellulose (43 %), and lignin (7.6 %), with xylose being the major constituent of hemicellulose.
To increase the value of corncobs, they have been used to produce fermentable sugars via acid pretreatment and enzymatic saccharification. Among the acids studied for the pretreatment step, diluted sulfuric acid is the most commonly used. Through the pretreatment step, small amounts of cellulose and most of the hemicellulose can be hydrolyzed into monomeric sugars, which can then be further fermented into butanol.
Therefore, the present study deals with the optimization of fermentation conditions for butanol production by C. acetobutylicum TISTR 1462 and C. beijerinckii TISTR 1461 with respect to their ability to produce ABE, in addition to considering product enhancement with ammonium acetate in batch fermentation with glucose as the carbon source, since, until now, there has been no report on the supplementation of ammonium acetate to the hydrolysate of biomass. Since sugars from biomass often contain mixed C5 and C6 sugars, modification of the medium in the preculture step with various ratios of glucose and xylose has also been studied (Ounine et al. 1985; Wayman and Yu 1985). Finally, the selected strain was employed, under suitable conditions, to produce ABE from acid-pretreated corncobs.
Materials and methods
Strain and inoculum development
The C. acetobutylicum TISTR 1462 and C. beijerinckii TISTR 1461 strains were obtained from the Thailand Institute of Scientific and Technological Research. The spores were maintained in cooked meat medium (CMM; Difco Laboratories, Detroit, MI, USA) at 4 °C. The spores (1.8 mL) were transferred to cooked meat medium (CMM) and heat-shocked at 75 °C for 2 min (Qureshi et al. 2008b). The heat-shocked spores were then transferred to 16.2 mL CMM for spore germination at 37 °C until the optical density (600 nm) reached 0.8. Then, 8 mL of active growth cells were transferred into 72 mL of tryptone glucose yeast (TGY) medium (Areesirisuk 2007) for 6 h at 37 °C.
Corncob pretreatment and overliming
Corncobs were supplied as complimentary samples by the Betagro Company, Thailand. The composition was determined using the NREL (National Renewable Energy Laboratory) method for the determination of structural carbohydrates and lignin in biomass (Sluiter et al. 2008). The average particle size of the corncob was 1.6 mm when homogenized in a single lot. The corncobs were dried in a 65 °C oven for 2 days. Then, 34 g of the corncobs were presoaked in 0.5 L of 0.75 % (v/v) H2SO4 in a 1-L glass screw-cap bottle (1 g of biomass per 15 mL of solution) at 50 °C for 30 min. For pretreatment, 0.5 L of the suspended corncobs in diluted acid were heated in an autoclave at 121 °C for 1 h, then left to cool to room temperature. Dilute acid pretreatment is generally well known to produce a significant amount of fermentation inhibitors during the process; therefore, the overliming process was also conducted by using Ca(OH)2 to detoxify the inhibitors contained in dilute acid hydrolysates of lignocelluloses prior to fermentation (Martinez et al. 2001). The hydrolysate was adjusted with Ca(OH)2 to pH 10, which was followed by the addition of 1 g/L of NaSO3 to reduce the redox potential, and then heating to 90 °C for 30 min (Qureshi et al. 2010). After cooling to room temperature, the pH was adjusted to 6.6 by H2SO4. Before being used as a substrate, the hydrolysate was filtered twice to remove sediments and sterilized with a 0.2-μm pore size membrane. Samples with and without overliming treatment were analyzed for sugar composition and furfural content.
Fermentation
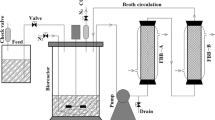
Fermentations were performed in a 100-mL screw cap Duran® glass bottle with 2 stainless tubes inserted for purging ultra-high nitrogen gas (to provide anaerobic conditions), sample collecting, and pressure release. The incubation temperature was maintained at 37 °C with a 150-rpm orbital shaking rate without pH control. The P2 medium was chosen as the production medium (Qureshi and Blaschek 1999). Glucose, xylose, and corncob hydrolysates were utilized as the carbon source at various concentrations. To prepare the P2 medium, sugar-containing distilled water and 80 g/L of yeast extract were autoclaved separately to prevent a Maillard reaction (Jaeger et al. 2010). The fermentation process was initiated by mixing all the substances to reach an exact concentration: 5 % (v/v) actively grown cells obtained from TGY, 1 g/L yeast extract, acetate buffer (0.5 g/L K2HPO4 and KH2PO4 and 2.2 g/L ammonium acetate), vitamins (0.001 g/L p–amino–benzoic acid and thiamine and 0.00001 g/L biotin), minerals (0.01 g/L MnSO4·H2O, FeSO4·7H2O, and NaCl and 0.2 g/L MgSO4·7H2O) and glucose or xylose at various concentrations. The stock solutions were sterilized by passing through a 0.2-μm pore size syringe filter.
