Abstract
Although oyster mushroom (Pleurotus spp.) is a valuable food, cultivated worldwide on an industrial scale, still very little is known about the microbial dynamics during oyster mushroom substrate preparation. Therefore, the characterization of the microbial dynamics by chemical and biological tools was the objective of this study. During substrate preparation, enzymatic digestibility of the substrate improved by 77%, whereas the cellulose and hemicellulose to lignin ratios decreased by 9% and 19%, respectively. Fluorescein diacetate hydrolysis reached its minimum value at the temperature maximum of the process during the composting phase and exceeded the initial level at the end of the process. Fungal species played part in the initial mesophilic phase of the substrate preparation process, but they disappeared after pasteurization in tunnels at constant elevated temperatures. Changes in the microbiota showed a marked bacterial community succession during substrate preparation investigated by 16S ribosomal deoxyribonucleic acid-based terminal restriction fragment length polymorphism (T-RFLP). Mature samples represented the least variance, which indicated the effect of the standardized preparation protocol. The relation between mushroom yield and the bacterial community T-RFLP fingerprints was investigated, but the uniformity of mushroom yields did not support any significant correlation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oyster mushroom (Pleurotus spp.) is a valuable food with high protein and vitamin contents and low fat content (Bonatti et al. 2004). It is cultivated around the world: it was the third most popularly cultivated edible mushroom in 1997 after button mushroom (Agaricus bisporus) and shiitake (Lentinula edodes) (Chang and Miles 2004). Most beneficial characteristic of oyster mushroom is its conversion rate of substrate mass into mushroom biomass, which is some of the greatest among cultivated mushrooms. Biological efficiency, the relation between the fresh weight of the mushroom and the dry weight of the compost, often exceed 100% (Stamets 2000). In addition, the production uses a wide array of agricultural and forestry waste products, including straw, corn stalks, sugar cane bagasse, coffee pulp (Stamets 2000), sawdust, paddy straw, waste cotton, stalks, and banana leaves (Chang and Miles 2004). All of these waste products could be used for Pleurotus mushroom production without the need for expensive processing methods and substrate amendments, although, in most cases, industrial scale cultivation applies a more or less sophisticated composition of the abovementioned substrates (Chang and Miles 2004).
Publications on oyster mushroom production deal with the type of utilizable substrates, the suitable concentrations of nitrogen and manganese (Baysal et al. 2003; Curvetto et al. 2002; Baldrian et al. 2005), the physico-chemical parameters, and the productivity of oyster mushroom production (Hernández et al. 2003). Most of these studies were carried out under model conditions using sterilized or pasteurized substrates (Baysal et al. 2003; Curvetto et al. 2002; Salmones et al. 2005; Zhang et al. 2002). However, these processes are expensive and increase the chances of subsequent infections with competitive species. As a consequence, usually longer composting and only short pasteurization without sterilization are applied in industrial scale cultivation.
Microbial dynamics of composting in general has been well studied with various techniques (Klamer and Bååth 1998; Peters et al. 2000; Takaku et al. 2006), but composting procedures are rather different from the faster and better regulated mushroom compost preparation. Song et al. (2001) isolated thermophilic actinomycetes and Thermoactinomyces spp. from different mushroom compost, and performed their phylogenetic analysis. Microbiota of spent mushroom compost was investigated by Ntougias et al. (2004), revealing the dominance of gram-positive bacterial isolates. Microbial changes during the substrate preparation of white button mushroom (Agaricus bisporus) were monitored with molecular fingerprinting methods by Székely et al. (2009). Principal component analysis (PCA) of the fingerprints showed intensive shifts in community composition during substrate preparation and patterns of mature compost were almost identical. They revealed the dominance of a supposedly cellulose-degrading consortium of mature compost composed of phylotypes related to Pseudoxanthomonas, Thermobifida, and Thermomonospora. However, the microbiology of oyster mushroom substrate preparation is lesser-known. The objective of the present study was to investigate the microbial community dynamics during this process. Since the use of culture-dependent methods alone is inadequate to describe bacterial diversity (Amann et al. 1990) and the construction of large clone-libraries is time-consuming and expensive (Hartmann and Wilder 2006), we used terminal restriction fragment length polymorphism (T-RFLP; Liu et al. 1997) as a molecular fingerprinting method, which has already been successfully applied to reveal the bacterial diversity of composting environments (Tiquia et al. 2005; Székely et al. 2009). It is proved to be an appropriate tool for the comparison of communities at a longer time-span, especially when analysis is supplemented with physico-chemical data and fungal cultivation analysis, as in our case.
