Abstract
Agaricus bisporus is the most widely cultivated mushroom species in the world. Cultivation is commenced by inoculating beds of semi-pasteurised composted organic substrate with a pure spawn of A. bisporus. The A. bisporus mycelium subsequently colonises the composted substrate by degrading the organic material to release nutrients. A layer of peat, often called “casing soil”, is laid upon the surface of the composted substrate to induce the development of the mushroom crop and maintain compost environmental conditions. Extensive research has been conducted investigating the biochemistry and genetics of A. bisporus throughout the cultivation process; however, little is currently known about the wider microbial ecology that co-inhabits the composted substrate and casing layers. The compost and casing microbial communities are known to play important roles in the mushroom production process. Microbial species present in the compost and casing are known for (1) being an important source of nitrogen for the A. bisporus mycelium, (2) releasing sugar residues through the degradation of the wheat straw in the composted substrate, (3) playing a critical role in inducing development of the A. bisporus fruiting bodies and (4) acting as pathogens by parasitising the mushroom mycelium/crop. Despite a long history of research into the mushroom cropping process, an extensive review of the microbial communities present in the compost and casing has not as of yet been undertaken. The aim of this review is to provide a comprehensive summary of the literature investigating the compost and casing microbial communities throughout cultivation of the A. bisporus mushroom crop.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agaricus bisporus is the most widely cultivated mushroom crop in the world, with an estimated annual worth of up to US$ 4.7 billion as of 2009 (Sonnenberg et al. 2011). Commercial cultivation of A. bisporus crops is performed worldwide, with production mostly concentrated in North America, Europe, India and China (Kabel et al. 2017). Currently, cultivation of A. bisporus is dominated by a single hybrid strain produced in the Netherlands in the 1980s by cross-breeding the Horst-U1 and U3 strains (Savoie et al. 2013). This lack of genetic diversity in A. bisporus cultivars has resulted in worldwide A. bisporus production facing similar threats from pathogenic species, which appear to originate from the mushroom growing medium (Largeteau and Savoie 2010). Despite this, in-depth investigations of the microbial communities co-habiting the growing medium have, until recently, been relatively unexplored. However, in the past few years studies using advanced techniques for characterising microbial communities, such as molecular fingerprinting techniques, next generation sequencing (NGS) and phospholipid fatty acid analysis (PLFA), have revealed new insights into the microbial dynamics present in the mushroom growing medium (McGee et al. 2017a; McGee et al. 2017b; Siyoum et al. 2016; Szekely et al. 2009; Pecchia et al. 2014; Vos et al. 2017a; Zhang et al. 2014). The aim of this review is to summarise the current understanding of the activities and dynamics of the microbial communities inhabiting the mushroom growing medium throughout the mushroom cropping process.
Preparation of the mushroom growing medium and the mushroom cropping process
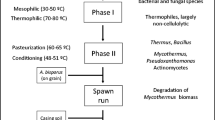
Large-scale commercial cultivation of A. bisporus using pure inoculants was first performed by the Pasteur Institute in 1894 using substrates of partially fermented horse manure and straw from stables (Van Griensven and Van Roestel 2004). Modern cultivation of A. bisporus is predominantly performed using a substrate of semi-pasteurised composted organic material, the main components of which are generally gypsum, manure (horse or chicken) and wheat straw (Straatsma et al. 1994). The growing medium substrate is a mixture that is optimal for growth and production of the A. bisporus crop. Typically, the composted substrate is covered by a layer of “casing” soil, which is usually peat-based, prior to inducing production of the mushroom crop (Berendsen et al. 2012). The mushroom composted substrate undergoes three distinct phases during its production and preparation prior to being used for producing the mushroom crop (Kabel et al. 2017). Phase 1 of mushroom compost production is a short composting process of the raw ingredients usually performed outdoors in windrow stacks (Derikx et al. 1990). In phase 2 of production, the mushroom compost is conditioned by heat treatment to enable the development of thermophilic microbial communities and to remove insects prior to A. bisporus inoculation (Mouthier et al. 2017; Zhang et al. 2014). Phase 3, sometimes known as the “spawn run”, involves the inoculation of phase 2 compost, with an inoculation/spawn of A. bisporus, which subsequently colonises the substrate (Van Griensven and Van Roestel 2004). Upon completion of phase 3, the colonised compost is generally transferred to a mushroom production house/unit where the compost surface is covered with a peat-based casing layer (Sharma and Kilpatrick 2000). The initial mushroom crop or “flush” is induced by controlling environmental conditions such as temperature, CO2 and moisture inside the cropping house (Sharma et al. 2005). A graphical representation of the colonisation of the mushroom growing medium is presented in Fig. 1. The mushroom cropping process consists of harvesting a series of two to three flushes from the compost bed before the substrate is considered spent (spent mushroom compost: SMC), and the process is terminated (Royse et al. 2008). Successive mushroom crop flushes produce diminishing crop yields, despite the substantial levels of nutrients remaining in the compost substrate (Pecchia et al. 2014). A substantial body of research has been conducted into investigating the capacity of the A. bisporus mycelium to release carbohydrate nutrients from the straw fibres present in the compost substrate. The genetics and biochemistry relating to carbohydrate degradation of A. bisporus has been extensively reviewed in Kabel et al. (2017). This review will focus on the activity and composition of the wider microbial community present in the mushroom growing medium.
