Abstract
Mushrooms are an important food crop for many millions of people worldwide. The most important edible mushroom is the button mushroom (Agaricus bisporus), an excellent example of sustainable food production which is cultivated on a selective compost produced from recycled agricultural waste products. A diverse population of bacteria and fungi are involved throughout the production of Agaricus. A range of successional taxa convert the wheat straw into compost in the thermophilic composting process. These initially break down readily accessible compounds and release ammonia, and then assimilate cellulose and hemicellulose into compost microbial biomass that forms the primary source of nutrition for the Agaricus mycelium. This key process in composting is performed by a microbial consortium consisting of the thermophilic fungus Mycothermus thermophilus (Scytalidium thermophilum) and a range of thermophilic proteobacteria and actinobacteria, many of which have only recently been identified. Certain bacterial taxa have been shown to promote elongation of the Agaricus hyphae, and bacterial activity is required to induce production of the mushroom fruiting bodies during cropping. Attempts to isolate mushroom growth-promoting bacteria for commercial mushroom production have not yet been successful. Compost bacteria and fungi also cause economically important losses in the cropping process, causing a range of destructive diseases of mushroom hyphae and fruiting bodies. Recent advances in our understanding of the key bacteria and fungi in mushroom compost provide the potential to improve productivity of mushroom compost and to reduce the impact of crop disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cultivated mushrooms are an important food source for many people around the world, with global production estimated at over 10 million tons per year (Food and Agriculture Organization of the United Nations 2014). Over two thirds of the production of edible mushrooms are harvested in mainland China, where mushrooms form a more traditional role in food and medicine than they do in many Western countries, and where they provide a living for over 25 million mushroom farmers (Zhang et al. 2014b). The most important edible mushroom genus grown commercially is Agaricus (mainly Agaricus bisporus, the button mushroom), which makes up about 30% of the global market (Royse 2014). Other important edible genera include Pleurotus (5–6 species of oyster mushrooms that are cultivated commercially), Lentinula (shiitake), Auricularia (3–4 species of woodear mushrooms), Flammulina (enoki), and Volvariella (paddy straw). Edible mushroom production is dominated by a few species where the technology for large-scale industrial cultivation has been optimized (Chang and Miles 2004), but in many countries large numbers of small-scale farms also exist, and there are increasing attempts to domesticate local wild mushrooms for production purposes (Mwai and Muchane 2016).
Cultivated mushrooms are saprophytes which grow by degrading natural lignocellulosic substrates, which are commonly available in large volumes as agricultural or industrial byproducts. The methods for commercial cultivation of mushrooms on these substrates can be divided into three broad groups. The first group includes cultivation of many wood-degrading mushrooms that were traditionally grown on wood logs or harvested from trees. Some of these, such as Lentinula, are now grown on artificial logs of compacted, sterilized sawdust, while others, such as Flammulina, Pholiota (nameko), or Auricularia are cultivated on a partly composted mixture of sawdust and other components (bran, straw, corncobs), which is sterilized at high temperature (121 °C) before inoculation with mycelium (Chang and Miles 2004; Sanchez 2010). Because of the rigorous sterilization process, these mushrooms are essentially grown in axenic culture. The second group of cultivation methods either uses uncomposted substrates directly, or uses partially composted substrates that have not been subjected to a rigorous sterilization process. This includes methods commonly used for Pleurotus and Volvariella species, though Pleurotus is also sometimes grown on sterilized sawdust substrates. These are fast-growing and adaptable genera capable of rapid bioconversion of a broad range of substrates (e.g., rice straw, bagasse, cornstalks, waste cotton, stalks, and leaves of bananas (Chang and Miles 2004; Thongklang and Luangharn 2016). The substrates are usually not sterilized before inoculation, though a pasteurization step may be included in the process, and the mushrooms therefore grow in competition with other microorganisms on the substrate. The most industrially complex process is the cultivation of Agaricus, which is grown on a pasteurized straw-based compost which requires lengthy preparation, but allows selective growth of the Agaricus mycelium over competitor organisms.
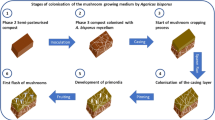
For mushrooms grown in fully sterilized substrates, such as Lentinula, the rate of mycelium growth is dependent on enzymatic degradation of lignocellulose by the mushroom itself and is independent of other microbes. Addition of particular bacteria or other fungi could potentially stimulate either growth or fruiting, but this has not been investigated in any depth. For Pleurotus and Agaricus, by contrast, growth of the mycelium and production of the commercial fruiting body are dependent not only on the mushroom itself, but also on bacteria and other fungi in the substrate. These bacteria and fungi play critical roles at several different stages of production (Fig. 1), including (i) conversion of the lignocellulose feedstocks into a selective, nutrient-rich compost for mushroom growth; (ii) interactions with the fungal mycelium during hyphal elongation and proliferation through the substrate; and (iii) induction of fruiting body formation during cropping. In addition, several bacterial and fungal taxa act as pathogens of the mushroom crop, causing either a reduction in yield or severe loss of quality.
This review will focus primarily on the importance of bacteria and fungi in mushroom compost during the production of Agaricus bisporus and Pleurotus ostreatus. Several excellent reviews are available that discuss bacterial-fungal interactions in a range of other environmental, agricultural, and clinical areas (Frey-Klett et al. 2011; Kobayashi and Crouch 2009; Scherlach et al. 2013). A number of other studies provide overviews of the composting of general municipal and agricultural wastes (Chandna et al. 2013; De Gannes et al. 2013a, b; Hultman et al. 2010; Partanen et al. 2010), and highlight the influence of feedstocks and process parameters on fungal and bacterial diversity and succession in the resulting composts (Neher et al. 2013).
Diversity and succession of bacteria and fungi in mushroom compost
A. bisporus is commercially grown on a composted substrate prepared in a thermophilic, microbial process from wheat straw and/or horse stable bedding, nitrogen-containing additives, the most common which are poultry manure, seed meal, or synthetic nitrogen (urea or ammonium nitrate), and gypsum (Chang and Miles 2004; Royse and Beelman 2016; Straatsma et al. 2000) (Fig. 1). The wheat straw is usually soaked for 3–10 days before mixing with the other feedstocks (Noble and Gaze 1996), and then subjected to a period of aerobic, thermophilic composting (phase I) during which the compost temperature rapidly rises to 80 °C due to microbial activity (Straatsma et al. 2000; Zhang et al. 2014a). Phase I can take up to 14 days to complete (Noble et al. 2002) but can be completed as quickly as 6 days (Weil et al. 2013), and serves primarily for growth of the microbial population at the expense of soluble components of the feedstocks, since there is relatively little decrease in the total content of complex carbohydrates (cellulose, hemicellulose) or lignin (Jurak et al. 2015). In phase II, the compost is held at 58–60 °C for 2 days in tunnels that are designed to provide uniform temperature and airflow into the compost (Noble and Gaze 1996), followed by a “conditioning” or “curing” period, in which the compost is maintained at 48–51 °C for 2–3 days (Straatsma et al. 2000). This is a period of intense microbial activity, and by the end of phase II, 50–60% of the cellulose and hemicellulose in the original feedstocks has been degraded (Jurak et al. 2015). During the same period, the excess ammonia released during the thermophilic phase has been assimilated by the microbial biomass in the compost (Miller et al. 1991; Wiegant et al. 1992). The microbial community present at the end of phase II represents a climax community, into which A. bisporus is introduced, usually as grain spawn grown on millet or rye. The mycelium of Agaricus takes approximately 16 days to fully colonize the mature compost (Jurak 2015; Royse and Beelman 2016), initially utilizing bacterial and fungal biomass as a key source of nutrition and then progressively breaking down over 50% of the lignin (Jurak et al. 2015).