Materials and methods
Oyster mushroom substrate preparation process
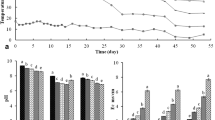
Crude wheat straw (Triticum aestivum), stored in bales after wheat harvest, supplemented with alfalfa (Medicago sativa, 5% w/w of dry straw) was chopped into 1–6-cm pieces, followed by moistening to a moisture content of 75% w/w. The wet straw was kept in heaps (approximately 5 by 3 m in cross-section) for composting for 7 days. The heap was mixed daily; the inside temperature rose to 65–70°C. On day 7, the pre-composted substrate was filled into tunnels. During filling, the substrate cooled down; therefore, it has to be heated to 65°C with aerated steam inside the tunnel (warming-up phase). This process was followed by pasteurization (65°C for 18 h), after which the substrate was cooled down for conditioning (48°C for 48 h). Before the substrate was taken out of the tunnel, it was left to cool to approximately 25°C (Fig. 1). Upon emptying, the substrate was spawned by a spawn applicator with Pleurotus strain HK35 obtained from Sylvan Hungary Ltd. (Dunaharaszti, Hungary; 20 L of spawn per wet substrate ton). The spawned substrate was pressed, wrapped into plastic foil and finally perforated. Wrapped substrate blocks (23–24 kg) were transported to temperature-, humidity-, and light-controlled production houses, where mushroom production was closely monitored until the end of the third flush. Mushroom yield was determined as a percentage of wet weights of kilogram fresh mushroom per 100-kilogram substrate block.
Temperature changes during oyster mushroom substrate preparation. Numbers are depicting the sampling points (stages 1–7 samples), described in Table 1. Vertical line in the middle separates open-air and in-door phases
Sampling
Samples were taken from different substrate production series in 2006 and 2007 designated as: 6-19, 6-55, 6-61, 6-77, 6-93, 6-99, 6-104, 6-110, 7-19, 7-118, 7-138, where the first numbers indicate the year of production and the second numbers stand for production series. Each substrate production series were characterized by seven samples representing the consecutive stages of substrate preparation (Table 1). All samples were composite of three subsamples (approximately 0.5 kg), which were taken from 6–10 m wide apart along the composting heap or the tunnel of the core of the substrate (1–1.5 m depth), and these subsamples were carefully mixed and stored adequately until further analysis.
Physico-chemical analysis of the samples
The composition of the substrate was analyzed using the two-step acid hydrolysis method of Hägglund (1951) as modified by Kaar et al. (1991), and a sequential gravimetric method as described by Goering and van Soest (1970). Briefly, the acid method employed 72% H2SO4 for 1 h at 30°C, then for 1 h at 121°C in the case of the 30-time-diluted reaction mixture. The resulting monosaccharides (glucose, xylose, and arabinose) were quantified from the acid hydrolyzate with HPLC with refracting index detection (Shimadzu, Kyoto, Japan) after separation on an Aminex HPX-87H column (Bio-Rad Laboratories, Hercules, CA) at 63°C using 4 mM H2SO4 as the mobile phase at 0.6 mL/min flow rate. Conversion factor for cellulose to glucose was 162/180, for arabinoxylan to xylose and arabinose was 132/150. The solid residue separated by filtration from the hydrolyzate was reported as Klason lignin. In brief, the gravimetric method employed sequential neutral detergent washing to remove soluble components (lipids, protein, free sugars, and water-soluble minerals), acidic detergent washing to remove hemicellulose, and oxidation with potassium permanganate to remove lignin, leaving cellulose and ash as solid residue. The net ash content was determined from the intact samples after ignition at 550°C for 3 h.