Stages of colonisation of the mushroom growing medium by Agaricus bisporus. Stage 1: Phase 2 semi-pasteurised compost is inoculated with a pure spawn of A. bisporus. Stage 2: Colonised phase 3 compost is transferred to a mushroom growing facility where the casing layer is applied to the surface commencing the initiation of the mushroom cropping process. Stage 3: The compost A. bisporus mycelium colonises the casing layer i.e. Spawn run. Stage 4: A. bisporus mycelium in casing layer condenses into “pins” which develop into primordia. Stage 5: Primordial pins develop into full mushroom fruiting bodies for harvesting in stage 6
Scope of review
Mushroom compost and casing is a very heterogeneous environment which is inhabited by a broad range of microbial species including archaea (Derikx et al. 1989), bacteria (McGee et al. 2017b), fungi (McGee et al. 2017a) and viruses (Eastwood et al. 2015). Regardless of this, investigations of microbial communities in the mushroom cropping process have generally been limited to bacteria and fungi. Therefore, the scope of this review is limited to focusing on the bacterial and fungal communities of the compost and casing substrates, as the larger body of work conducted in these areas allows for determination of certain distinguishing trends. This review also considers studies which report compost and casing enzyme activities that reflect activity of the whole microbial community present. The review aims to address the microbial commutes present during the mushroom cropping process only, and does not cover the earlier stages of mushroom growing medium production. A list of the relevant mushroom cropping process microbial ecology literature is presented in Table 1. A substantial body of literature has been published on microbial pathogens of the A. bisporus crop; these have been well reviewed in Largeteau and Savoie (2010). This review only refers to known microbial pathogens when it is pertinent, and reported in surveys of microbial communities in mushroom compost and casing.
Enzymatic activity of the mushroom compost microbial community
Microbial degradation of straw fibres in the mushroom compost is critical in releasing carbohydrate nutrition that feeds the A. bisporus mycelium (Savoie 1998). However, large amounts of lignin and hemicellulose have been found to remain in the spent mushroom compost after the mushroom cropping process has been completed (Jurak et al. 2015; Vos et al. 2017b). As a result of this, a large body of work has been conducted investigating the microbial community enzymatic activities involved in degrading the complex carbohydrates in the mushroom compost substrate (Bonnen et al. 1994; Jurak et al. 2015; Savoie 1998; Vos et al. 2017b), though little or no work has reported enzyme activities in the casing substrate. The carbohydrate-degrading enzymes which have been most commonly studied are (1) the lignin-degrading enzymes laccase and manganese peroxidise (MnP), and (2) the hemicellulose-degrading enzymes hemicellulase, xylanase and β-xylanase (Bonnen et al. 1994; Jurak et al. 2015; Kabel et al. 2017; Savoie 1998; Vos et al. 2017a).
Investigations of enzyme activities present in the compost layer indicate that activity of the A. bisporus mycelium dominates in the degradation of lignin straw residues. The study of Savoie (1998) indicated that laccase and MnP activities in the compost fraction were associated with A. bisporus inoculation, as activities of these enzymes were below limits of detection (LOD) in the microbial community present in uninoculated mushroom compost. The absence of laccase activity in uninoculated mushroom compost was supported by the study of Vos et al. (2017a). There are indications that the degradation of mushroom compost lignin by MnP activity is limited by low levels of the co-factor H2O2 in the mushroom compost (Vos et al. 2017b). The results from these studies indicate that the wider microbial community plays little or no part in the degradation of lignin in mushroom cropping compost. Fungi are known to be more active decomposers of lignin than bacteria (Brown and Chang 2014). It is probable that the phase 1 composting process and phase 2 semi-pasteurisation effectively suppress fungi capable of degrading lignin in the mushroom compost substrate which may compete with A. bisporus in phase 3 and the mushroom cropping process.
In distinct contrast to lignin degradation, production of xylanase enzymes for degrading hemicellulose in mushroom compost appears to be highly associated with the wider mushroom compost microbial community in addition to A. bisporus. The study of Savoie (1998) found xylanase and β-xylanase activities to be higher in uninoculated mushroom compost, compared to mushroom compost inoculated with a spawn of A. bisporus. Their study found that after the application of a casing layer on the 14th day of the spawn run period, xylanase and β-xylanase activities markedly decreased in the A. bisporus-inoculated mushroom compost compared to the uninoculated mushroom compost. Xylanase activity of the A. bisporus mycelium appears to decrease throughout the mushroom cropping process, with lower xylanase activity observed in successive mushrooms flushes (Patyshakuliyeva et al. 2013, 2015). This may be as a result of the A. bisporus mycelium lacking the ability to degrade the highly substituted xylan residues that build up in the mushroom compost as the cropping process progresses (Jurak et al. 2015).