Although it is possible to grow A. bisporus mycelium on non-composted wheat straw, yields are low and the process is not used commercially (Mamiro et al. 2007). The composting process provides specificity for Agaricus cultivation because it converts the wheat straw into a mixture of microbial biomass and humus-lignin complexes which are not available to competing fungi, but which Agaricus can access by degrading living and dead thermophilic fungal and bacterial biomass (Bilay and Lelley 1997; Fermor et al. 1991; Straatsma et al. 1994b; Vos et al. 2017). The selectivity for Agaricus can be removed by artificial lysis of phase II compost biomass (chemical treatment or prolonged high temperatures), which allows many other contaminant ascomycetes to grow. However, if the sterilized compost is reconditioned by adding microbes that bind the released nutrients into fresh microbial biomass, then the selectivity for Agaricus can be restored (Ross and Harris 1983a; Straatsma et al. 1989).
The microbes in mushroom compost are introduced with the feedstocks, though they can also be enriched by the use of recycled compost leachate (sometimes called “goody water”) in the wetting process (Kertesz and Safianowicz 2015). A clear succession is seen in the mushroom compost community, which has been studied by cultivation-based analysis (Hayes 1968; Ryckeboer et al. 2003; Singh et al. 2012; Siyoum et al. 2016) and by a number of cultivation-independent techniques, including fingerprinting methods (Wang et al. 2016) coupled to DNA sequencing methods (Siyoum et al. 2016; Szekely et al. 2009; Vajna et al. 2010, 2012) and, most recently, by metagenomic or amplicon sequencing of bacterial and fungal communities (Kertesz et al. 2016; Langarica-Fuentes et al. 2014; McGee et al. 2017; Souza et al. 2014; Zhang et al. 2014a). Most of these studies have been done on Agaricus compost, though partially composted Pleurotus compost has also been examined (Vajna et al. 2010, 2012). Cultivation-based analysis of phase I compost has yielded largely actinobacterial and Bacillus isolates [reviewed in (Ryckeboer et al. 2003)], but this method provides a very limited window on the microbial diversity present, since many compost bacteria and fungi are not readily cultivable, Many studies have also focused on single time points within the composting process, providing a limited overview. More recent DNA sequencing studies have provided evidence for the presence of a wealth of other microbes in compost and their succession, with a broad range of phyla involved (Fig. 2). The most dramatic changes in microbial populations in compost occur during the initial wetting period and during phase I (Kertesz et al. 2016). This period sees rapid assimilation of easily accessible nutrients such as free sugars and amino acids from the compost, and the changes in the most abundant bacteria probably reflect the rapid succession of bacteria and fungi that can use these compounds. Initially, these are mesophilic pioneer organisms such as Solibacillus, Comamonas, Acinetobacter, Pseudomonas, and Sphingomonas (Kertesz et al. 2016; Vajna et al. 2012).
Bacterial phylum succession in mushroom compost during composting, spawn run, and cropping of Agaricus bisporus. Bacterial communities were determined by Illumina Miseq sequencing of the 16S rRNA gene (Kertesz et al. 2016). The heat map shows relative sequence abundance of the indicated phyla at different stages of the process
As temperatures begin to increase in phase I, peaks are seen in populations of thermophilic Bacillus, Paenibacillus, and uncharacterized Clostridia and Proteobacteria. The most abundant actinobacteria present are Corynebacterium and Streptomyces (Zhang et al. 2014a) and, although they make up only a small fraction of the total bacterial population, analysis of the cellulase gene diversity reveals that these actinobacteria are cellulolytic (Zhang et al. 2014a). The taxon evenness in the compost increases to reach a maximum at the end of phase I, with an increase in thermophilic taxa. Thermus thermophilus is a key member of the bacterial population at this point (Kertesz et al. 2016; Szekely et al. 2009), and Thermi are relatively important, though they disappear again later during composting (Fig. 2).
The dynamics of fungal diversity are similar to the bacteria changes during phase I. Initial pioneers such as Lewia, Rhizomucor, and Aspergillus are overgrown by Talaromyces, Thermomyces, Thermus, and unclassified taxa as temperature and pH rise (Kertesz et al. 2016; Straatsma et al. 1994b). Most of these organisms are not cellulolytic although some of them will break down hemicellulose, and cellulose and hemicellulose are indeed only marginally depleted during phase I (Jurak et al. 2014). Pioneer fungi grow by utilizing other carbon sources in the compost, and once these alternative carbon sources are exhausted, the fungal population is replaced by cellulose degraders such as Mycothermus thermophilus and Chaetomium thermophilum, with M. thermophilus being the climax species in phase II compost (Kertesz et al. 2016; Souza et al. 2014).
Mycothermus (Scytalidium) and other thermophilic microbes—nutrition for Agaricus
During phase II, the microbial community dynamics change completely with rapid succession of different genera appearing in phase I being replaced by a comparatively stable microbial community during conditioning. The dominant fungal species during the conditioning process is M. thermophilus [previously Scytalidium thermophilum or Torula thermophila (Natvig et al. 2015)]. This species is a thermophilic, cellulolytic ascomycete which is a dominant component of many composting systems and plays an important role in degradation of polymeric carbohydrates. Isolates of this species from compost secrete a suite of over 60 different cellulases, hemicellulases, and other glycosyl hydrolases (Basotra et al. 2016).
The importance of thermophilic bacteria and fungi in mushroom compost production was recognized very early (Chanter and Spencer 1974; Eicker 1977; Stanek 1972). Thermophilic fungi, in particular, grow rapidly during the conditioning period, removing free nutrients from the compost and assimilating the ammonia released by ammonification (Ross and Harris 1983b). This physiological activity provides a selective environment for growth of Agaricus by immobilizing nutrients in a form unavailable to competitor molds (Ross and Harris 1983a). Unlike competing ascomycetes, the Agaricus mycelium aggressively decomposes both living and dead bacterial and fungal biomass to obtain the nutrients it requires (Fermor and Wood 1991). This has been shown by growth of Agaricus on [14C]-labeled Bacillus subtilis cells leading to direct uptake of the isotope into the fungal mycelium (Fermor et al. 1991). Agaricus also disintegrated Mycothermus cultures on agar medium even through a layer of cellophane, causing complete loss of viability in the latter fungus (Op den Camp et al. 1990). In a cropping setting, the amount of living bacterial biomass in phase II compost (measured as phospholipid fatty acids) decreases by about 75% after addition of Agaricus and the abundance of thermophilic fungal biomass also decreases dramatically (Vos et al. 2017). The mycelium of Agaricus seems to be partly selective for Mycothermus and does not degrade other thermophilic fungi so effectively (Straatsma et al. 1994b), but the reasons for this are not known.
Bacterial diversity in phase II compost has traditionally been thought to be dominated by cellulolytic actinomycetes and bacilli (Ryckeboer et al. 2003). Recent cultivation-independent studies have shown a peak in Actinobacteria at this stage (Fig. 2) and have identified Thermomonospora, Thermobispora, Thermopolyspora, Thermobifida, and Microbispora as key genera (Silva et al. 2009; Szekely et al. 2009; Vajna et al. 2012; Zhang et al. 2014a). However, the most abundant bacterial taxon in both Agaricus and Pleurotus compost is Pseudoxanthomonas taiwanensis, together with Thermus and several bacilli (Bacillus, Geobacillus, Ureibacillus) (Kertesz et al. 2016; Vajna et al. 2012).
P. taiwanensis is only present in low numbers in phase I, because although it is thermophilic, its optimum growth is at 50 °C, and it does not grow above 65 °C (Chen et al. 2002). The population of P. taiwanensis increases as the temperature falls during compost conditioning and it is the most abundant bacterial species in mature compost (Kertesz et al. 2016; Szekely et al. 2009; Vajna et al. 2012). As a heterotrophic nitrifier, it is able to convert ammonia into N2O (Chen et al. 2002), but how important this activity is in removing the abundant ammonia in phase II compost is not yet known. Unexpectedly, P. taiwanensis does not appear to break down cellulose, though it produces β-glucosidase and can utilize cellobiose (Chen et al. 2002; Kato et al. 2005). Nevertheless, it has been detected repeatedly as an essential component of stable cellulose-degrading consortia isolated from a variety of sources (Du et al. 2015; Kato et al. 2004, 2005; Wang et al. 2011), and efficient degradation of lignocellulose in bioethanol production, for example, relied on optimization of the P. taiwanensis abundance (Du et al. 2015). In one of these consortia, it seems to play its essential role by interacting with a cellulolytic species of Clostridium (Kato et al. 2004, 2005), and in mushroom compost, it may well be interacting in a similar way with Mycothermus. This is underlined by the fact that its abundance in phase II compost appears to increase in parallel to that of Mycothermus (Kertesz et al. 2016), but further studies are required to confirm the relevance of this finding.