The digestibility of substrate as a function of oyster mushroom substrate preparation was followed through measuring the enzymatic digestibility of samples using Celluclast 1.5 L (mixture of various cellulases from T. reesei) and Novozym 188 (β-glucosidase from A. niger) both obtained from Novozymes (Bagsværd, Denmark). Hydrolysis experiments were carried out in 100-mL capped Pyrex flasks at a final volume of 80 mL with 3% (w/v) dry matter (DM) content in 0.05 M sodium acetate buffer (pH 4.8) using 10 filter paper units per gram of substrate DM Celluclast together with 10 international units per gram of substrate DM Novozym at 50°C with shaking at 180 rpm. The applied enzyme loadings were based on activity determinations according to Ghose (1987) for the overall cellulase activity (filter paper activity) of Celluclast, and Berghem and Pettersson (1974) for the β-glucosidase activity of Novozym. DM was determined by drying the samples at 105°C overnight.
Measurement of microbial activity
Viable microbial activity was monitored using fluorescein diacetate hydrolysis (FDA) during substrate preparation. FDA hydrolysis rate was measured as described by Adam and Duncan (2001) using an incubation period of 30 min at 12°C. FDA hydrolysis values were expressed as milligrams of fluorescein released per gram of sample (DM).
Cultivation based techniques to detect fungal diversity
A 5 g portion of each sample was suspended in 50 ml physiological saline solution and shaken for 10 min at 180 rpm, from which a tenfold dilution series was prepared using physiological saline solution. Fifty-microliter aliquots from the diluted samples were plated on fungal selective YEG medium (in 1 L: glucose, 2.0 g; yeast extract, 2.0 g; Bacto agar, 20.0 g; supplemented with chloramphenicol, 0.1 mg; streptomycin, 0.1 g; and oxytetracyclin, 0.1 g, to suppress bacteria). Plates were incubated at 22–24°C and 45–50°C for up to 1 week to isolate mesophilic and thermotolerant/thermophilic fungi. The number of colony forming units per gram of substrate DM was calculated for total mycota and for different morphotypes. Fungal genera were identified conventionally according to their macroscopic and microscopic features (Barron 1968; Arx 1981; Hanlin 1990). Species level identification was made using ITS sequence comparisons of polymerase chain reaction (PCR) products (using ITS5 forward [5′-GGA AGT AAA AGT CGT AAC AAG G-3′] and ITS4 reverse [5′-TCC TCC GCT TAT TGA TAT GC-3′] primers) according to the techniques described by Wu et al. (2002). In order to check the Trichoderma isolates for the presence of the harmful “green mould” species (Trichoderma aggressivum, T. pleurotum and T. pleuroticola), PCR investigations were performed with specific primers regarding the β-tubulin and the tef1 genes (Chen et al. 1999; Hatvani et al. 2007, Komon-Zelazowska et al. 2007). The nucleotide sequences of the ITS regions were deposited in GenBank with accession numbers described in Table 2.
Molecular fingerprinting techniques to detect bacterial diversity changes
Frozen samples were powderized by quick grinding in liquid nitrogen in a sterile mortar, without allowing the sample to thaw. At each time, three subsamples (300 mg) were transferred into microcentrifuge tubes containing 1 mL of CLS-Y lysis buffer (Bio 101, La Jolla, CA, USA) and 50 mg polyvinylpolypyrrolidone (Sigma-Aldrich, St. Louis, MO, USA) to remove co-extracted humic acids (Cullen and Hirsch 1998). The debris were pelleted with centrifugation at 12,000 × g for 5 min. The crude DNA extract was purified with G-spin For Bacteria, Genomic DNA Extraction Kit (iNtRON Biotechnology, Seongnam, South-Korea) according to the manufacturer's protocol. Extracted DNA from three subsamples was pooled for each sample.