Distinguishing the difference between enzyme activity of the A. bisporus mycelium and the wider microbial community is difficult. Few studies have investigated comparisons between inoculated and uninoculated mushroom compost. The study of Savoie (1998) indicated that in addition to xylanase activity, the wider microbial community in uninoculated mushroom compost had higher potential to produce endocellulases and laminarases for degrading cellulose fibres in the mushroom compost. The wider microbial community therefore clearly contributes substantially to the release of carbohydrate nutrients through the degradation of the straw fibres in the mushroom compost.
Microbial ecology of the compost substrate
Compost fungal community
The mushroom compost substrate has been shown to be dominated by the A. bisporus mycelium throughout the cropping process (Vos et al. 2017a). PLFA conducted on mushroom compost found the A. bisporus mycelium to make up 6.8% w/w of the mushroom compost after complete colonisation, though less than half of the mycelium was found to be active (Vos et al. 2017a). This work was supported in the study of McGee et al. (2017a) which investigated fungal activity based on cDNA internal transcribed spacer (ITS) transcripts. Activity of the A. bisporus mycelium was found to dominate the fungal compost community up to the first mushroom crop flush (> 90%), before decreasing substantially thereafter to generally < 30%, with activity peaking slightly during the second and third mushroom crop flushes (McGee et al. 2017a). The decrease in activity of the A. bisporus mycelium resulted in the fungal community becoming dominated by other fungal species, particularly the pathogen Lecanicillium fungicola and an unidentified fungal species (McGee et al. 2017a). Fungal pathogens affecting the A. bisporus mycelium, such as L. fungicola, Trichoderma aggressivum, Mycogone perniciosa and Cladobotryum dendroides are typically associated with the later flushes of the mushroom cropping process (Largeteau and Savoie 2010). The decreased activity of the A. bisporus mycelium after the first flush may be a critical factor allowing fungal pathogens to gain an increased niche in the compost environment and an opportunity to attack the mushroom fruiting crop and mycelium.
In-depth studies investigating the composition of the fungal mushroom compost community have been limited to date. The study of Zhang et al. (2014) used NGS to target the DNA-derived compost fungal community at one point during the mushroom cropping process, in a survey aimed at phase 1, 2 and 3 and mushroom cropping. Their study detected only a single unclassifiable fungal species in addition to A. bisporus, in the mushroom compost substrate at the budding stage of the first flush of the mushroom crop. Isolation of fungal species using culturing techniques in the study of Siyoum et al. (2016) identified a greater diversity of fungi in mushroom compost, particularly yeast species. The study of Siyoum et al. (2016) isolated two species of Penicillium (meleafrinum and brevicompactum) from the compost along with five yeasts: Cystofilobasidium infirmominiatum, Rhodotorula mucilaginosa, Candida glaebosa, Trichosporon moniliforme and Trichosporon cutaneum from two sampling points (pinning and first flush). However, the isolation of cultivatable fungal species does not indicate whether the species are active or dormant. The study of McGee et al. (2017a) identified a broad diversity of fungal species (211) in the mushroom cropping compost over 11 sampling points using NGS to target the DNA-derived community. However, analysis of the cDNA-derived community identified only 51 active species over the same period, indicating that the majority of species detected in the DNA-derived community were dormant and possibly legacy species from phases 1, 2 and 3. Determining microbial communities using culturing and DNA-derived methodologies has the limitation of not distinguishing between dormant species and species that are active when the substrate is sampled. Interestingly, the DNA-derived community was found to be dominated by Ascomycota species, with only one other Basidiomycete species detected in addition to A. bisporus. In contrast to this, the active cDNA-derived community was dominated by a mixture of Ascomycota and Basidomycota (McGee et al. 2017a). Some similarities were observed between the active fungal species identified from the cDNA-derived community and the fungal species isolated in the study of Siyoum et al. (2016) such as the presence of yeast species such as Trichosporon cutaneum, Candida and Rhodotorula. The mushroom cropping compost has a high level of extractable simple sugars (Jurak et al. 2015) and tends to be quite moist at > 60% (McGee et al. 2017b), both factors which are highly associated with yeast-rich environments (Nagy et al. 2017). Yeast species are known to be highly active in thermophilic composting processes; however, little is currently known about their influence in the mushroom compost cropping process.
Certain fungal pathogens which attack the A. bisporus mycelium, such as Trichoderma aggressivum which causes the green mould disease, are known to typically originate from the mushroom compost (Samuels et al. 2002). However, reports on the compost fungal community have rarely detected the presence of this pathogen. It is possible that published studies on the microbial community have tended to use pathogen-free or relatively free cropping cycles for the basis of their studies.