Because the presence of thermophilic fungi can promote the growth rate of Agaricus mycelium up to two fold (Straatsma et al. 1994a), this fungal group, and in particular M. thermophilus, has been studied extensively as an inoculum to promote compost preparation and accelerate Agaricus growth (Bilay 2000; Sanchez et al. 2008; Sanchez and Royse 2009; Straatsma et al. 1994a; Wiegant et al. 1992). Although M. thermophilus has shown the greatest stimulation of Agaricus growth of a range of fungi tested, in its absence several other fungal species had similar effects (Straatsma et al. 1994b). Anecdotal evidence from mushroom farmers suggests that inoculation with Mycothermus may stimulate compost productivity on a farm scale, but in general, it is not necessary to add Mycothermus unless the compost has been pasteurized at too high a temperature, since naturally occurring strains of the fungus are always present (Ross and Harris 1983b; Straatsma et al. 1994a).
A number of studies have examined the addition of bacterial inocula to increase compost productivity and mushroom yield (Table 1). Inoculation with Bacillus megaterium or a thermophilic strain of Staphylococcus has been shown to promote mushroom production and advance cropping by several days (Ahlawat and Vijay 2010) and Pseudomonas putida also promoted hyphal extension of Agaricus in vitro (Rainey 1991). However, in another study, addition of B. subtilis or B. megaterium to compost along with the Agaricus spawn did not affect yield (Ekinci and Dursun 2014). A more thorough search for bacteria that promote compost productivity was also unsuccessful (Straatsma et al. 1994b). It should be noted, however, that these were all small-scale screening experiments using compost that was first sterilized at high temperature to selectively inactivate thermophilic fungi. It seems likely that promotion of Agaricus may require an interacting consortium of both bacteria and fungi for effective lignocellulose breakdown.
Bacterial involvement in formation of mushroom primordia and fruiting bodies
Formation of mushroom fruiting bodies is controlled by a range of environmental factors. Fruiting body primordia are initiated in response to a reduction in temperature compared with mycelial growth conditions and greater aeration to reduce levels of CO2 (Chang and Miles 2004). The optimum ranges of these two factors vary for different species of mushrooms (Stamets and Chilton 1983). Transcriptomic studies of Agaricus have shown that a reduction in temperature is essential for further differentiation of primordia, and the level of CO2 exerts quantitative control on the number of fruiting bodies formed (Eastwood et al. 2013). Many mushroom species also show a requirement for a change in nutrient availability, with high levels of nutrition favoring mycelial growth over primordia formation (Chang and Miles 2004). Nutrient supply governs outgrowth of the primordia, with the appearance of mushroom fruiting bodies in separate flushes governed by depletion of specific nutrients required by the primordia (Straatsma et al. 2013). Some species also require a change in pH or light conditions (Chang and Miles 2004).
For commercial cultivation of Agaricus, formation of fruiting bodies is induced by overlaying the colonized compost with a layer of “casing,” usually a mixture of peat and lime. This casing layer contains a diverse bacterial population, and the presence of these bacteria is essential for primordia formation as fruiting does not occur with sterilized casing (Eger 1972; Hayes et al. 1969). Initiation of primordia in autoclaved or fumigated casing can be partially restored by addition of a bacterial inoculum (Eger 1972) with the best studied examples being P. putida or a related pseudomonad (Colauto et al. 2016; Hayes et al. 1969; Rainey et al. 1990). Alternatively, addition of adsorptive carbon-based materials such as activated charcoal will also restore fruit body formation (Noble et al. 2003). These results suggested that the stimulatory role of the bacteria is to remove an inhibitor of primordia formation. This compound has been identified as 1-octen-3-ol (Noble et al. 2009), which is a volatile compound produced by the Agaricus mycelium, that controls the early differentiation of vegetative hyphae to multicellular knots (Eastwood et al. 2013). A considerable proportion of bacterial isolates from the casing layer are were found to be tolerant to high levels of this compound and many of these isolates were also able to promote mushroom yields by up to 10% (Zarenejad et al. 2012). 1-Octen-3-ol is also important for other mushroom species; it is the dominant flavor component in Pleurotus (Misharina et al. 2009; Venkateshwarlu et al. 1999), and bacteria isolated from Pleurotus growing on Pangola grass (including Bacillus cereus, B. megaterium, Enterobacter asburiae, Enterobacter cloacae, Kurthia gibsonii, Pseudomonas pseudoalcaligenes, and Meyerozyma guilliermondii), were found to grow on 1-octen-3-ol and both promote mycelial growth and induce fruiting of P. ostreatus in vitro (Torres-Ruiz et al. 2016).
Other bacterial metabolic pathways may also be important for stimulation of mushroom growth. For example, an ACC deaminase-producing strain of P. putida stimulated primordia formation in Agaricus but this property was lost in knockout mutants in the ACC deaminase gene (Chen et al. 2013). This suggests that ethylene, which is produced by Agaricus hyphae (Turner et al. 1975), acts as an inhibitor of mycelial growth and primordia formation (Zhang et al. 2016), and that P. putida can encourage Agaricus fruiting by lowering the levels of ethylene produced by Agaricus.
The bacterial population in casing increases dramatically after initiation of the first primordia but decreases during later flushes (Cai et al. 2009; Pardo et al. 2002). The species present are mostly proteobacteria related to Pseudomonas, Pedobacter, and Caulobacter (Fermor et al. 2000; Kertesz et al. 2016; Siyoum et al. 2010). As bacteria are required for initiation and growth of mushroom fruiting bodies, a number of investigators have screened compost or casing for “mushroom growth-promoting bacteria” that promote either mycelial growth or fruiting body formation. Most of the organisms identified have been applied to casing in an attempt to stimulate fruiting, and most of the growth-promoting strains found were Pseudomonas or Bacillus species (Table 1). The prevalence of these two genera probably reflects the culturing strategies used, since cultivation-independent methods reveal a very high diversity of bacteria present, many of which are uncharacterized (Kertesz et al. 2016; Siyoum et al. 2010). There has been little systematic study of the mechanisms by which inocula stimulate mycelial extension or initiation of primordia, or indeed whether they actively colonize Agaricus hyphae or are active on the casing itself.
Not unexpectedly, the fungal community found in button mushroom casing is greater than 90% A. bisporus (McGee et al. 2017), but over 200 other fungal species have also been detected, with highest diversity during first flush. Mycothermus is not detected at all, though other thermophilic fungi such as Thermomyces and Myceliophthora are still present, confirming the role of Mycothermus as nutrient source for growth of Agaricus. The dominant known taxa in casing are Lecanicillium fungicola, Thermomyces lanuginosus, Aspergillus spp., Myceliophthora spp., Sordaria spp., Candida subhashii, Paecilomyces niveus, and Cercophora spp. (McGee et al. 2017). The overall abundance of unknown fungal taxa is more than 20-fold higher than known taxa (excluding Agaricus) (McGee et al. 2017) suggesting that much remains to be learnt about the role of these fungi in this part of mushroom production. Interestingly, Agaricus activity (measured as ITS region cDNA abundance) reaches a maximum at first flush and then decreases substantially, and a range of unclassified taxa dominate the cDNA community for the remainder of the cropping period. During this period, the active taxa include L. fungicola, the causal agent of dry bubble disease (see below), suggesting that it can co-exist with Agaricus without causing disease, and that disease induction may require specific environmental factors (McGee et al. 2017).