PCR reactions, enzymatic digestions, purification of enzymatic digests, and electrophoresis of labeled fragments were carried out as reported in an earlier study (Székely et al. 2009). The only differences were that the 27F forward primer was hexachlorofluorscein (HEX)-labeled during the PCR, and AluI and Hin6I restriction endonucleases were used for enzymatic digestion.
Analysis of the T-RFLP results was conducted briefly by (1) the selection of the adequate runs, than (2) the alignment of TRFs of parallel samples and different samples, and finally (3) the visualization of the result by PCA. (1) The T-RFLP chromatograms were analyzed with the GeneMapper® Software v3.7 (Applied Biosystems, Foster City, CA, USA). Only runs with total peak areas between 200,000 and 400,000 fluorescent units were accepted. The following criteria were used to define T-RFs: fragment size between 50 and 500 bp and fluorescence intensity higher than 50 fluorescence units (peak height). (2) For consensus profiles, two parallel runs from each sample were aligned with the T-Align program (Smith et al. 2005) using 0.5 bp confidence interval omitting peaks below 0.2% relative abundance. In order to get a more robust result, AluI and Hin6I profiles were combined for each sample and these profiles were aligned with the T-Align program for all samples. (3) The resulted matrix was visualized with PCA. Diversity indices and box-plots were also calculated. As PCA was used here only for visualization, a statistical tool was needed for verifying the significance of the separation among the bacterial community stages. This was tested with multivariate analysis of variance (MANOVA) using the first nine principal coordinates from PCA according to Defernez and Kemsley (1997). In case of significant separation, post-hoc analyses were carried out to explore the significance of pairwise separation of the groups with Hotelling's pairwise comparison (Legendre and Legendre 1998), which is the multivariate equivalent of the t test. Statistical and multivariate analyses were carried out using the PAST: Paleontological Statistics Software Package (Hammer et al. 2001).
The relationship between composition of bacterial community and mushroom yield of the adequate production series was investigated with redundancy discriminate analysis (RDA). RDA allows the ordination of samples with species variables (composition of bacterial community) and environmental variables (mushroom yield). To test significant relationship between them, Monte Carlo permutation test was carried out on the RDA coordinates based on 199 random permutations of the data using mushroom yield as environmental data (Legendre and Legendre 1998). For RDA and Monte Carlo permutation test, CANOCO for Windows version 4 (Microcomputer Power, Ithaca, USA) was used.
Results
Yield of oyster mushroom, physico-chemical and FDA hydrolysis activity changes
Only three yields fell outside the mean ± standard deviation range (Fig. 2): two were below (7-118 and 6-93) and one was above (6-110) the range. The partial degradation of wheat straw polysaccharides during oyster mushroom substrate preparation was detected as a light decrease of the cellulose/lignin (9%) and hemicellulose/lignin (19%) ratio (Fig. 3). The accessibility of oyster mushroom substrate for microbial degradation improved by 77% during the maturation process, as indicated by the convertibility of cellulose content by cellulases. FDA hydrolysis activity of stages 1, 3, and 7 were 0.126 ± 0.099, 0.112 ± 0.057, and 0.190 ± 0.062 mg fluorescein per gram of dry matter, respectively.
Mushroom yield of the investigated production series. Yield was determined as a percentage of wet weights of kilogram fresh mushroom per 100-kilogram substrate block. Bars indicate standard deviation for individual production series, based on yield values of different mushroom production houses. The thick lines show the mean ± standard deviation range for the entire dataset
Succession of fungal community
In the initial phase, different Penicillium, Fusarium, Aspergillus, Cladosporium, and Trichoderma strains were detected most frequently, in addition to Alternaria and Mucor species. Their presence could not be demonstrated after 3 days of composting (Fig. 4). After the third day, a substantial part of the fungal community in most of the samples consisted of two mesophilic basidiomycota yeast species, Rhodotorula mucilaginosa and Trichosporon moniliiforme. In the succession process, when the temperature was between 40–65°C, groups of thermophilic and thermotolerant filamentous fungi (Thermomyces lanuginosus, Myceliophthora thermophile, and Rhizomucor pusillus) colonized the straw (Fig. 4). A human pathogenic thermophilic fungus, Aspergillus fumigatus, was also present but with a low CFU value. After pasteurization, no fungi could be isolated with the applied methods.