Compost bacterial community
While growth of the A. bisporus mycelium may dominate the mushroom growing medium, the bacterial community present in the mushroom cropping compost is known to be highly diverse (McGee et al. 2017a; Siyoum et al. 2016; Szekely et al. 2009; Vos et al. 2017a; Zhang et al. 2014). Several studies have indicated that bacterial diversity is higher than fungal diversity in the compost substrate throughout the mushroom cropping process (Siyoum et al. 2016; Zhang et al. 2014). Colonisation of the mushroom compost by the A. bisporus mycelium has been shown to affect the bacterial community structure throughout the cropping process (Cai et al. 2009). A comparison between A. bisporus-inoculated and uninoculated mushroom compost found abundance of the bacterial community to be significantly reduced by the presence of the A. bisporus mycelium (Vos et al. 2017a). Studies have shown that the A. bisporus mycelium feeds on bacteria present in the mushroom compost utilising them as a source of nitrogen (Fermor and Wood 1981; Fermor et al. 1991). There is some indication that gram-negative bacteria may be more susceptible to the A. bisporus mycelium than gram-positive bacteria. Gram-positive compost bacterial relative abundance has been shown to increase in the compost substrate compared to gram-positive bacteria (Vos et al. 2017a). However, an NGS characterisation of the DNA-derived bacterial community at budding of the first flush of mushrooms found the bacterial community to be dominated by gram-negative Actinobacteria such as Streptomyces (60%), Ilumatobacter (8%), Microbispora (2%), Saccharopolyspora (2%) and an unknown gram-negative bacterial species (19%) (Zhang et al. 2014). NGS characterisation of the DNA and cDNA-derived bacterial communities after sampling 11 points throughout the mushroom cropping process has indicated that there is a highly dynamic community present in the compost substrate (McGee et al. 2017b). Characterisation of the bacterial community using NGS indicated that the mushroom compost is dominated by Actinobacteria, Firmicutes, Proteobacteria, Planctomycetes and Deinococcus-Thermus (McGee et al. 2017b). In total, 273 and 274 species exceeding 0.1% relative abundance were identified in the DNA and cDNA-derived bacterial communities, respectively. A substantial change in the composition of the bacterial compost community structures was found to occur after the first flush of mushrooms (McGee et al. 2017b). As the mushroom cropping process progressed, abundance of the Deinococcus-Thermus and Proteobacteria was found to decrease in relative abundance as the Actinobacteria and Firmicutes inversely increased (McGee et al. 2017b).
Culturing techniques have shown thermophilic bacterial species to play prominent roles in phase 1 composting, but were found to be below LOD or become dormant at the mesophilic temperatures of phase 3 (Szekely et al. 2009). Inoculation of phase 1 mushroom compost with thermophilic bacteria capable of degrading lignocellulosic material has been shown to improve the efficacy of the composting process (Ahlawat and Vijay 2010). Thermophilic bacteria of the phyla Deinococcus-Thermus and Thermodesulfobacteria were shown to be highly active in the mushroom cropping compost until the first flush of the mushroom crop, based on cDNA transcripts (McGee et al. 2017b). However, little is known about the possible role thermophilic bacteria may play in the mushroom compost throughout the cropping process.
The study of McGee et al. (2017b) found bacteria with nitrifying properties, such as Rhizobium and Stenotrophomonas, to become more active in the mushroom compost after the first flush. The increase in activity of nitrifying bacteria was found to correlate with increased levels of extractable nitrate in the mushroom compost, which steadily increased until the termination of the cropping process. Interestingly, the addition of cyanobacteria with nitrifying properties, such as Nostoc, to mushroom compost during the cropping process has been shown to increase crop yield (Riahi et al. 2011). Spent mushroom compost (SMC) has been shown to be a good source of nitrogen for plants when added to soil-based systems (Hackett 2015; Stewart et al. 1998). This may possibly be a result of the increasing levels of extractable nitrate production in compost media throughout the cropping process; however, more research is needed to establish whether nitrification is a consistent property related to the mushroom compost substrate during the cropping process.
The study of Siyoum et al. (2016) found cultivatable compost bacterial isolates to be dominated by three species of Proteobacteria—Citrobacter kosen, Serratia marcescens, Pseudomonas putida—and one Actinobacteria, Arthrobacter arilaitensis, though sampling was limited to two points in the mushroom cropping process. In contrast to this, the bacterial community they characterised using denaturing gradient gel electrophoresis (DGGE) targeting extracted DNA identified a much more diverse community. In the Siyoum et al. (2016) study DGGE identified Bacteroidetes (Flavisolibacter sp., Rhodothermus obamensis) and Firmicutes (Bacillus badius, Cellulomonas sp., Microbispora sp. and Blastococcus sp.), in addition to Proteobacteria (Bordetella sp., Luteimonas sp. and Pseudomonas sp.). The study of McGee et al. (2017b) found Pseudomonas species to be present throughout the mushroom cropping process, with activity appearing to peak during the first flush of mushrooms. The presence of certain Pseudomonas species in the peat casing layer have been shown to aid in inducing the development of the mushroom crop; however, other species of Pseudomonas such as P. gingeri, P. aeruginosa, P. fluorescens and P. tolaasii are known to be pathogens of the fruiting mushroom cap (Largeteau and Savoie 2010).
Microbial ecology of the casing layer
Casing fungal community
The fungal casing community has rarely been studied in detail. The majority of studies investigating fungal species associated with the mushroom casing layer generally relate to pathogenic species attacking the A. bisporus mycelium and fruiting bodies. Fungal pathogens which have been observed to colonise the mushroom casing layer and attack fruiting mushroom bodies include C. dendroides (Carrasco et al. 2017), L. fungicola (Berendsen et al. 2010), M. perniciosa (Fu et al. 2016) and Trichoderma spp. (Samuels et al. 2002) all of which belong to the phylum Ascomycota. The occurrence and epidemiology of these pathogens been extensively reviewed in Largeteau and Savoie (2010).