Fungal and bacterial pathogens of Agaricus
While many of the bacteria and fungi described in the previous section have been shown to promote hyphal elongation or fruiting body formation of Agaricus, there has been little work to distinguish whether these organisms are actively colonizing the surface of the hyphae or living in the casing itself. Mushroom fruiting bodies, by contrast, have their own distinctive microflora. The cultivable population of microorganisms on button mushroom fruiting bodies includes pseudomonads, bacilli, and coagulase-negative staphylococci, together with the yeast Rhodotorula and several species of actinomycetes (Rossouw and Korsten 2017; Xiang et al. 2017). While the levels of human pathogens found on fruiting bodies are low (Venturini et al. 2011), bacterial contamination can cause postharvest deterioration (Beelman et al. 1989). A specific study of enterobacteria on button mushrooms at the point of harvest has revealed a considerable load of Ewingella americana (Reyes et al. 2004), which was confirmed in a recent report on microbial succession on healthy mushrooms at point of harvest (Siyoum et al. 2016). This organism is a potential human pathogen (Hassan et al. 2012), but it is better known as the cause of mushroom stipe necrosis in both Agaricus and Pleurotus (Gonzalez et al. 2012). The presence of E. americana on healthy postharvest mushrooms highlights the susceptibility of edible mushrooms to a variety of microbial diseases that can devastate crop production (Largeteau and Savoie 2010).
The most important of these are three fungal diseases, two of which have been reviewed recently (Berendsen et al. 2010; Carrasco et al. 2017). Cobweb disease (Cladobotryum dendroides) grows as a web-like mycelium over the surface of the fruiting bodies and produces large numbers of conidia that are readily dispersed by aerial means (Carrasco et al. 2017). Dry bubble disease (L. fungicola) produces severe outbreaks leading to formation of misshapen sporophores (Berendsen et al. 2010), and a similar effect is seen for wet bubble disease (Mycogone perniciosa), although this disease is less widespread and has not been studied as extensively (Khanna et al. 2003). Green mold disease (Trichoderma aggressivum) caused extensive crop losses in both America and Europe in the 1990s and continues to be a problematic disease worldwide. This disease is caused by two slightly different strains in America and Europe (T. aggressivum f. aggressivum and T. aggressivum f. europaeum) (Samuels et al. 2002), although the American pathovar has recently also been reported in Europe (Hatvani et al. 2017). Pleurotus is also affected by green mold disease, but the disease is caused by a related but phylogenetically different species of Trichoderma (Kredics et al. 2009). Spores of both L. fungicola and T. aggressivum are sticky in nature, and are therefore mainly transmitted by insect or human vectors or in water (Fletcher and Gaze 2008). Interestingly, spore germination and growth of L. fungicola, M. perniciosa, and T. aggressivum are inhibited by 1-octen-3-ol, the Agaricus metabolite described previously that acts as a potent inhibitor of fruiting body formation, possibly allowing the pathogens to time their growth to coincide with appearance of the mushroom fruiting bodies (Berendsen et al. 2013).
Pseudomonas species play a variety of different roles in mushroom casing. As described above, P. putida has been identified as the key casing organism that breaks down 1-octen-3-ol and induces fruiting body formation (Noble et al. 2009). Pseudomonas isolates have also been shown to antagonize Lecanicillium in casing, competing for iron and releasing antifungal compounds, though this is not sufficient to protect Agaricus effectively against dry bubble disease (Berendsen et al. 2012). Pseudomonas also cause a range of blotch diseases of Agaricus which cause severe crop losses. Brown blotch disease is caused by Pseudomonas tolaasii, a species which is endemic in compost and induces symptoms through production of an extracellular lipodepsipeptide toxin, tolaasin [see reviews by (Largeteau and Savoie 2010) and (Soler-Rivas et al. 1999)]. Pseudomonas reactans causes similar symptoms, releasing a related but distinct toxin to that produced by P. tolaasii (Wells et al. 1996). Because P. tolaasii is ubiquitous in the compost environment, it is very difficult to control, since it can switch rapidly between nonvirulent and virulent forms in response to environmental changes and possibly also in response to metabolites produced by Agaricus (Largeteau and Savoie 2010). P. tolaasii also attacks Pleurotus, causing yellow discolorations (Lo Cantore and Iacobellis 2014) and Flammulina (Han et al. 2012), causing black rot. Several other Pseudomonas species also cause commercially important diseases. Pseudomonas gingeri is the main causal agent of ginger blotch (Wong et al. 1982), and many different pseudomonads have similar effects, causing a range of discolorations (Godfrey et al. 2001). Pseudomonas agarici is responsible for drippy gill disease, degrading the extracellular matrix of the fruiting body and producing droplets of bacterial ooze on the lamellae of Agaricus (Gill and Cole 2000). A range of soft rot diseases of mushrooms are also caused by bacterial pathogens. These include Burkholderia gladioli pv agaricicola, which attacks a range of different edible mushroom species (Chowdhury and Heinemann 2006; Gill and Tsuneda 1997; Lee et al. 2010), Janthinobacterium agaricidamnosum, which causes soft rot in Agaricus (Chowdhury and Heinemann 2006; Lincoln et al. 1999), and Pantoea species that affect Pleurotus (Kim et al. 2015).
Control of these bacterial and fungal mushroom diseases is essential for the mushroom industry, as outbreaks can destroy a large proportion of the crop. Effective prevention has traditionally relied on maintaining good production hygiene, together with the strategic use of biocides and antifungals (Fletcher and Gaze 2008). A major restriction is that only a limited range of products have been approved for application onto mushroom crops. Alternative methods that are being developed include the use of essential oils and antagonistic bacterial species as biocontrol agents (Berendsen et al. 2012; Sokovic and van Griensven 2006), but improved molecular methods for early detection of infection and the selection of resistant mushroom varieties (Savoie et al. 2016) are also important in reducing the impact of these widespread pathogens.
Conclusions and outlook
Research into mushroom compost goes back at least to the work of Waksman in the early 1930s (Waksman and Nissen 1932), with the aim of optimizing microbiological and process parameters to maximize mushroom yields. Most of our present understanding of mushroom compost microbiology has come from cultivating isolates of thermophilic and cellulolytic bacteria and fungi from compost, and it is only recently that sequencing efforts have revealed that some of the most abundant and important organisms in compost have been overlooked by this method. High-yielding sustainable production of edible mushrooms is currently primarily hampered by inconsistency of the compost, caused by variability in the quality and composition of the feedstocks, and by changes in the microbial communities present. Our improved understanding of the microbiology of compost provides renewed potential to design consortia of bacteria and fungi that can be used in bioaugmentation to optimize composting of lower quality feedstocks, and to identify and validate biomarkers that can be used to assess the quality of a compost before cropping commences. More detailed studies are also required to explore the relationship between microbial activity and diversity in compost and casing during cropping. Most of the nutrients in mushroom compost are left untouched by the mushroom crop, illustrated by the fact that spent mushroom compost is a valued soil conditioner. Manipulation of microbial activity and nutrient availability during cropping may allow higher yields of mushrooms in later crop flushes. Finally, a more thorough understanding of the biocontrol of mushroom pathogens has the potential to increase the quality of the mushrooms produced. Mushroom compost is a completely recycled product produced from agricultural wastes, and the fungi and bacteria that define it allow us to enjoy mushrooms as truly sustainable foods.