Analyses
The centrifuged, filtered fermentation products were analyzed by a gas chromatograph (GC; PR2100; Perichrom) equipped with a DB–FFAP column and a flame ionization detector (FID). The initial and final temperatures were 60 and 157 °C with a heating rate of 15 °C/min. The detector and injector temperatures were 220 °C. The reducing sugars were measured using the 3,5-dinitrosalicylic acid (DNS) method (Miller 1959). The DNS was mixed with the samples and heated for 5 min. After the samples were cooled, they were observed with a UV–VIS spectrophotometer at a wavelength of 540 nm and calculated with a standard curve of glucose concentration and absorbance. For the sugars and furfural concentrations, HPLC was applied with Biorad Aminex HPX–87H column (300 × 7.8 mm). The mobile phase was 5 mM H2SO4 filtered through 0.45 μm and degassed before use. The flow rate was adjusted to 0.7 mL/min and the temperature was set isothermally at 40 °C for efficient separation (Buday et al. 1990). Cell growth was measured directly by the UV–VIS spectrophotometer at a wavelength of 600 nm.
ABE productivity was calculated as total ABE produced in g/L divided by the incubation period and is expressed as g/L/h while the incubation period is defined as the fermentation time. ABE yield was calculated as total ABE produced divided by the total sugar utilized. Carbon recovery was approximately calculated as decribed by Chen and Blaschek (1999a). The total carbon in the utilized substrates was obtained from the difference in sugars types (glucose, xylose, and arabinose) prior to and after fermentation though acetate was present in both substrates and products. The total carbon was acquired from the acetone, butanol, ethanol, and butyrate in the products. It was calculated by multiplying the number of carbons of each compound by the moles of each compound.
Statistical analysis
The data of butanol concentration from ABE fermentation were analyzed by one-way analysis of variance (ANOVA) to inspect the effect of ammonium acetate addition. The comparison test was performed by using Tukey HSD and Scheffe methods. Statistical significance was set at the 0.05 probability level.
Results and discussion
Composition of corncob
The corncob composition was determined using the NREL method to compare the composition change during the acid pretreatment. Xylan and arabinan were reduced due to the solubilization of hemicellulose by dilute sulfuric pretreatment. The composition of corncob prior to the acid pretreatment was 34.25 ± 0.48 % w/w glucan, 23.14 ± 0.06 % w/w xylan, and 4.06 ± 0.24 % w/w arabinan, while, after the pretreatment, they were 59.33 ± 1.63 % w/w glucan, 2.20 ± 0.79 % w/w xylan, and 0.05 ± 0.01 % w/w arabinan. The results indicate that acid pretreatment is an effective method to hydrolyze hemicellulose to monomeric sugars and leave an amount of cellulose as an increase of glucan.
Comparative ABE production of C. acetobutylicum TISTR 1462 and C. beijerinckii TISTR 1461
In order to achieve the main objective of this work, C. acetobutylicum TISTR 1462 and C. beijerinckii TISTR 1461 were compared for their ability to produce ABE when using the same production medium, incubation temperature, and shaking rate. A glucose concentration of 20 g/L was used as a carbon source and extended to 60 g/L to define the excess amount of glucose. The effect of varying the strain type and glucose concentration on the ABE concentration, acid concentration, productivity, yield, and utilized glucose is shown in Fig. 1. The results show that C. acetobutylicum TISTR 1462 cannot utilize glucose proficiently even at a low concentration (2 % w/v).