The study of Cai et al. (2009) characterised the abundance of microbial casing communities through the cropping process until the second flush of a mushroom crop. Their study found the fungal casing community to have a higher abundance than the bacterial casing community throughout the cropping process. The fungal casing community abundance was found to increase almost exponentially until the inter-flush period between the first and second mushroom flushes, and was most likely dominated by A. bisporus. However, upon completion of the first mushroom flush, the ratio of fungal to bacterial biomass was found to decrease during the second flush primordia formation period and into the second mushroom flush.
To date, the fungal community present in the mushroom casing layer has been best characterised in the study of Siyoum et al. (2016). Their study characterised fungal isolates cultivated from the mushroom casing layer during the cropping process. Similar to their findings in the mushroom compost layer, they found a broad selection of fungal yeast species belonging to the phylum Ascomycota; three Candida spp. and one Pichia sp. In addition to A. bisporus, several filamentous fungal species were identified in the casing layer, which also belonged to the phylum Ascomycota: Bionectria sp., Chaetomium sp., Chromelosporium sp., Pseudallescheria sp., Trichoderma sp. and three Penicillium spp.
Similar to the compost substrate, the majority of fungal species in the casing layer appear to belong to the phylum Ascomycota. This is a somewhat perplexing property, given the optimisation of the growing medium to favour a basidiomycete species. Knowledge of the casing community is severely limited at present due to a lack in studies and sufficient sampling throughout the mushroom cropping process. In order to more fully understand how and why these pathogens or potential competitors for nutritional resources come to establish themselves in the casing layer, a more complete knowledge of the casing fungal community dynamics is required in future studies.
Casing bacterial community
Characterisation of bacterial casing community structures has received substantially more attention than the fungal community. The bacterial casing community abundance has been shown to be lower than fungal casing community abundance throughout the cropping process (Cai et al. 2009). However, bacterial abundance, as measured in PLFAs, has been shown to increase in the casing layer during primordia formation of the first and second mushroom flushes, decreasing during other stages of mushroom growth and development (Cai et al. 2009). The study of Cai et al. (2009) indicated that gram-negative bacteria were in higher abundance than gram-positive bacteria throughout a mushroom cropping process (Cai et al. 2009).
The study of Pecchia et al. (2014) investigated the bacterial casing community throughout the cropping process using NGS and determined that bacteria were dominated by species from four phyla: Actinobacteria, Bacteriodetes, Firmicutes and Proteobacteria. Their study indicated that as the mushroom cropping process progressed, relative abundance of the Actinobacteria (Actinomycetales and Coriobacteriales) and Firmicutes (Bacillales and Clostridiales) increased successively after both the first and second mushroom flushes. Relative abundance of the Proteobacteria (Rhizobiales, Caulobacteriales, Sphingomonadeles; Burkholderiales; Xanthomonadales and Pseudomonadales) decreased after the first mushroom flush, while relative abundance of the Bacteriodetes (Flavobacteriales and Sphingobacteriales) increased between the first and second flush, but decreased between the second flush and third flush. The study of Siyoum et al. (2016) identified cultivatable bacterial isolates from the same four phyla detected in the study of Pecchia et al. (2014): Actinobacteria (Rhodococcus sp. and Microbacterium sp.), Bacteriodetes (Terrimonas sp. and Sphingobacterium sp.), Firmicutes (Trichococcus collinsii) and Protobacteria (Acidovorax temperans, Aminobacter sp., Bosea thiooxidans, Devosia hwasunensis, Mesorhizobium sp., Paracoccus koreensis, Pseudaminobacter salicylatoxidans, Rhizobium sp. and Shinella kummerowiae) in the casing layer throughout a mushroom cropping process. However, when the bacterial casing community was characterised using DGGE, only Proteobacteria were identified such as Alpha-Proteobacteria (Chelativorans multitrophicus, Rhizobium sp., Shinella sp. and Sphingomonas sp.), Beta-Proteobacteria (Curvibacter sp.) and four Pseudomonas of the Gamma-Proteobacteria (P. nitroreducens, P. plecoglossicida, P. putida and P. reactans). The study of Choudhary (2011) also found cultivable species of bacteria in casing to be dominated by strains of Acinetobacter and Pseudomonas throughout the mushroom cropping process.
The Pseudomonadales were observed to decrease throughout the mushroom cropping process monitored in the Pecchia et al. (2014) study. The Pseudomonadales contain the genera Pseudomonas, which play influential roles in the development of mushroom fruiting bodies cropping process (Noble et al. 2003). It has been indicated that Pseudomonas species in the casing layer can aid in the development of the mushroom crop by metabolising volatile compounds that are inhibitory to the formation of A. bisporus primordia (Noble et al. 2009). Pseudomonas species are also known to cause numerous cavity diseases on mushroom fruiting bodies (Jolivet et al. 1998).
