Abstract
Laccases are green oxidases with a number of potential industrial applications. In this study, recombinant Bacillus subtilis CotA laccase was secreted by Escherichia coli via both the α-hemolysin secretion system and the YebF secretion system after microaerobic induction. Meanwhile, we discovered a much simpler approach for extracellular production of recombinant CotA laccase from E. coli, involving alternation of induction conditions to release recombinant CotA following intracellular expression. By optimizing the induction parameters, the extracellular yield of recombinant CotA laccase was improved from 157.4 to 2401.3 U/L after 24 h of induction. This strategy could be suitable for large-scale production of CotA laccase for industrial use. Recombinant CotA laccase was purified by Ni2+ affinity chromatography in a single step and showed similar biochemical properties to wild-type laccase. Purified as well as crude recombinant CotA laccase efficiently decolorized seven structurally different dyes. The decolorization capability of recombinant CotA laccase under harsh conditions was investigated by incubation of the enzyme with a simulated textile effluent (STE) with pH 11.6, 3.5 % salinity and peak absorbance of 10.42. Recombinant CotA laccase efficiently decolorized 77.0 % of STE after 48 h reaction, demonstrating the potential of this enzyme for industrial dye effluent treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laccases (benzenediol:oxygen oxidoreductases; EC 1.10.3.2) which belong to the multi-copper oxidase (MCO) group, have received considerable attention since their discovery because of their broad substrate specificity and great biotechnological potential in green chemistry (Pezzella et al. 2015). Laccases contain a type 1 mononuclear copper center and a trinuclear copper cluster which includes two type 3 copper centers and one type 2 copper center. The copper centers mediate four single-electron oxidations of substrate, with concomitant reduction of oxygen to water (Enguita et al. 2004). Past century has witnessed significant progress in the discovery of laccases with applications in diverse industrial fields (Pezzella et al. 2015). In recent years, novel laccases with robust properties are being constantly discovered to expand the applications of laccases in industrial fields that require harsh treatment condition (Ausec et al. 2015; Hildén et al. 2009; Uthandi et al. 2012). Bacillus spore coat protein (CotA) was reported to show high activity and stability under elevated temperature, alkaline pH and in the presence of metal ions (Guan et al. 2014; Lu et al. 2012; Sondhi et al. 2014). To date, CotA laccases have been applied in dye decolorization (Brissos et al. 2009; Lončara et al. 2013; Pereira et al. 2009), degradation of insecticides (Ulčnik et al. 2013), bioethanol production from lignocelluloses (Chang et al. 2014; Furtado et al. 2013), surface functionalization (Kudanga et al. 2009), and biofuel cell applications (Beneyton et al. 2013), positioning them as outstanding industrial enzymes. However, commercial preparation of laccases from native sources is complicated by the spore-bound nature of CotA protein (Martins et al. 2002), as well as the low level of enzyme expression.
To solve the problem, current research has focused on the production of CotA laccase by genetic engineering. Escherichia coli is the first-choice host for CotA laccase expression because it contains the conditions for prokaryotic post-translational modification and has a high growth rate and straightforward scalability in a simple, inexpensive culture medium (Rosano and Ceccarelli 2014). Till now, CotA laccases from different Bacillus species have been successfully expressed in the cytoplasm of E. coli (Beneyton et al. 2014; Brander et al. 2014; Ihssen et al. 2015). However, this expression pattern often results in the formation of inclusion bodies (Martins et al. 2002), although emerging strategies are developed by researchers to overcome the phenomenon (Fang et al. 2014; Mohammadian et al. 2010; Mollania et al. 2013). Besides, cell disruption is required to release the target protein, which decreases the yield (Mergulhão et al. 2005). By contrast, extracellular expression has the advantage of simplifying the purification process, reducing the formation of inclusion bodies and increasing the stability of active proteins (Ni and Chen 2009). However, no extracellular production of recombinant CotA protein in E. coli has been reported up to date.
In this study, two secretion systems were applied to achieve the secretion of recombinant CotA into the culture medium. The uropathogenic E. coli α-hemolysin (HlyA) secretion system is the most widely used secretion system for recombinant protein production (Ni and Chen 2009). Three components, HlyB (an ATP-binding cassette [ABC] protein), HlyD (a membrane fusion protein), and TolC (an outer membrane protein), together form a translocator that spans the cell envelope. The C-terminal secretion signal located at the C-terminal 50–60 amino acids of substrate HlyA (HlyAs) is responsible for activating the translocator, leading to the direct translocation of cytoplasmic HlyA into the extracellular medium in one step (Kanonenberg et al. 2013). The two-step YebF secretion system was discovered in commonly used laboratory strains of E. coli (Zhang et al. 2006). The 13 kDa YebF protein, via its Sec-leader sequence, is translocated into the periplasm through the Sec pathway and cleaved to a 10.8 kDa mature form, which is then translocated across the outer membrane in a process that involves OmpF and OmpC (Prehna et al. 2012). We successfully achieved extracellular production of Bacillus subtilis CotA from E. coli via both of these secretion systems, but we also found a much simpler approach for the extracellular production of CotA laccase by altering the induction conditions to release recombinant CotA laccase intracellularly expressed by E. coli. The enzyme production process was optimized. Recombinant CotA laccase was purified and characterized. The effect of recombinant laccase on dye decolorization was investigated.