Production of ABE, acid concentration, ABE productivity, yield, and percentage of utilized glucose where (I) C. acetobutylicum TISTR 1462, 2 % initial glucose, (II) C. beijerinckii TISTR 1461, 2 % initial glucose, (III) C. beijerinckii TISTR 1461, 4 % initial glucose, and (IV) C. beijerinckii TISTR 1461, 6 % initial glucose collected at 48 h of fermentation
Unlike C. acetobutylicum TISTR 1462, C. beijerinckii TISTR 1461 utilized the glucose more efficiently in P2 medium (∼100 %) at 18 h and produced mainly solventogenic products. This corresponds with the higher ABE concentration and productivity of the C. beijerinckii TISTR 1461 strain. Therefore, C. acetobutylicum TISTR 1462 is considered an acid producer due to the volume of the accumulated acids (acetic acid and butyric acid) of ∼5 g/L. It is generally accepted that acetic acid and butyric acid cause the acid crash during ABE fermentation. This result can be explained by the acid crash that occasionally occurs in uncontrolled pH batch fermentations in which high concentrations of undissociated acids in broth exceed 57–60 mM (Maddox et al. 2000). In this case—that is, when C. acetobutylicum TISTR 1462 is used—metabolism cannot be shifted to the solventogenic phase due to high concentrations of undissociated acids (58 mM). Consequently, we chose C. beijerinckii TISTR 1461 for further study by increasing the glucose concentration to determine the excess concentration.
The results show that C. beijerinckii TISTR 1461 can grow in P2 medium containing 2–6 % glucose (Fig. 1). The increase in the glucose concentration raised the ABE concentration and total solvent productivity; however, the effect of the glucose concentration on the ABE productivity and yield were not much different from those of 2 % glucose. Thus, it can be concluded that C. beijerinckii TISTR 1461 can utilize glucose to produce solventogenic products efficiently. Using 6 % glucose concentration as the substrate (4.6 % utilized), C. beijerinckii TISTR 1461 can produce 4.84, 7.98, 0.26, and 13.07 g/L of acetone, butanol, ethanol, and total ABE, respectively, with an A:B:E ratio at 3:6:1. Then, the proper amount of initial glucose for C. beijerinckii TISTR 1461 was indicated for solventogenic enhancement by ammonium acetate since the excessive glucose combined with acetate may alter the acetone/butanol ratio (Jones and Woods 1986).
Effect of ammonium acetate on production
The two most important parameters affecting butanol formation are the pH condition and the concentrations of the acids produced. There have been studies on the addition of acids to promote solventogenic products for some strains (Chen and Blaschek 1999b; Ezeji et al. 2007), and on the addition of ammonium acetate to improve the solvent yield when using cassava as a fermentation medium (Gu et al. 2009). In this study, ammonium acetate was present in the P2 medium at 30 mM, which is an optimum concentration. However, the effect of ammonium acetate on the production of ABE fermentation by C. beijerinckii TISTR 1461 has not previously been reported. Therefore, testing was carried out on the effect of ammonium acetate on product enhancement by varying concentrations of ammonium acetate from 30 to 80 mM with 6 % glucose concentration. The acidity of the fermentation broth is shown in Fig. 2, where each ammonium acetate concentration is significantly different due to a high buffer capacity (Bryant and Blaschek 1988).
A pH of 5.0 is usually suitable for ABE fermentation; however, the pH range may vary widely depending on the type of strain and the conditions used. The results showed that 30 mM ammonium acetate may lack the buffer capacity necessary since the pH decreased during the fermentation period. In order to maintain the pH at an optimum level, the addition of ammonium acetate in P2 medium was monitored. The addition of ammonium acetate could maintain the pH above 5.0 after the exponential phase, suggesting that acids produced from the culture did not have much effect on the broths. It has been reported that the addition of ammonium acetate can increase transcription of acidogenic (ask and buk) and solventogenic genes (adc, ctfAB, adhE and bdhB) observed by reverse transcription–PCR analysis (Gu et al. 2009).