Conclusions
The fungal community within the mushroom growing medium is highly dynamic and responsive to the stages of the mushroom cropping process. The A. bisporus mycelium dominates the mushroom cropping substrate (Vos et al. 2017a), but also appears to have significantly reduced activity after the first flush of mushrooms, based on compost laccase activity (Savoie 1998; Vos et al. 2017a) and cDNA synthesis (McGee et al. 2017a). The reduced A. bisporus activity after the first flush of mushrooms results in increased activity of other fungal species within the compost substrate, and possibly facilitates the development of some fungal pathogens (McGee et al. 2017a). Apart from the basidiomycete A. bisporus, most fungal species found in the mushroom growing medium tend to belong to the phylum Ascomycota (McGee et al. 2017a; Siyoum et al. 2016), particularly the pathogenic species associated with the mushroom cropping process (Largeteau and Savoie 2010). A considerable amount of yeast species have been identified in the mushroom substrate, though as of yet their influence on the cropping process is unknown.
The abundance (Vos et al. 2017a) and structure (McGee et al. 2017b) of the compost bacterial community appear to be very responsive to growth of the A. bisporus mycelium throughout the mushroom cropping process. The compost and casing bacterial communities are very diverse and predominantly composed with members of the phyla Actinobacteria, Bacteriodetes, Firmicutes and Proteobacteria (Pecchia et al. 2014; McGee et al. 2017b; Siyoum et al. 2016). Members of the Pseudomonadales tend to be frequently detected in both compost and casing (Choudhary 2011; McGee et al. 2017b; Siyoum et al. 2016; Pecchia et al. 2014) and are highly associated with development of A. bisporus primordia, fruiting bodies (Noble et al. 2003, 2009) and fruiting body diseases (Jolivet et al. 1998; Largeteau and Savoie 2010).
The understanding of microbial communities in the mushroom cropping process is still far from being fully understood. Though most fungal pathogens of the A. bisporus crop appear to colonise and inhabit the casing layer, no attempt has yet been reported that characterises the fungal casing community using advanced molecular techniques. The archaeal community has still as of yet received no characterisation. In order to more fully understand the influence of microbial community dynamics on the mushroom cropping process, more advanced studies are required, particularly those sampling the cropping process at higher frequencies. The use of advanced molecular techniques will also help facilitate the complexities of the microbial communities influencing the A. bisporus mushroom cropping process.
References
Ahlawat OP, Vijay B (2010) Potential of thermophilic bacteria as microbial inoculant for commercial scale white button mushroom (Agaricus bisporus) compost production. J Sci Ind Res 69:948–955
Berendsen RL, Baars JJP, Kalkhove SIC, Lugones LG, Wosten HAB, Bakker PAHM (2010) Lecanicillium fungicola: causal agent of dry bubble disease in white-button mushroom. Mol Plant Pathol 11(5):585–595. https://doi.org/10.1111/j.1364-3703.2010.00627.x
Berendsen RL, Kalkhove SIC, Lugones LG, Wosten HAB, Bakker PAHM (2012) Germination of Lecanicillium fungicola in the mycosphere of Agaricus bisporus. Environ Microbiol Rep 4(2):227–233. https://doi.org/10.1111/j.1758-2229.2011.00325.x
Bonnen AM, Anton LH, Orth AB (1994) Lignin-degrading enzymes of the commercial button mushroom, Agaricus bisporus. Appl Environ Microbiol 60(3):960–965
Brown M, Chang MCY (2014) Exploring bacterial lignin degradation. Curr Opin Chem Biol 19:1–7. https://doi.org/10.1016/j.cbpa.2013.11.015
Cai WM, Yao HY, Feng WL, Jin QL, Liu YY, Li NY, Zheng Z (2009) Microbial community structure of casing soil during mushroom growth. Pedosphere 19(4):446–452. https://doi.org/10.1016/S1002-0160(09)60137-5
Carrasco J, Navarro MJ, Gea FJ (2017) Cobweb, a serious pathology in mushroom crops: a review. Span J Agric Res 15(2):e10R01. https://doi.org/10.5424/sjar/2017152-10143
Choudhary DK (2011) First preliminary report on isolation and characterization of novel Acinetobacter spp. in casing soil used for cultivation of button mushroom, Agaricus bisporus (Lange) Imbach. Int J Microbiol 2011:1–6. https://doi.org/10.1155/2011/790285
Derikx PJL, de Jong GAH, Op den Camp HJM, Van der Drift C, Van Griensven LJLD, Vogels GD (1989) Isolation and charactelization of thermophilic methanogenic bacteria from mushroom compost. FEMS Microbiol Ecol 62(4):251–258. https://doi.org/10.1111/j.1574-6968.1989.tb03699.x