With Trichoderma-specific PCR primers no harmful “green mould” species were detected amongst the isolates. In stage 1 samples, T. atroviride and non-aggressive T. harzianum strains and in stage 2 samples, traces of T. longibrachiatum were present.
Results of the T-RFLP studies on bacteria
Only samples of stages 1, 3, and 7 were investigated for molecular fingerprints, as these phases represent the key steps of substrate preparation. Two low-diversity samples (19-3 and 99-1) were outliers based on the Box-plot of Simpson indices (data not shown) and therefore were omitted from further statistical analyses (Fig. 5a). The PCA ordination of the T-RFLP results showed succession during substrate preparation, showing a noticeable trend from stage 1 through stage 3 all the way to stage 7 (Fig. 5b). The samples of the end-product (stage 7) formed a distinct group independent of the initial stages. There was an overall significant difference (p < 0.001) among the bacterial communities of the three phases based on the MANOVA results using the first nine principal coordinates of PCA. In addition, all of the post-hoc Hotelling's pairwise comparison were significant (p < 0.05).
Two-dimensional plot of principal component analysis based on the T-RFLP data set of the bacterial communities. a Including the two outlier samples (6-19-3 and 6-99-1). b After the exclusion of the outliers. Filled square stage 1 samples; empty circle stage 3 samples; filled triangle stage 7 samples. Circles represent 85% confidence intervals for each type of samples separately calculated with the PAST program
The Monte Carlo test showed that mushroom yield as canonical axe was not significant (p > 0.05) in RDA for T-RFLP results of stages 3 or 7, indicating that no significant relation between mushroom yield and composition of bacterial community existed.
Discussion
Oyster mushroom yield distribution was rather uniform according to the coefficient of variation (22.9%). Comparisons with other studies were hindered by the fact that most of them were carried out using laboratory model conditions. Labuschagne et al. (2000), who worked at a moderately higher scale (with 10 kg substrate bags), revealed a nearly two times bigger coefficient of variation (38.5%). These data support that our substrate and mushroom production was quite balanced. Hence it does not allow to draw conclusions between production series with higher and lower yields, but the basic characterization of our mushroom substrate preparation could be established.
For the chemical composition of wheat straw the acid method (primarily developed for wood composition analysis) and the gravimetric method (specially recommended for the analysis of agricultural residues) gave highly similar results (data not shown), therefore we propose to use the less demanding and less time consuming acid procedure of Hägglund for raw material analysis. Changes in straw composition during substrate preparation were in agreement with the results of Iiyama et al. (1994) and Lyons et al. (2006). However, changes in the composition remained low. Hemicellulose and cellulose content slightly decreased during the preparation, while, without substantial degradation, the structure of lignin was altered mainly by the formation of lignin-humus complex. As the polysaccharid composition of wheat straw is highly inhomogenous (Liu et al. 2005) and the absolute changes in the amounts of the various components are less pronounced, we propose an alternative measure, enzymatic degradability, to evaluate the microbial accessibility of the mushroom substrate.
The starting level of microbial activity (stage 1 samples) decreased after the composting treatment (stage 3 samples). The FDA activity exceeded the initial level by the completion of conditioning (stage 7 samples). Results suggest that FDA could be a fast method to monitor the microbial activity during mushroom substrate preparation. It reached its minimum value at the temperature maximum of the composting phase, which was in accordance with the study of Ryckeboer et al. (2003).