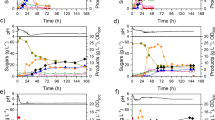
When ammonium acetate was introduced, the ABE concentration, ABE productivity, and yield increased as the ammonium acetate concentration increased, as shown in Fig. 3a. It can be suggested that the supplementation of ammonium acetate into hydrolysate is required. However, further increases in the ammonium acetate concentration did not result in a higher yield of solvent. The optimum ammonium acetate concentration of 70 mM was confirmed by statistical analysis using one-way analysis of variance (ANOVA, P < 0.05). For ABE product concentration, the addition of 70 mM of ammonium acetate was considered as the same group of homogeneous subset of 80 mM ammonium acetate in both Tukey HSD (honestly significant difference) and Scheffe methods. In the experiment, the addition of 70 mM ammonium acetate elevated the ABE concentration, total solvent productivity, and yield to 17.82 g/L, 0.37 g/L/h, and 0.35, respectively, after 48 h of fermentation.
At 80 mM of ammonium acetate, the ABE concentration, total solvent productivity, and yield were 17.63 g/L, 0.37 g/L/h, and 0.33, respectively, which were approximately the same as for 70 mM of ammonium acetate. The C. beijerinckii TISTR 1461 in this work can produce butanol at a rate of 12.01 g/L compared with 13.90 g/L for cultures grown in MP2 medium containing 60 mM sodium acetate and C. beijerinckii NCIMB 8052 (Chen and Blaschek 1999b). The highest butanol yield (0.24) and productivity (0.25) was obtained after 48 h of fermentation by C. beijerinckii TISTR 1461. Meanwhile, Chen and Blaschek (1999b) used C. beijerinckii NCIMB 8052 with the addition of 60 mM sodium acetate for butanol production which led to butanol yield and productivity of 0.39, and 0.29, respectively.
There was an improvement in glucose utilization and also an increase in the level of the products, except at 80 mM, as displayed in Fig. 3b. The %ABE value increased rapidly during the initial addition of ammonium acetate. At higher concentrations of ammonium acetate, the utilized glucose in the presence of 80 mM ammonium acetate was comparable to that observed for 70 mM ammonium acetate. Yet, this result was inconsistent with the results of studies of C. acetobutylicum EA 2018 (Gu et al. 2009) and C. beijerinckii NCIMB 8052 (Lee et al. 2008). For C. acetobutylicum EA 2018, either the sodium acetate or ammonium sulfate addition alone is not sufficient to enhance solvent production in cassava medium, which is different from C. beijerinckii NCIMB 8052, which uses only acetate. Other researchers state that the acetate addition can change the ratio of A:B (conventionally 1:2) to 2:3 due to the metabolic shift toward acetone production/ since the improvement in CoA–transferase activity consequently positively affects the conversion of acetate to acetyl–CoA and further converts to acetone by a metabolic pathway (Nölling et al. 2001).
Effect of sugar composition in preculture medium (TGY) on ABE fermentation
Sugars obtained from pretreated biomass usually contain both C5 (mainly from hemicellulose) and C6. Many studies of sugar uptake from glucose and xylose have been reported. One prior study observed diauxic growth by means of glucose consumption until depletion, after which xylose utilization occurred (Kanouni et al. 1998). There have also been studies about the effect of xylose–pregrown cells in production medium (Soni et al. 1982) that differed from the finding with C. beijerinckii TISTR 1461 in the present study. Since growth in P2 medium was relatively low, an adjustment of the amount of xylose in the preculture step (TGY) by varying the ratio of the carbon sources (glucose/xylose) and using mixed glucose (30 g/L) and xylose (30 g/L) as a substrate was investigated. The results of ABE production, shown in Table 1, indicate that ABE fermentation occurred in all the samples except for the medium inoculated with glucose–xylose–pregrown cells at the ratio of 30/70. Although there was not much difference under the 100/0 and 70/30 conditions, the latter produced a higher total ABE concentration and higher accuracy for replication results. Moreover, the utilization of glucose and xylose in the fermentation broth was also observed. Since glucose was completely utilized, it was found that glucose was a preferable sugar for C. beijerinckii TISTR 1461 compared to xylose in all cases. With the fixed incubation period of 24 h, the fastest glucose utilization rate occurred in the pregrown cells inoculated with glucose/xylose at the ratio of 100/0. For the 30/70 condition, the highest amount of xylose concentration in preculture medium resulted in a low rate of sugar uptake and produced mainly ethanol. In the batch fermentation containing a mixture of glucose and xylose, the highest amount of fermentable sugars of 50 g/L was obtained. This result is comparable to the work of Ounine et al. (1985) and Wayman and Yu (1985). It has been reported that the suppression of glucose in xylose utilization may be caused by glucose competition with xylose in the transport system (Zyl et al. 1993) and catabolite repression in the xylose metabolism pathway (Blencke et al. 2003).