Derikx PJL, Op den Camp HJM, Van der Drift C, Van Griensven LJLD, Vogels GD (1990) Biomass and biological activity during the production of compost used as a substrate in mushroom cultivation. Appl Environ Microbiol 56(10):3029–3034
Eastwood D, Green J, Grogan H, Burton K (2015) Viral agents causing brown cap mushroom disease of Agaricus bisporus. Appl Microbiol Ecol 81(20):7125–7134
Fermor TR, Wood DA (1981) Degradation of bacteria by Agaricus bisporus and other fungi. J Gen Microbiol 126:377–387
Fermor TR, Wood DA, Lincoln SP, Fenlon JS (1991) Bacteriolysis by Agaricus bisporus. J Gen Microbiol 137(1):15–22. https://doi.org/10.1099/00221287-137-1-15
Fu Y, Wang X, Li D, Liu Y, Song B, Zhang C, Wang Q, Chen M, Zhang Z, Li Y (2016) Identification of resistance to wet bubble disease and genetic diversity in wild and cultivated strains of Agaricus bisporus. Int J Mol Sci 17(10):1568. https://doi.org/10.3390/ijms17101568
Hackett R (2015) Spent mushroom compost as a nitrogen source for spring barley. Nutr Cycl Agroecosys 102(2):253–263. https://doi.org/10.1007/s10705-015-9696-3
Jolivet S, Arpin N, Wichers HJ, Pellon G (1998) Agaricus bisporus browning: a review. Mycol Res 102(12):1459–1483. https://doi.org/10.1017/S0953756298006248
Jurak E, Patyshakuliyeva A, de Vries RP, Gruppen H, Kabel MA (2015) Compost grown Agaricus bisporus lacks the ability to degrade and consume highly substituted xylan fragments. PLoS One 10(8):e0134169. https://doi.org/10.1371/journal.pone.0134169
Kabel MA, Jurak E, Makela MR, de Vries RP (2017) Occurrence and function of enzymes for lignocellulose degradation in commercial Agaricus bisporus cultivation. Appl Microbiol Biotechnol 101(11):4363–4369. https://doi.org/10.1007/s00253-017-8294-5
Largeteau ML, Savoie JM (2010) Microbially induced diseases of Agaricus bisporus: biochemical mechanisms and impact on commercial mushroom production. Appl Microbiol Biotechnol 86(1):63–73. https://doi.org/10.1007/s00253-010-2445-2
McGee CF, Byrne H, Irvine A, Wilson J (2017a) Diversity and dynamics of the DNA and cDNA-derived compost fungal communities throughout the commercial cultivation process for Agaricus bisporus. Mycologia 109(3):475–484. https://doi.org/10.1080/00275514.2017.1349498
McGee CF, Byrne H, Irvine A, Wilson J (2017b) Diversity and dynamics of the DNA and cDNA-derived bacterial compost communities throughout the Agaricus bisporus mushroom cropping process. Ann Microbiol 67(11):751–761. https://doi.org/10.1007/s13213-017-1303-1
Mouthier TMB, Kilic B, Vervoort P, Gruppen H, Kabel MA (2017) Potential of a gypsum-free composting process of wheat straw for mushroom production. PLoS One 12(10):e0185901. https://doi.org/10.1371/journal.pone.0185901
Nagy LG, Tóth R, Kiss E, Slot J, Gácser A, Kovács GM (2017) Six key traits of fungi: their evolutionary origins and genetic bases. Microbiol Spectr 5(4): FUNK-0036-2016
Noble R, Fermor TR, Lincoln S, Dobrovin-Pennington A, Evered C, Mead A, Li R (2003) Primordia initiation of mushroom (Agaricus bisporus) strains on axenic casing materials. Mycologia 95(4):620–629. https://doi.org/10.1080/15572536.2004.11833066
Noble R, Dobrovin-Pennington A, Hobbs PJ, Pederby J, Rodger A (2009) Volatile C8 compounds and pseudomonads influence primordium formation of Agaricus bisporus. Mycologia 101(5):583–591. https://doi.org/10.3852/07-194
Patyshakuliyeva A, Jurak E, Kohler A, Baker A, Battaglia E, de Bruijn W, Burton KS, Challen MP, Coutinho PM, Eastwood DC, Gruben BS, Mäkelä MR, Martin F, Nadal M, van den Brink J, Wiebenga A, Zhou M, Henrissat B, Kabel M, Gruppen H, de Vries RP (2013) Carbohydrate utilization and metabolism is highly differentiated in Agaricus bisporus. BMC Genomics 14(1):663
Patyshakuliyeva A, Post H, Zhou M, Jurak E, Heck AJ, Hildén KS, Kabel MA, Mäkelä MR, Altelaar MA, de Vries RP (2015) Uncovering the abilities of Agaricus bisporus to degrade plant biomass throughout its life cycle. Environ Microbiol 17(8):3098–3109. https://doi.org/10.1111/1462-2920.12967
Pecchia J, Cortese R, Albert I (2014) Investigation into the microbial community changes that occur in the casing layer during cropping of the white button mushroom, Agaricus bisporus. In: Singh M (ed) Proceedings of the 8th International Conference on Mushroom Biology and Mushroom Products, New Delhi, pp 309–313
Riahi H, Eskash A, Shariatmadari Z (2011) Effect of bacterial and cyanobacterial culture on growth, quality and yield of Agaricus bisporus. Mushroom biology and mushroom products. In: Savoie JM, Foulongne-Oriol M, Largeteau M, Barroso G (eds) Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products, Arcachon, France, Vol 1, pp 406–411