Fungal species could have persisted through the open-air phase in the outer, colder zones, while the inner temperature reached 50–60°C. After the third day, a substantial part of the fungal community consisted of two mesophilic basidiomycota yeast species, R. mucilaginosa and T. moniliiforme, with limited extracellular enzyme producing abilities. They were not able to degrade biopolymers, but were able to use d-xylose and cellobiose, proposing they were present in the composting straw as commensalists. During the thermophilic open-air phase, filamentous fungi were present from the genera Thermomyces, Myceliophthora, and Rhizomucor. Several strains of T. lanuginosus secrete high levels of xylanases, which are very active and stable at elevated temperatures. M. thermophila is well characterized with regard to its cellulolytic activity and a promising producer of thermostable amylases. Rhizomucor is a cosmopolitan genus found in soil and decaying fruit and vegetables, and they are also often isolated from fermenting and composting organic matter (Salar and Aneja 2007). Rhizomucor spp. other than R. variabilis are thermophilic in nature and can grow at temperatures as high as 54°C (Salar and Aneja 2007). Harmful Trichoderma “green mould” species were not detected amongst the isolates, which means that the T. pleurotum species (Komon-Zelazowska et al. 2007) possibly colonized the mushroom substrate in a later phase. No fungi were detected at constant elevated temperatures in the tunnels, which is consistent with previous findings of Klamer and Bååth (1998) and Dees and Ghiorse (2001). Accordingly, fungal species may catalyze important processes in the initial mesophilic phase of substrate preparation. Due to their versatile extracellular enzyme system, they take part in the decomposition of straw-polysaccharides.
A succession of the bacterial community was observed on the PCA ordination of the T-RFLP results during the substrate preparation (Fig. 5), which is quite similar to the findings of Herrmann and Shann (1997) based on phospholipid fatty acid analysis and Székely et al. (2009) based on T-RFLP and denaturing gradient gel electrophoresis (DGGE). Stage 1 samples constituted the most variable group indicated by the biggest confidence circle on the PCA-plot. A more dramatic change in the bacterial community occured in the heap phase along the first PC. Pasteurization and conditioning had an obvious but smaller effect on community composition. Therefore, stage 3 and 7 samples were less variable, which indicated the effect of the standardized protocol on substrate preparation, again in accordance with Székely et al. (2009).
There was no significant correlation between mushroom yield (Fig. 2) and the bacterial community T-RFLP fingerprints. In our case, the uniformity of mushroom yields did not support conclusions for “better” and “worse” bacterial community in the sense of mushroom production. This could be accounted for the so-called functional redundancy (Pérez-Piqueres et al. 2006), which means that community function can be carried out by several different bacteria. Thus changes in community structure might not directly lead to changes in community functionality and thus to altered mushroom yields. Another suggestion to consider is that the composition of the bacterial community is a highly important, but not sufficient enough characteristic to ensure good mushroom yield. Conditions of cultivation are also very determinative for the mushroom yield. Thus, a “good” bacterial community of the substrate might not have its beneficial effect on mushroom yield if the conditions of the cultivation are not satisfactory. Labuschagne et al. (2000) got similar results investigating the influence of crude straw quality on oyster mushroom yield.
According to our knowledge, this is the first report that gives a deeper, complex insight into oyster mushroom substrate preparation. Our results showed that a definite succession in microbial communities followed substrate preparation, accompanied by a stable trend of physico-chemical parameters. Further studies need to be carried out to identify bacterial species, which could help to describe the functional structure of the community. In addition to chemical measurements during substrate preparation, we also suggest the use of cellulose conversion and FDA hydrolysis activity for regular monitoring