References
Ahlawat OP, Rai RD (2000) Bacterial inoculants and their effect on the pinning, yield and false truffle disease incidence in Agaricus bitorquis. Scie Cultivation Edible Fungi 15:695–699
Ahlawat OP, Vijay B (2010) Potential of thermophilic bacteria as microbial inoculant for commercial scale white button mushroom (Agaricus bisporus) compost production. J Sci Ind Res 69:948–955
Basotra N, Kaur B, Di Falco M, Tsang A, Chadha BS (2016) Mycothermus thermophilus (Syn. Scytalidium thermophilum): repertoire of a diverse array of efficient cellulases and hemicellulases in the secretome revealed. Bioresour Technol 222:413–421. https://doi.org/10.1016/j.biortech.2016.10.018
Beelman RB, Guthrie BD, Royse DJ (1989) Influence of bacterial populations on postharvest deterioration of fresh mushrooms. Mushroom Sci 12:655–666
Berendsen RL, Baars JJP, Kalkhove SIC, Lugones LG, Wosten HAB, Bakker P (2010) Lecanicillium fungicola: causal agent of dry bubble disease in white-button mushroom. Mol Plant Pathol 11:585–595. https://doi.org/10.1111/j.1364-3703.2010.00627.x
Berendsen RL, Kalkhove SIC, Lugones LG, Baars JJP, Wosten HAB, Bakker P (2012) Effects of fluorescent Pseudomonas spp. isolated from mushroom cultures on Lecanicillium fungicola. Biol Control 63(2):210–221. https://doi.org/10.1016/j.biocontrol.2012.07.012
Berendsen RL, Kalkhove SIC, Lugones LG, Baars JJP, Wosten HAB, Bakker P (2013) Effects of the mushroom-volatile 1-octen-3-ol on dry bubble disease. Appl Microbiol Biotechnol 97(12):5535–5543. https://doi.org/10.1007/s00253-013-4793-1
Bilay VT (2000) Growth of Agaricus bisporus on grain pre-colonized by Humicola insolens and growth of mushroom mycelium from this spawn on compost. Science and cultivation of edible fungi 15:425–429
Bilay VT, Lelley JI (1997) Growth of mycelium of Agaricus bisporus on biomass and conidium of Humicola insolens. Angew Bot 71:21–23
Cai WM, Yao HY, Feng WL, Jin QL, Liu YY, Li NY, Zheng Z (2009) Microbial community structure of casing soil during mushroom growth. Pedosphere 19(4):446–452. https://doi.org/10.1016/S1002-0160(09)60137-5
Carrasco J, Navarro MJ, Gea FJ (2017) Cobweb, a serious pathology in mushroom crops: a review. Span J Agric Res 15(2):11. https://doi.org/10.5424/sjar/2017152-10143
Chandna P, Nain L, Singh S, Kuhad RC (2013) Assessment of bacterial diversity during composting of agricultural byproducts. BMC Microbiol 13(1):14. https://doi.org/10.1186/1471-2180-13-99
Chang S-T, Miles PG (2004) Mushrooms: cultivation, nutritional value, medicinal effect and environmental impact, 2nd edn. CRC Press, Boca Raton. https://doi.org/10.1201/9780203492086
Chanter DP, Spencer DM (1974) The importance of thermophilic bacteria in mushroom compost fermentation. Sci Hortic 2(3):249–256. https://doi.org/10.1016/0304-4238(74)90033-8
Chen MY, Tsay SS, Chen KY, Shi YC, Lin YT, Lin GH (2002) Pseudoxanthomonas taiwanensis sp nov., a novel thermophilic, N2O-producing species isolated from hot springs. Int J Syst Evol Microbiol 52(6):2155–2161. https://doi.org/10.1099/ijs.0.02306-0
Chen SC, Qiu CW, Huang T, Zhou WW, Qi YC, Gao YQ, Shen JW, Qiu LY (2013) Effect of 1-aminocyclopropane-1-carboxylic acid deaminase producing bacteria on the hyphal growth and primordium initiation of Agaricus bisporus. Fungal Ecol 6(1):110–118. https://doi.org/10.1016/j.funeco.2012.08.003
Cho YS, Kim JS, Crowley DE, Cho BG (2003) Growth promotion of the edible fungus Pleurotus ostreatus by fluorescent pseudomonads. FEMS Microbiol Lett 218(2):271–276. https://doi.org/10.1016/s0378-1097(02)01144-8
Chowdhury PR, Heinemann JA (2006) The general secretory pathway of Burkholderia gladioli pv. agaricicola BG164R is necessary for cavity disease in white button mushrooms. Appl Environ Microbiol 72(5):3558–3565. https://doi.org/10.1128/aem.72.5.3558-3565.2006
Coello-Castillo MM, Sanchez JE, Royse DJ (2009) Production of Agaricus bisporus on substrates pre-colonized by Scytalidium thermophilum and supplemented at casing with protein-rich supplements. Bioresour Technol 100(19):4488–4492. https://doi.org/10.1016/j.biortech.2008.10.061
Colauto NB, Fermor TR, Eira AF, Linde GA (2016) Pseudomonas putida stimulates primordia on Agaricus bitorquis. Curr Microbiol 72(4):482–488. https://doi.org/10.1007/s00284-015-0982-8
De Gannes V, Eudoxie G, Hickey WJ (2013a) Insights into fungal communities in composts revealed by 454-pyrosequencing: implications for human health and safety. Front Microbiol 4:164. https://doi.org/10.3389/fmicb.2013.00164
De Gannes V, Eudoxie G, Hickey WJ (2013b) Prokaryotic successions and diversity in composts as revealed by 454-pyrosequencing. Bioresour Technol 133:573–580. https://doi.org/10.1016/j.biortech.2013.01.138
Du R, Yan JB, Li SZ, Zhang L, Zhang SR, Li JH, Zhao G, Qi PL (2015) Cellulosic ethanol production by natural bacterial consortia is enhanced by Pseudoxanthomonas taiwanensis. Biotechnol Biofuels 8(1):10. https://doi.org/10.1186/s13068-014-0186-7
Eastwood DC, Herman B, Noble R, Dobrovin-Pennington A, Sreenivasaprasad S, Burton KS (2013) Environmental regulation of reproductive phase change in Agaricus bisporus by 1-octen-3-ol, temperature and CO2. Fung Genet Biol 55:54–66. https://doi.org/10.1016/j.fgb.2013.01.001
Eger G (1972) Experiments and comments on the action of bacteria on sporophore initiation in Agaricus bisporus. Mushroom Sci 8:719–725
Eicker A (1977) Thermophilic fungi associated with the cultivation of Agaricus bisporus. J South Afr Bot 43:193–208
Ekinci M, Dursun A (2014) The effects of compost added bacteria, organic fertilizer and their mixtures on yield and quality of mushroom (Agaricus bisporus). C R Acad Bulg Sci 67:1441–1450
Fermor TR, Wood DA (1991) Mushroom compost microbial biomass: a review. Sci Cultivation Edible Fungi 13:191–200
Fermor TR, Wood DA, Lincoln SP, Fenlon JS (1991) Bacteriolysis by Agaricus bisporus. J Gen Microbiol 137(1):15–22. https://doi.org/10.1099/00221287-137-1-15
Fermor T, Lincoln S, Noble R, Dobrovin-Pennington A, Colauto N (2000) Microbiological properties of casing. Sci Cultivation Edible Fungi 15:447–454
Fletcher JT, Gaze RH (2008) Mushroom pest and disease control: a colour handbook. Academic Press, San Diego
Food and Agriculture Organization of the United Nations (2014) Mushrooms and truffles. FAOSTAT. http://www.fao.org/faostat/en/#data/QC. Accessed 20 Oct 2017
Frey-Klett P, Burlinson P, Deveau A, Barret M, Tarkka M, Sarniguet A (2011) Bacterial-fungal interactions: hyphens between agricultural, clinical, environmental, and food microbiologists. Microbiol Mol Biol Rev 75(4):583–609. https://doi.org/10.1128/mmbr.00020-11
Gill W, Cole T (2000) Aspects of the pathology and etiology of 'drippy gill' disease of the cultivated mushroom Agaricus bisporus. Can J Microbiol 46(3):246–258. https://doi.org/10.1139/w99-133
Gill WM, Tsuneda A (1997) The interaction of the soft rot bacterium Pseudomonas gladioli pv agaricicola with Japanese cultivated mushrooms. Can J Microbiol 43(7):639–648. https://doi.org/10.1139/m97-091