Moreover, this study indicates the amount of butanol that can inhibit the growth of C. beijerinckii TISTR 1461. A previous study (Ounine et al. 1985) reported that glucose and xylose permease were inhibited when butanol concentration reached 12 and 8 g/L, respectively. As found in this study, C. beijerinckii TISTR 1461, with an ammonium acetate 70 mM supplement, can tolerate butanol toxicity to 12 and 11 g/L for glucose and xylose permease, respectively.
ABE fermentation using acid pretreated corncobs
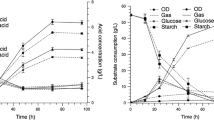
The substrate obtained from acid-pretreated corncobs contains mainly glucose (1.60 g/L), xylose (15.85 g/L), and arabinose (3.43 g/L) as a carbon source with a sugar concentration of 20.88 g/L (Table 2). Detoxification by the overliming process was implemented in order to lower the amount of furfural. Previous reports suggest that furfural and hydroxymethyl furfural (HMF) at a level less than 3 g/L have no effect as an inhibitor (Ezeji et al. 2007). However, in this study, C. beijerinckii TISTR 1461 was not able to grow in the acid-pretreated corncobs without the overliming process (no ABE was detected after 48 h of fermentation). Table 2 shows the amount of sugars, acetic acid, and furfural of the pretreated corncobs before and after the overliming process. Lime treatment can lower furfural concentration twofold and slightly increase other substances due to the high temperature of the reaction and loss of water. Acetic acid contained in the hydrolysate occurs from the deacetylation of xylan, which also has a significant effect on microbial fermentation (Martinez et al. 2000). In the initial phase of the fermentation, there is a high amount of acetic acid, 4.80 ± 0.93 g/L, compared with that found in glucose/xylose fermentation.
To investigate the ABE fermentation capability of C. beijerinckii TISTR 1461 with acid-pretreated corncobs, a glucose addition of up to 50 g/L total sugars was applied. At 48 h of fermentation, a productivity and yield of ABE at 0.44 g/L/h and 0.45 were found to have significantly higher level compared to the control experiment (Table 3). Butanol toxicity produced in ABE fermentation is often encountered in butanol production. Therefore, the amount of utilized sugar (with approximately 3 g/L total sugars) at 96 h of fermentation (Fig. 4) may be due to butanol toxicity. It was lower compared with the effect of sugar composition in the preculture medium (TGY) on ABE fermentation since the culture can tolerate only 9.61 g/L butanol compared to 11 g/L as previously mentioned. According to the literature, the best results ever obtained for ABE fermentation in terms of butanol concentration have been less than 20 g/L since butanol can significantly inhibit cell growth and fermentation. Liu et al. (2010) found that 6.3 ± 0.5 g/L of butanol were produced in 72 h by C. beijerinckii 550251. On the other hand, C. beijerinckii NCIMB 8052 and C. beijerinckii BA101 grown in semidefined P2 medium containing 6 % glucose produced 9.2 and 18.8 g/L of butanol, respectively, in batch fermentation (Formanek et al. 1997). C. beijerinckii BA101 was developed from C. beijerinckii NCIMB 8052; therefore, C. beijerinckii BA101 produces a higher concentration of total solvents.
Although C. beijerinckii TISTR 1461 can produce ABE at a rate of up to 20 g/L, the main component is acetone (11.14 g/L), which is affected by the ABE ratio changing from 3:6:1 to 5:4:1. Nevertheless, there was an enhancement of butanol production since butanol yield and productivity were elevated from 0.16 and 0.17 to 0.20 and 0.20, respectively, compared to the control experiment. This was confirmed after comparison to the butanol yield and productivity, using the reducing sugar content of Ca(OH)2-detoxified corncob residue hydrolysate, as they were 0.16 and 0.17, respectively (Zhang et al. 2012). The high concentration of acetone may be due to the acetate from the hydrolysate and ammonium acetate since the acetate can reversibly change in the solventogenic phase. Moreover, the carbon recovery, shown in Table 4, indicates that the amount of carbon source was not limited.