References
Adam G, Duncan H (2001) Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol Biochem 33:943–951
Amann RI, Binder B, Chisholm SW, Olsen R, Devereux R, Stahl DA (1990) Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microb 56:1919–1925
von Arx JA (1981) The genera of fungi sporulating in pure culture. Cramer, Germany
Baldrian P, Valaskova V, Merhautova V, Gabriel J (2005) Degradation of lignocellulose by Pleurotus ostreatus in the presence of copper, manganese, lead and zinc. Res Microb 156:670–676
Barron GL (1968) The genera of hyphomycetes from soil. Williams & Wilkins, Baltimore
Baysal E, Peker H, Yalinkilic MK, Temiz A (2003) Cultivation of oyster mushroom on waste paper with some added supplementary materials. Bioresour Technol 89:95–97
Berghem LER, Pettersson LG (1974) The mechanism of enzymatic cellulose degradation: isolation and some properties of a β-glucosidase from Trichoderma viride. Eur J Biochem 46:295–305
Bonatti M, Karnopp P, Soares HM, Furlan SA (2004) Evaluation of Pleurotus ostreatus and Pleurotus sajor-caju nutritional characteristics when cultivated in different lignocellulosic wastes. Food Chem 88:425–428
Chang ST, Miles PG (2004) Mushrooms: cultivation, nutritional value, medicinal effect, and environmental impact. CRC Press, Boca Raton
Chen X, Romaine CP, Ospina-Giraldo MD, Royse DJ (1999) A polymerase chain reaction-based test for the identification of Trichoderma harzianum biotypes 2 and 4, responsible for the worldwide green mold epidemic in cultivated Agaricus bisporus. Appl Microb Biotechnol 52:246–250
Cullen DW, Hirsch PR (1998) Simple and rapid method for direct extraction of microbial DNA from soil for PCR. Soil Biol Biochem 30:983–993
Curvetto NR, Figlas D, Devalis R, Delmastro S (2002) Growth and productivity of different Pleurotus ostreatus strains on sunflower seed hulls supplemented with N-NH +4 and/or Mn(II). Bioresour Technol 84:171–176
Dees PM, Ghiorse WC (2001) Microbial diversity in hot synthetic compost as revealed by PCR-amplified rRNA sequences from cultivated isolates and extracted DNA. FEMS Microb Ecol 35:207–216
Defernez M, Kemsley EK (1997) The use and misuse of chemometrics for treating classification problems. Trends Anal Chem 16:216–221
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59:257–268
Goering HK, Soest PJ van (1970) Forage fiber analyses (apparatus, reagents, procedures, and some applications). Agricultural handbook No. 379 US Agricultural Research Service, Washington
Hägglund E (1951) Chemistry of wood. Academic Press, New York
Hammer O, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4:1–9
Hanlin RT (1990) Illustrated genera of ascomycetes. The American Phytophatological Society, St. Paul, Minnesota
Hartmann M, Wilder F (2006) Community structure analyses are more sensitive to differences in soil bacterial communities than anonymous diversity indices. Appl Environ Microbiol 72:7804–7812
Hatvani L, Antal Z, Manczinger L, Szekeres A, Druzhinina IS, Kubicek CP, Nagy A, Nagy E, Vágvölgyi C, Kredics L (2007) Green mould diseases of Agaricus and Pleurotus spp. are caused by related but phylogenetically different Trichoderma species. Phytopathol 97:532–537
Herrmann RF, Shann JF (1997) Microbial community changes during the composting of municipal solid waste. Microb Ecol 33:78–85
Hernández D, Sánchez JE, Yamasaki K (2003) A simple procedure for preparing substrate for Pleurotus ostreatus cultivation. Bioresour Technol 90:145–150
Iiyama K, Stone BA, Macauley BJ (1994) Compositional changes in compost during composting and growth of Agaricus bisporus. Appl Environ Microb 60:1538–1546
Kaar WE, Cool LG, Merriman MM, Brink DL (1991) The complete analysis of wood polysaccharides using HPLC. J Wood Chem Technol 11:447–463
Klamer M, Bååth E (1998) Microbial community dynamics during composting of straw material studied using phospholipid fatty acid analysis. FEMS Microbiol Ecol 27:9–20