Godfrey SAC, Harrow SA, Marshall JW, Klena JD (2001) Characterization by 16S rRNA sequence analysis of pseudomonads causing blotch disease of cultivated Agaricus bisporus. Appl Environ Microbiol 67(9):4316–4323. https://doi.org/10.1128/aem.67.9.4316-4323.2001
Gonzalez AJ, Gea FJ, Navarro MJ, Fernandez AM (2012) Identification and RAPD-typing of Ewingella americana on cultivated mushrooms in Castilla-La Mancha, Spain. Eur J Plant Pathol 133(3):517–522. https://doi.org/10.1007/s10658-012-9952-1
Han HS, Jhune CS, Cheong JC, Oh JA, Kong WS, Cha JS, Lee CJ (2012) Occurrence of black rot of cultivated mushrooms (Flammulina velutipes) caused by Pseudomonas tolaasii in Korea. Eur J Plant Pathol 133(3):527–535. https://doi.org/10.1007/s10658-012-9941-4
Hassan S, Amer S, Mittal C, Sharma R (2012) Ewingella americana: an emerging true pathogen. Case Rep Infect Dis 2012. https://doi.org/10.1155/2012/730720
Hatvani L, Kredics L, Allaga H, Manczinger L, Vagvolgyi C, Kuti K, Geosel A (2017) First report of Trichoderma aggressivum f. aggressivum green mold on Agaricus bisporus in Europe. Plant Dis 101(6):1052–1053. https://doi.org/10.1094/pdis-12-16-1783-pdn
Hayes WA (1968) Microbiological changes in composting wheat straw/horse manure mixtures. Mushroom Sci 7:173–186
Hayes WA, Randle PE, Last FT (1969) Nature of microbial stimulus affecting sporophore formation in Agaricus bisporus (Lange) Sing. Ann Appl Biol 64(1):177–187. https://doi.org/10.1111/j.1744-7348.1969.tb02867.x
Hultman J, Vasara T, Partanen P, Kurola J, Kontro MH, Paulin L, Auvinen P, Romantschuk M (2010) Determination of fungal succession during municipal solid waste composting using a cloning-based analysis. J Appl Microbiol 108(2):472–487. https://doi.org/10.1111/j.1365-2672.2009.04439.x
Jurak E (2015) How mushrooms feed on compost: conversion of carbohydrates and lignin in industrial wheat straw based compost enabling the growth of Agaricus bisporus. Ph. D., Wageningen University
Jurak E, Kabel MA, Gruppen H (2014) Carbohydrate composition of compost during composting and mycelium growth of Agaricus bisporus. Carbohydr Polym 101:281–288. https://doi.org/10.1016/j.carbpol.2013.09.050
Jurak E, Punt AM, Arts W, Kabel MA, Gruppen H (2015) Fate of carbohydrates and lignin during composting and mycelium growth of Agaricus bisporus on wheat straw based compost. PLoS One 10(10):e0138909. https://doi.org/10.1371/journal.pone.0138909
Kato S, Haruta S, Cui ZJ, Ishii M, Igarashi Y (2004) Effective cellulose degradation by a mixed-culture system composed of a cellulolytic Clostridium and aerobic non-cellulolytic bacteria. FEMS Microbiol Ecol 51(1):133–142. https://doi.org/10.1016/j.femsec.2004.07.015
Kato S, Haruta S, Cui ZJ, Ishii M, Igarashi Y (2005) Stable coexistence of five bacterial strains as a cellulose-degrading community. Appl Environ Microbiol 71(11):7099–7106. https://doi.org/10.1128/aem.71.11.7099-7106.2005
Kertesz M, Safianowicz K (2015) The biology of recycled process water – the goody, the bad and the ugly? AMGA J Winter 2015:18–22
Kertesz M, Safianowicz K, Bell T (2016) New insights into the microbial communities and biological activities that define mushroom compost. Sci Cultivation Edible Fungi 19:161–165
Khanna PK, Sodhi HS, Kapoor S (2003) Diseases of Agaricus bisporus and their management. Annu Rev Plant Pathol 2:163–205
Kim MK, Math RK, Cho KM, Shin KJ, Kim JO, Ryu JS, Lee YH, Yun HD (2008) Effect of Pseudomonas sp P7014 on the growth of edible mushroom Pleurotus eryngii in bottle culture for commercial production. Bioresour Technol 99(8):3306–3308. https://doi.org/10.1016/j.biortech.2007.06.039
Kim MK, Lee SH, Lee YH, Kim H, Lee J, Rho IR (2015) Characterization and chemical control of soft rot disease caused by Pantoea sp strain PPE7 in Pleurotus eryngii mushroom crops. Eur J Plant Pathol 141(2):419–425. https://doi.org/10.1007/s10658-014-0538-y
Kobayashi DY, Crouch JA (2009) Bacterial/fungal interactions: from pathogens to mutualistic endosymbionts. Annu Rev Phytopathol 47(1):63–82. https://doi.org/10.1146/annurev-phyto-080508-081729
Kredics L, Kocsube S, Nagy L, Komon-Zelazowska M, Manczinger L, Sajben E, Nagy A, Vagvolgyi C, Kubicek CP, Druzhinina IS, Hatvani L (2009) Molecular identification of Trichoderma species associated with Pleurotus ostreatus and natural substrates of the oyster mushroom. FEMS Microbiol Lett 300(1):58–67. https://doi.org/10.1111/j.1574-6968.2009.01765.x
Langarica-Fuentes A, Handley PS, Houlden A, Fox G, Robson GD (2014) An investigation of the biodiversity of thermophilic and thermotolerant fungal species in composts using culture-based and molecular techniques. Fungal Ecol 11:132–144. https://doi.org/10.1016/j.funeco.2014.05.007
Largeteau ML, Savoie JM (2010) Microbially induced diseases of Agaricus bisporus: biochemical mechanisms and impact on commercial mushroom production. Appl Microbiol Biotechnol 86(1):63–73. https://doi.org/10.1007/s00253-010-2445-2
Lee CJ, Yun HS, Jhune CS, Cheong JC, Yoo YB (2010) Occurrence of bacterial soft rot of Pleurotus ostreatus caused by Burkholderia gladioli pv. agaricicola in Korea. J Plant Pathol 92:235–240
Lincoln SP, Fermor TR, Tindall BJ (1999) Janthinobacterium agaricidamnosum sp. nov., a soft rot pathogen of Agaricus bisporus. Int J Syst Bacteriol 49(4):1577–1589. https://doi.org/10.1099/00207713-49-4-1577
Lo Cantore P, Iacobellis NS (2014) Characterization of fluorescent pseudomonads responsible for the yellowing of oyster mushroom (Pleurotus ostreatus). Phytopathol Mediterr 53:54–65
Mamiro DP, Royse DJ, Beelman RB (2007) Yield, size, and mushroom solids content of Agaricus bisporus produced on non-composted substrate and spent mushroom compost. World J Microbiol Biotechnol 23(9):1289–1296. https://doi.org/10.1007/s11274-007-9364-0
McGee CF, Byrne H, Irvine A, Wilson J (2017) Diversity and dynamics of the DNA- and cDNA-derived compost fungal communities throughout the commercial cultivation process for Agaricus bisporus. Mycologia 109(3):475–484. https://doi.org/10.1080/00275514.2017.1349498
Miller FC, Macauley BJ, Harper ER (1991) Investigation of various gases, pH and redox potential in mushroom composting phase I stacks. Aust J Exp Agric 31(3):415–425. https://doi.org/10.1071/ea9910415
Misharina TA, Muhutdinova SM, Zharikova GG, Terenina MB, Krikunova NI (2009) The composition of volatile components of cepe (Boletus edulis) and oyster mushrooms (Pleurotus ostreatus). Appl Biochem Microbiol 45(2):187–193. https://doi.org/10.1134/s0003683809020124
Mwai S, Muchane N (2016) Domestication of wild edible mushrooms in eastern Africa: a review of research advances and future prospects. Sci Cultivation Edible Fungi 19:384–388
Natvig DO, Taylor JW, Tsang A, Hutchinson MI, Powell AJ (2015) Mycothermus thermophilus gen. et comb. nov., a new home for the itinerant thermophile Scytalidium thermophilum (Torula thermophila). Mycologia 107(2):319–327. https://doi.org/10.3852/13-399
Neher DA, Weicht TR, Bates ST, Leff JW, Fierer N (2013) Changes in bacterial and fungal communities across compost recipes, preparation methods, and composting times. PLoS One 8(11):e79512. https://doi.org/10.1371/journal.pone.0079512