Conclusions
In efforts to eliminate the obstacle of high substrate prices, corncobs present a promising alternative due to their high sugar content. The present study found that acid-pretreated corncobs are a potential substrate for ABE fermentation since they can produce total sugars up to 20 g/L (294 mg sugar/g corncobs) and be used practically as a carbon source for C. beijerinckii TISTR 1461. Both the glucose and xylose in the pregrown medium and the addition of ammonium acetate have a significant effect on the ABE concentration, ABE productivity, and yield. Butanol toxicity remains a major problem when the concentration reaches ∼10 g/L. Future work should concentrate on enhancing extraction efficiency to increase the amount of sugar obtained from the biomass by using both acid pretreatment and enzymatic saccharification, or on investigating techniques to collect butanol during fermentation.
References
Areesirisuk A (2007) The study of butanol production from sweet sorghum stem juice by Clostridium beijerinckii JCM 1390. Dissertation, Khon Kaen University
Blencke HM, Homuth G, Ludwig H, Mäder U, Hecker M, Stülke J (2003) Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways. Metab Eng 5:133–149
Bryant DL, Blaschek HP (1988) Buffering as a means for increasing growth and butanol production by Clostridium acetobutylicum. J Ind Microbiol 3:49–55
Buday Z, Linden JC, Karim MN (1990) Improved acetone-butanol fermentation analysis using subambient HPLC column temperature. Enzyme Microb Tech 12:24–27
Chen CK, Blaschek HP (1999a) Acetate enhances solvent production and prevents degeneration in Clostridium beijerinckii BA101. Appl Microbiol Biot 52:170–173
Chen CK, Blaschek HP (1999b) Effect of acetate on molecular and physiological aspects of Clostridium beijerinckii NCIMB 8052 solvent production and strain degeneration. Appl Environ Microb 65:499–505
Ezeji T, Qureshi N, Blaschek HP (2007) Butanol production from agricultural residues: Impact of degradation products on Clostridium beijerinckii growth and butanol fermentation. Biotechnol Bioeng 97:1460–1469
Formanek J, Mackie R, Blaschek HP (1997) Enhanced butanol production by Clostridium beijerinckii BA101 grown in semidefined P2 medium containing 6 percent Maltodextrin or Glucose. Appl Environ Microb 63:2306–2310
Gu Y, Hu S, Chen J, Shao L, He H, Yang Y, Yang S, Jiang W (2009) Ammonium acetate enhances solvent production by Clostridium acetobutylicum EA 2018 using cassava as a fermentation medium. J Ind Microbiol Biot 36:1225–1232
Jones DT, Woods DR (1986) Acetone-butanol fermentation revisited. Microbiol Rev 50:484–524
Jaeger H, Janositz A, Knorr D (2010) The Maillard reaction and its control during food processing. The potential of emerging technologies. Pathol Biol 58:207–213
Jieun L, Seo E, Kweon D, Park K, Jin Y (2009) Fermentation of rice bran and defatted rice bran for butanol production using Clostridium beijerinckii NCIMB 8052. J Microbiol Biotechn 19:482–490
Kanouni AE, Zerdani I, Zaafa S, Znassni M, Loutfi M, Boudouma M (1998) The improvement of glucose/xylose fermentation by Clostridium acetobutylicum using calcium carbonate. World J Microb Biot 14:431–435
Keis S, Shaheen R, Jones DT (2001) Emended descriptions of Clostridium acetobutylicum and Clostridium beijerinckii, and descriptions of Clostridium saccharoperbutylacetonicum sp. nov. and Clostridium saccharobutylicum sp. nov. Int J Syst Evol Micr 51:2095–2103
Ladisch MR (1991) Fermentation–derived butanol and scenarios for its uses in energy-related applications. Enzyme Microb Tech 13:280–283
Lee SM, Cho MO, Park CH, Chung Y, Kim JH, Sang B, Um Y (2008) Continuous butanol production using suspended and immobilized Clostridium beijerinckii NCIMB 8052 with supplementary butyrate. Energ Fuel 22:3459–3464
Liu Z, Ying Y, Li F, Ma C, Xu P (2010) Butanol production by Clostridium beijerinckii ATCC 55025 from wheat bran. J Ind Microbiol Biot 37:495–501