Komon-Zelazowska M, Bissett J, Zafari D, Hatvani L, Manczinger L, Woo SI, Lorito M, Kredics L, Kubicek CP, Druzhinina IS (2007) Genetically closely related but phenotypically divergent Trichoderma species cause world-wide green mould disease in oyster mushroom farms. Appl Environ Microbiol 73:7415–7426
Labuschagne PM, Eicker A, Aveling TAS, de Meillon S, Smith MF (2000) Infuence of wheat cultivars on straw quality and Pleurotus ostreatus cultivation. Bioresour Technol 71:71–75
Legendre P, Legendre L (1998) Numerical ecology. Elsevier, Amsterdam
Liu W, Marsh TL, Cheng H, Forney LJ (1997) Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl Environ Microbiol 63:4516–4522
Liu R, Yu H, Huang Y (2005) Structure and morphology of cellulose in wheat straw. Cellulose 12:25–34
Lyons GA, Sharma HSS, Kilpatrick M, Cheung L, Moore S (2006) Monitoring of changes in substrate characteristics during mushroom compost production. J Agric Food Chem 54:4658–4667
Ntougias S, Zervakis GI, Kavroulakis N, Ehaliotis C, Papadopoulou KK (2004) Bacterial diversity in spent mushroom compost assessed by amplified rDNA restriction analysis and sequencing of cultivated isolates. Syst Appl Microbiol 27:746–754
Pérez-Piqueres A, Edel-Hermann V, Alabouvette C, Steinberg C (2006) Response of soil microbial communities to compost amendments. Soil Biol Biochem 38:460–470
Peters S, Koschinsky S, Schwieger F, Tebbe CC (2000) Succession of microbial communities during hot composting as detected by PCR-single-strand-conformation polymorphism-based genetic profiles of small-subunit rRNA genes. Appl Environ Microbiol 66:930–936
Ryckeboer J, Mergaert J, Coosemans J, Deprins K, Swings J (2003) Microbial aspects of biowaste during composting in a monitored compost bin. J Appl Microbiol 94:127–137
Salar RK, Aneja KR (2007) Thermophilic fungi: taxonomy and biogeography. J Agric Technol 3:77–107
Salmones D, Mata G, Waliszewski KN (2005) Comparative culturing of Pleurotus spp. on coffee pulp and wheat straw: biomass production and substrate biodegradation. Bioresour Technol 96:537–544
Smith CJ, Danilowicz BS, Clear AK, Costello FJ, Wilson B, Meijer WG (2005) T-Align, a web-based tool for comparison of multiple terminal restriction fragment length polymorphism profiles. FEMS Microbiol Ecol 54:375–380
Song J, Weon HY, Yoon SH, Park D, Go SSJ, Suh JW (2001) Phylogenetic diversity of thermophilic actinomycetes and Thermoactinomyces spp. isolated from mushroom composts in Korea based on 16S rRNA gene sequence analysis. FEMS Microbiol Lett 202:97–102
Stamets P (2000) Growing gourmet and medicinal mushrooms. Ten Speed Press, Berkeley
Székely AJ, Sipos R, Berta B, Vajna B, Cs H, Márialigeti K (2009) DGGE and T-RFLP analysis of bacterial succession during mushroom compost production and sequence-aided T-RFLP profile of mature compost. Microb Ecol 57:522–533
Takaku H, Kodaira S, Kimoto A, Nashimoto M, Takagi M (2006) Microbial communities in the garbage composting with rice hull as an amendment revealed by culture-dependent and -independent approaches. J Biosci Bioeng 101:42–50
Tiquia SM, Ichida JM, Keener HM, Elwell DL, Burtt EH, Michel FC (2005) Bacterial community profiles on feathers during composting as determined by terminal restriction fragment length polymorphism analysis of 16S rDNA genes. Appl Microbiol Biotechnol 67:412–419
Wu Z, Wang XR, Blomquist G (2002) Evaluation of PCR primers and PCR conditions for specific detection of common airborne fungi. J Environ Monit 4:377–382
Zhang R, Li X, Fadel JG (2002) Oyster mushroom cultivation with rice and wheat straw. Bioresour Technol 82:277–284
Acknowledgements
This study was supported by the grant OMFB-00977/2005 from the Ministry of Agriculture and Rural Development (Hungary). We would like to thank Rita Sipos for proofreading in English.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vajna, B., Nagy, A., Sajben, E. et al. Microbial community structure changes during oyster mushroom substrate preparation. Appl Microbiol Biotechnol 86, 367–375 (2010). https://doi.org/10.1007/s00253-009-2371-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2371-3