Noble R, Gaze RH (1996) Preparation of mushroom (Agaricus bisporus) composts in controlled environments: factors influencing compost bulk density and productivity. Int Biodeterior Biodeg 37(1-2):93–100. https://doi.org/10.1016/0964-8305(95)00072-0
Noble R, Hobbs PJ, Mead A, Dobrovin-Pennington A (2002) Influence of straw types and nitrogen sources on mushroom composting emissions and compost productivity. J Ind Microbiol Biotechnol 29(3):99–110. https://doi.org/10.1038/sj.jim.7000292
Noble R, Fermor TR, Lincoln S, Dobrovin-Pennington A, Evered C, Mead A, Li R (2003) Primordia initiation of mushroom (Agaricus bisporus) strains on axenic casing materials. Mycologia 95(4):620–629. https://doi.org/10.1080/15572536.2004.11833066
Noble R, Dobrovin-Pennington A, Hobbs PJ, Pederby J, Rodger A (2009) Volatile C8 compounds and pseudomonads influence primordium formation of Agaricus bisporus. Mycologia 101(5):583–591. https://doi.org/10.3852/07-194
Op den Camp HJM, Stumm CK, Straatsma G, Derikx PJL, Van Griensven LJLD (1990) Hyphal and mycelial interactions between Agaricus bisporus and Scytalidium thermophilum on agar media. Microb Ecol 19(3):303–310. https://doi.org/10.1007/BF02017174
Pardo A, De Juan JA, Pardo JE (2002) Bacterial activity in different types of casing during mushroom cultivation (Agaricus bisporus (Lange) Imbach). Acta Aliment 31(4):327–342. https://doi.org/10.1556/AAlim.31.2002.4.3
Park JY, Agnihotri VP (1969) Sporophore production of Agaricus bisporus in aseptic environments. Antonie Leeuwenhoek 35(1):523–528. https://doi.org/10.1007/bf02219169
Partanen P, Hultman J, Paulin L, Auvinen P, Romantschuk M (2010) Bacterial diversity at different stages of the composting process. BMC Microbiol 10(1):94. https://doi.org/10.1186/1471-2180-10-94
Rainey PB (1991) Effect of Pseudomonas putida on hyphal growth of Agaricus bisporus. Mycol Res 95(6):699–704. https://doi.org/10.1016/S0953-7562(09)80817-4
Rainey PB, Cole ALJ, Fermor TR, Wood DA (1990) A model system for examining involvement of bacteria in basidiome initiation of Agaricus bisporus. Mycol Res 94(2):191–195. https://doi.org/10.1016/S0953-7562(09)80612-6
Reyes JE, Venturini ME, Oria R, Blanco D (2004) Prevalence of Ewingella americana in retail fresh cultivated mushrooms (Agaricus bisporus, Lentinula edodes and Pleurotus ostreatus) in Zaragoza (Spain). FEMS Microbiol Ecol 47(3):291–296. https://doi.org/10.1016/s0168-6496(03)00283-6
Ross RC, Harris PJ (1983a) An investigation into the selective nature of mushroom compost. Sci Hortic 19(1-2):55–64. https://doi.org/10.1016/0304-4238(83)90044-4
Ross RC, Harris PJ (1983b) The significance of thermophilic fungi in mushroom compost preparation. Sci Hortic 20(1):61–70. https://doi.org/10.1016/0304-4238(83)90112-7
Rossouw W, Korsten L (2017) Cultivable microbiome of fresh white button mushrooms. Lett Appl Microbiol 64(2):164–170. https://doi.org/10.1111/lam.12698
Royse DJ (2014) A global perspective on the high five: Agaricus, Pleurotus, Lentinula, Auricularia and Flammulina. In: Proceedings of the 8th international conference on mushroom biology and mushroom products, New Delhi, p 1–6
Royse DJ, Beelman RB (2016) Six steps to mushroom farming. Penn State Extension, Pennsylvania State University. https://extension.psu.edu/six-steps-to-mushroom-farming. Accessed 1 Oct 2017
Ryckeboer J, Mergaert J, Vaes K, Klammer S, De Clercq D, Coosemans J, Insam H, Swings J (2003) A survey of bacteria and fungi occurring during composting and self-heating processes. Ann Microbiol 53:349–410
Samuels G, Dodd SL, Gams W, Castlebury LA, Petrini O (2002) Trichoderma species associated with the green mold epidemic of commercially grown Agaricus bisporus. Mycologia 94(1):146–170. https://doi.org/10.2307/3761854
Sanchez C (2010) Cultivation of Pleurotus ostreatus and other edible mushrooms. Appl Microbiol Biotechnol 85(5):1321–1337. https://doi.org/10.1007/s00253-009-2343-7
Sanchez JE, Royse DJ (2009) Scytalidium thermophilum-colonized grain, corncobs and chopped wheat straw substrates for the production of Agaricus bisporus. Bioresour Technol 100(4):1670–1674. https://doi.org/10.1016/j.biortech.2008.08.047
Sanchez JE, Mejia L, Royse DJ (2008) Pangola grass colonized with Scytalidium thermophilum for production of Agaricus bisporus. Bioresour Technol 99(3):655–662. https://doi.org/10.1016/j.biortech.2006.11.067
Savoie JM, Mata G, Largeteau M (2016) New prospects in pathogen control of button mushroom cultures. Academic Press Ltd-Elsevier Science Ltd, London
Scherlach K, Graupner K, Hertweck C (2013) Molecular bacteria-fungi interactions: effects on environment, food, and medicine. Annu Rev Microbiol 67(1):375–397. https://doi.org/10.1146/annurev-micro-092412-155702
Silva CF, Azevedo RS, Braga C, Rd S, Dias ES, Schwan RF, da Silva R (2009) Microbial diversity in a bagasse-based compost prepared for the production of Agaricus brasiliensis. Braz J Microbiol 40(3):590–600. https://doi.org/10.1590/S1517-83822009000300023
Singh AV, Sharma A, Johri BN (2012) Phylogenetic profiling of culturable bacteria associated with early phase of mushroom composting assessed by amplified rDNA restriction analysis. Ann Microbiol 62(2):675–682. https://doi.org/10.1007/s13213-011-0304-8
Siyoum NA, Surridge K, Korsten L (2010) Bacterial profiling of casing materials for white button mushrooms (Agaricus bisporus) using denaturing gradient gel electrophoresis. Sth Afr J Sci 106(9/10):49–54. https://doi.org/10.4102/sajs.v106i9/10.253
Siyoum NA, Surridge K, van der Linde EJ, Korsten L (2016) Microbial succession in white button mushroom production systems from compost and casing to a marketable packed product. Ann Microbiol 66(1):151–164. https://doi.org/10.1007/s13213-015-1091-4
Sokovic M, van Griensven L (2006) Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom, Agaricus bisporus. Eur J Plant Pathol 116(3):211–224. https://doi.org/10.1007/s10658-006-9053-0
Soler-Rivas C, Jolivet S, Arpin N, Olivier JM, Wichers HJ (1999) Biochemical and physiological aspects of brown blotch disease of Agaricus bisporus. FEMS Microbiol Rev 23(5):591–614. https://doi.org/10.1111/j.1574-6976.1999.tb00415.x
Souza TP, Marques SC, Santos D, Dias ES (2014) Analysis of thermophilic fungal populations during phase II of composting for the cultivation of Agaricus subrufescens. World J Microbiol Biotechnol 30(9):2419–2425. https://doi.org/10.1007/s11274-014-1667-3
Stamets P, Chilton JS (1983) The mushroom cultivator. A practical guide to growing mushrooms at home. Agarikon Press, Washington
Stanek M (1972) Microorganisms inhabiting mushroom compost during fermentation. Mushroom Sci 8:797–811
Straatsma G, Gerrits JPG, Augustijn MPAM, Op den Camp HJM, Vogels GD, van Griensven LJLD (1989) Population dynamics of Scytalidium thermophilum in mushroom compost and stimulatory effects on growth rate and yield of Agaricus bisporus. J Gen Microbiol 135:751–759
Straatsma G, Olijnsma TW, Gerrits JPG, Amsing JGM, Op den Camp HJM, Van Griensven LJLD (1994a) Inoculation of Scytalidium thermophilum in button mushroom compost and its effect on yield. Appl Environ Microbiol 60(9):3049–3054