Maddox IS, Steiner E, Hirsch S, Wessner S, Gutierrez A, Gapes JR, Schuster KC (2000) The cause of “acid crash” and “acidogenic fermentations” during the batch acetone-butanol- ethanol (ABE-) fermentation process. J Mol Microb Biotech 2:95–100
Martinez A, Rodriguez ME, Wells ML, York SW, Preston JF, Ingram LO (2001) Detoxification of dilute acid hydrolysates of lignocellulose with lime. Biotechnol Progr 17:287–293
Martinez A, Rodriguez ME, York SW, Preston JF, Ingram LO (2000) Effects of Ca(OH)2 treatments (“Overliming”) on the composition and toxicity of bagasse hemicellulose hydrolysates. Biotechnol Bioeng 69:526–536
Matta-el-ammouri G, Janati-idrissi R, Junelles A, Petitdemange H, Gay R (1987) Effects of butyric and acetic acids on acetone-butanol formation by Clostridium acetobutylicum. Biochimie 69:109–115
Miller GL (1959) Use of dinitrosalicylic reagent for the determination of reducing sugar. Anal Chem 31:426–428
Nölling J, Breton G, Omelchenko MV, Makarova KS, Zeng QD, Gibson R, Lee HM, Dubois J, Qiu DY, Hitti J, Wolf YI, Tatusov RL, Sabathe F, Doucette-Stamm L, Soucaille P, Daly MJ, Bennett GN, Koonin EV, Smith DR (2001) Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J Bacteriol 183:4823–4838
Ounine K, Petitdemange H, Raval G, Gay R (1985) Regulation and butanol inhibition of D-xylose and D-glucose uptake in Clostridium acetobutylicum. Appl Environ Microb 49:874–878
Qureshi N, Blaschek HP (1999) Butanol recovery from model solution/fermentation broth by pervaporation: evaluation of membrane performance. Biomass Bioenerg 17:175–184
Qureshi N, Ezeji TC, Ebener J, Dien BS, Cotta MA, Blaschek HP (2008a) Butanol production by Clostridium beijerinckii. Part I: Use of acid and enzyme hydrolyzed corn fiber. Bioresource Technol 99:5915–5922
Qureshi N, Saha BC, Dien B, Hector RE, Cotta MA (2010) Production of butanol (a biofuel) from agricultural residues: Part I – Use of barley straw hydrolysate. Biomass Bioenerg 34:559–565
Qureshi N, Saha BC, Hector RE, Hughes SR, Cotta MA (2008b) Butanol production from wheat straw by simultaneous saccharification and fermentation using Clostridium beijerinckii: Part I—Batch fermentation. Biomass Bioenerg 32:168–175
Soni B, Das K, Ghose T (1982) Bioconversion of agro-wastes into acetone butanol. Biotechnol Lett 4:19–22
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass. U.S. Department of Energy, Colorado
Talebnia F, Karakashev D, Angelidaki I (2010) Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresource Technol 101:4744–4753
Wayman M, Yu S (1985) Acetone-butanol fermentation of xylose and sugar mixtures. Biotechnol Lett 7:255–260
Zhang W, Liu Z, Liu Z, Li F (2012) Butanol production from corncob residue using Clostridium beijerinckii NCIMB 8052. Lett Appl Microbiol 55:240–246
Zyl CV, Prior BA, Kilian SG, Brandt EV (1993) Role of D-ribose as a cometabolite in D-xylose metabolism by Saccharomyces cerevisiae. Appl Environ Microb 59:1487–1494
Acknowledgments
We are grateful to the National Research University Project of CHE and the Ratchadaphiseksomphot Endowment Fund (EN269B), the Development and Promotion of Science and Technology Talents Project (DPST), and the Center of Excellence for Petrochemical and Materials Technology, Thailand, for supporting this research. The authors would also like to thank Betagro Public Company Limited for providing corncob samples used in this research. In addition, the authors thank Dr. Charnwit Kositanont for his valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boonsombuti, A., Komolpis, K., Luengnaruemitchai, A. et al. Enhancement of ABE fermentation through regulation of ammonium acetate and D–xylose uptake from acid-pretreated corncobs. Ann Microbiol 64, 431–439 (2014). https://doi.org/10.1007/s13213-013-0673-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-013-0673-2