Royse DJ, Sanchez JE, Beelman RB, Davidson J (2008) Re-supplementing and re-casing mushroom (Agaricus bisporus) compost for a second crop. World J Microbiol Biotechnol 24(3):319–325. https://doi.org/10.1007/s11274-007-9473-9
Samuels GJ, Dodd SL, Gams W, Castlebury LA, Petrini O (2002) Trichoderma species associated with the green mold epidemic of commercially grown Agaricus bisporus. Mycologia 94(1):146–170. https://doi.org/10.1080/15572536.2003.11833257
Savoie JM (1998) Changes in enzyme activities during early growth of the edible mushroom, Agaricus bisporus, in compost. Mycol Res 102(9):113–118
Savoie JM, Foulongne-Oriol M, Barroso G, Callac P (2013) Genetics and genomics of cultivated mushrooms, application to breeding of agarics. In: Kempken F (ed) Agricultural applications. The Mycota (a comprehensive treatise on fungi as experimental systems for basic and applied research), vol 11. Springer, Berlin, pp 3–33
Sharma HSS, Kilpatrick M (2000) Mushroom (Agaricus bisporus) compost quality factors for predicting potential yield of fruiting bodies. Can J Microbiol 46(6):515–519. https://doi.org/10.1139/w00-012
Sharma HSS, Kilpatrick M, Lyons G (2005) Monitoring mushroom compost quality during production and cropping. In: Tan Q, Zhang J, Chen M, Cao H, Buswell JA (eds) Fifth International Conference on Mushroom Biology and Mushroom Products, Shanghai Academy of Agricultural Sciences, Shanghai, China, pp 221–235
Sharma A, Singh AV, Johri BN (2013) Functional and genetic characterization of culturable bacteria associated with late phase of mushroom composting assessed by amplified rDNA restriction analysis. Int J Microbiol App Sci 2(6):162–175
Siyoum NA, Surridge K, van der Linde EJ, Korsten L (2016) Microbial succession in white button mushroom production systems from compost and casing to a marketable packed product. Ann Microbiol 66(1):151–164. https://doi.org/10.1007/s13213-015-1091-4
Sonnenberg ASM, Baars JJP, Hendrick PM, Lavrijssen B, Gao W, Weijn A, Mes, JJ (2011) Breeding and strain protection in the button mushroom Agaricus bisporus. In: Savoie JM, Foulongne-Oriol M, Largeteau M, Barroso G (eds) Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products, Genomics, Genetics and Breeding, Arcachon, France, Vol 7(9), pp 7–15
Stewart DPC, Cameroon KC, Cornforth IS (1998) Effects of spent mushroom substrate on soil chemical conditions and plant growth in an intensive horticultural system: a comparison with inorganic fertiliser. Aust J Soil Res 36(2):185–198. https://doi.org/10.1071/S97076
Straatsma G, Samson RA, Olijnsma TW, Camp HJMOD, Gerrits JPG, van Griensven LJLD (1994) Ecology of thermophilic fungi in mushroom compost, with emphasis on Scytalidium thermophilum and growth stimulation of Agaricus bisporus mycelium. Appl Environ Microbiol 60(2):454–458
Szekely AJ, Sipos R, Berta B, Vajna B, Hajdu C, Marialigeti K (2009) DGGE and T-RFLP analysis of bacterial succession during mushroom compost production and sequence-aided T-RFLP profile of mature compost. Microb Ecol 57(3):522–533. https://doi.org/10.1007/s00248-008-9424-5
Van Griensven LJLD, Van Roestel AJJ (2004) The cultivation of the button mushroom Agaricus bisporus in The Netherlands: a successful industry. Rev Mex Micol 19:95–102
Vos AM, Heijboer A, Boschker HTS, Bonnet B, Lugones LG, Wösten HAB (2017a) Microbial biomass in compost during colonization of Agaricus bisporus. AMB Express 7(1):12. https://doi.org/10.1186/s13568-016-0304-y
Vos AM, Jurak E, Pelkmans JF, Herman K, Pels G, Baars JJ, Hendrix E, Kabel MA, Lugones LG, Wösten HAB (2017b) H2O2 as a candidate bottleneck for MnP activity during cultivation of Agaricus bisporus in compost. AMB Express 7(1):124. https://doi.org/10.1186/s13568-017-0424-z
Zhang X, Zhong Y, Yang S, Zhang W, Xu M, Ma A, Zhuang G, Chen G, Liu W (2014) Diversity and dynamics of the microbial community on decomposing wheat straw during mushroom compost production. Bioresour Technol 170:183–195. https://doi.org/10.1016/j.biortech.2014.07.093
Acknowledgements
The author would like to thank Dr. Silvia Saloni and Dr. Noel Faherty for kindly taking the time to review the draft manuscripts during the preparation of this publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Rights and permissions
About this article
Cite this article
McGee, C.F. Microbial ecology of the Agaricus bisporus mushroom cropping process. Appl Microbiol Biotechnol 102, 1075–1083 (2018). https://doi.org/10.1007/s00253-017-8683-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8683-9