Straatsma G, Samson RA, Olijnsma TW, Op den Camp HJM, Gerrits JPG, Van Griensven LJLD (1994b) Ecology of thermophilic fungi in mushroom compost, with emphasis on Scytalidium thermophilum and growth stimulation of Agaricus bisporus mycelium. Appl Environ Microbiol 60(2):454–458
Straatsma G, Gerrits JPG, Thissen JTNM, Amsing JGM, Loeffen H, van Griensven LJLD (2000) Adjustment of the composting process for mushroom cultivation based on initial substrate composition. Bioresour Technol 72(1):67–74. https://doi.org/10.1016/s0960-8524(99)00088-7
Straatsma G, Sonnenberg ASM, Van Griensven L (2013) Development and growth of fruit bodies and crops of the button mushroom, Agaricus bisporus. Fungal Biol 117(10):697–707. https://doi.org/10.1016/j.funbio.2013.07.007
Szekely A, Sipos R, Berta B, Vajna B, Hajdu C, Marialigeti K (2009) DGGE and T-RFLP analysis of bacterial succession during mushroom compost production and sequence-aided T-RFLP profile of mature compost. Microb Ecol 57(3):522–533. https://doi.org/10.1007/s00248-008-9424-5
Thongklang N, Luangharn T (2016) Testing agricultural wastes for the production of Pleurotus ostreatus. Mycosphere 7:766–772. https://doi.org/10.5943/mycosphere/7/6/6
Torres-Ruiz E, Sanchez JE, Guillen-Navarro GK, Ramos-Perez DG, Royse DJ (2016) Microbial promoters of mycelial growth, fruiting and production of Pleurotus ostreatus. Sydowia 68:151–161. https://doi.org/10.12905/0380.sydowia68-2016-0151
Turner EM, Wright M, Ward T, Osborne DJ, Self R (1975) Production of ethylene and other volatiles and changes in cellulase and laccase activities during life cycle of cultivated mushroom, Agaricus bisporus. J Gen Microbiol 91(1):167–176. https://doi.org/10.1099/00221287-91-1-167
Vajna B, Nagy A, Sajben E, Manczinger L, Szijarto N, Kadar Z, Bordas D, Marialigeti K (2010) Microbial community structure changes during oyster mushroom substrate preparation. Appl Microbiol Biotechnol 86(1):367–375. https://doi.org/10.1007/s00253-009-2371-3
Vajna B, Szili D, Nagy A, Marialigeti K (2012) An improved sequence-aided T-RFLP analysis of bacterial succession during oyster mushroom substrate preparation. Microb Ecol 64(3):702–713. https://doi.org/10.1007/s00248-012-0063-5
Venkateshwarlu C, Chandravadana MV, Tewari RP (1999) Volatile flavour components of some edible mushrooms (Basidiomycetes). Flavour Frag J 14(3):191–194. https://doi.org/10.1002/(SICI)1099-1026(199905/06)14:3<191::AID-FFJ810>3.0.CO;2-7
Venturini ME, Reyes JE, Rivera CS, Oria R, Blanco D (2011) Microbiological quality and safety of fresh cultivated and wild mushrooms commercialized in Spain. Food Microbiol 28(8):1492–1498. https://doi.org/10.1016/j.fm.2011.08.007
Vos AM, Heijboer A, Boschker HTS, Bonnet B, Lugones LG, Wosten HAB (2017) Microbial biomass in compost during colonization of Agaricus bisporus. AMB Express 7(1):7. https://doi.org/10.1186/s13568-016-0304-y
Waksman SA, Nissen W (1932) On the nutrition of the cultivated mushroom, Agaricus campestris, and the chemical changes brought about by this organism in the manure compost. Am J Bot 19(6):514–537. https://doi.org/10.2307/2436074
Wang WD, Yan L, Cui ZJ, Gao YM, Wang YJ, Jing RY (2011) Characterization of a microbial consortium capable of degrading lignocellulose. Bioresour Technol 102(19):9321–9324. https://doi.org/10.1016/j.biortech.2011.07.065
Wang L, Mao JG, Zhao HJ, Li M, Wei QS, Zhou Y, Shao HP (2016) Comparison of characterization and microbial communities in rice straw- and wheat straw-based compost for Agaricus bisporus production. J Ind Microbiol Biotechnol 43(9):1249–1260. https://doi.org/10.1007/s10295-016-1799-6
Weil JD, Cutter CN, Beelman RB, LaBorde LF (2013) Inactivation of human pathogens during phase II composting of manure-based mushroom growth substrate. J Food Prot 76(8):1393–1400. https://doi.org/10.4315/0362-028x.jfp-12-508
Wells JM, Sapers GM, Fett WF, Butterfield JE, Jones JB, Bouzar H, Miller FC (1996) Postharvest discoloration of the cultivated mushroom Agaricus bisporus caused by Pseudomonas tolaasii, P. reactans, and P. gingeri. Phytopathology 86(10):1098–1104. https://doi.org/10.1094/Phyto-86-1098
Wiegant WM, Wery J, Buitenhuis ET, Debont JAM (1992) Growth-promoting effect of thermophilic fungi on the mycelium of the edible mushroom Agaricus bisporus. Appl Environ Microbiol 58(8):2654–2659
Wong WC, Fletcher JT, Unsworth BA, Preece TF (1982) A note on ginger blotch - a new bacterial disease of the cultivated mushroom Agaricus bisporus. J Appl Bacteriol 52(1):43–48. https://doi.org/10.1111/j.1365-2672.1982.tb04371.x
Xiang QJ, Luo LH, Liang YH, Chen Q, Zhang XP, Gu YF (2017) The diversity, growth promoting abilities and anti-microbial activities of bacteria isolated from the fruiting body of Agaricus bisporus. Pol J Microbiol 66(2):201–207
Young LS, Chu JN, Young CC (2012) Beneficial bacterial strains on Agaricus blazei cultivation. Pesqui Agropecu Bras 47(6):815–821. https://doi.org/10.1590/S0100-204X2012000600012
Young LS, Chu JN, Hameed A, Young CC (2013) Cultivable mushroom growth-promoting bacteria and their impact on Agaricus blazei productivity. Pesqui Agropecu Bras 48(6):636–644. https://doi.org/10.1590/s0100-204x2013000600009
Zarenejad F, Yakhchali B, Rasooli I (2012) Evaluation of indigenous potent mushroom growth promoting bacteria (MGPB) on Agaricus bisporus production. World J Microbiol Biotechnol 28(1):99–104. https://doi.org/10.1007/s11274-011-0796-1
Zhang X, Zhong YH, Yang SD, Zhang WX, Xu MQ, Ma AZ, Zhuang GQ, Chen GJ, Liu WF (2014a) Diversity and dynamics of the microbial community on decomposing wheat straw during mushroom compost production. Bioresour Technol 170:183–195. https://doi.org/10.1016/j.biortech.2014.07.093
Zhang YQ, Geng W, Shen YQ, Wang YL, Dai YC (2014b) Edible mushroom cultivation for food security and rural development in China: bio-innovation, technological dissemination and marketing. Sustainability 6(5):2961–2973. https://doi.org/10.3390/su6052961
Zhang CH, Huang T, Shen CH, Wang XT, Qi YC, Shen JW, Song AD, Qiu LY, Ai YC (2016) Downregulation of ethylene production increases mycelial growth and primordia formation in the button culinary-medicinal mushroom, Agaricus bisporus (Agaricomycetes). Int J Med Mushrooms 18(12):1131–1140. https://doi.org/10.1615/IntJMedMushrooms.v18.i12.80
Zhu HX, Zuo YT, Qiu CW, Wang XL, Zhang Y, Shen JW, Qiu LY (2013) Promotion of the growth and yield in Pleurotus ostreatus by Bradyrhizobium japonicum. J Pure Appl Microbiol 7:1087–1092
Acknowledgments
We thank Horticulture Innovation Australia and the Australian Mushroom Growers Association for their support of our research in to the microbial diversity of mushroom compost.
Funding
This work was partly supported by grant MU10021 from Horticulture Innovation Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kertesz, M.A., Thai, M. Compost bacteria and fungi that influence growth and development of Agaricus bisporus and other commercial mushrooms. Appl Microbiol Biotechnol 102, 1639–1650 (2018). https://doi.org/10.1007/s00253-018-8777-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-8777-z