Materials and methods
Strains, plasmids, and reagents
The strains, plasmids, and genomic DNA used in this study are shown in Table 1. The sequence of the CotA gene from LS02 has been previously deposited in GenBank (Accession No. GU972587). Oligonucleotide primers used in this study are listed in Supplementary Table S1. TransStart FastPfu DNA Polymerase, dNTP, DMT enzyme, T4 DNA ligase, and Blue Plus™ Protein Marker (14–100 kDa) were all from TransGen Biotech (Beijing, China). Restriction enzymes were supplied by Takara (Dalian, China). BugBuster Protein Extraction Reagent was obtained from Novagen, Merck Millipore (Billerica, MA, USA). DNA manipulation kits were supplied by Omega (Norcross, GA, USA). 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS), 2,6-dimethoxyphenol (2,6-DMP), N,N′-bis(3,5-dimethoxy-4-hydroxybenzylidene hydrazine) (syringaldazine), and 3′,5′-dimethoxy-4′-hydroxyacetophenone (acetosyringone) were purchased from Sigma-Aldrich (St. Louis, MO, USA). HisTrap HP affinity columns were from GE Healthcare (Piscataway, NJ, USA). Polyvinylidene difluoride (PVDF) membrane (0.45 μm) was from Millipore (Bedford, MA, USA). Anti-six-histidine (His6) mouse IgG was from Abgent (San Diego, CA, USA). Horseradish peroxidase (HRP)-labeled goat anti-mouse IgG (H+L) was from ZSGB-BIO (Beijing, China). Nonfat dry milk and SuperEnhanced chemiluminescence detection reagents were from Applygen Technologies (Beijing, China). BioMax MR film was purchased from Kodak (Rochester, NY, USA). Other reagents were of analytical grade.
Construction of engineered strains
Construction of the recombinant expression systems is detailed in the Supplementary Material. Recombinant vector construction diagrams are shown in Supplementary Figs. S1, S2, and S3. Recombinant plasmids were confirmed by DNA sequencing before further use. Expression vectors pET/CotA-HlyAs and pACYC1/HlyBD were simultaneously transferred into E. coli BL21 (DE3) to generate expression strain PSABD. The strain PCD carrying vector pET-20b(+) and pACYC-Deut-1 was used as the control for PSABD. Recombinant plasmid pYebFT7/CotA-His6 was transferred into E. coli BL21 (DE3) to generate expression strain YSD. The strain YD carrying vector pYebFH6/T7 was used as the control for YSD. Expression vector pET/CotA-His6 was transferred into E. coli BL21 (DE3) to generate expression strain PSD. The strain PD carrying vector pET-20b(+) was used as the control for PSD.
Expression of recombinant CotA laccase in E. coli
Single colonies of each engineered strain were inoculated into 5 mL Luria-Bertani (LB) medium supplemented with 50 mg/L ampicillin in a 50-mL flask and grown overnight at 37 °C, 180 rpm. Overnight culture (50 μL) was inoculated into 5 mL fresh LB medium supplemented with antibiotics in 50-mL flasks and incubated at 30 °C, 180 rpm. At OD600 = 0.8, 0.1 mM isopropyl-β-d-thiogalactoside (IPTG) and 0.1 mM CuSO4 were added to the medium. The culture was induced at 25 °C (aerobic culture temperature), 120 rpm, for 4 h. Then, microaerobic induction was achieved by cessation of shaking (0 rpm) and incubation at 30 °C (microaerobic culture temperature) for a further 20 h.
The effects of five culture parameters (induction temperature, induction point, inducer concentration, copper concentration, and shake flask volume) on the extracellular production of recombinant CotA laccase by the PSD strain were investigated after induction for 24 h.
Cell fractionation
After induction, 1 mL of culture medium was centrifuged at 15,000×g for 10 min. The supernatant was collected as the extracellular fraction. The cell pellet was disrupted with BugBuster Protein Extraction Reagent, the cell extract was centrifuged at 15,000×g for 10 min, and the supernatant was saved as the cytoplasmic fraction. Each fractionated sample was assayed for enzymatic activity or precipitated with trichloroacetic acid (TCA) prior to SDS-PAGE analysis.
Laccase activity assay
Agar plate assay was performed to test the activity of extracellular laccase expressed by the PSD strain. Freshly transformed colonies (PSD and PD) were spotted onto LB agar plates containing 50 mg/L ampicillin, 0.5 mM ABTS, 0.2 mM CuSO4, and 0.1 mM IPTG and incubated at 30 °C for 2 days to detect reaction zones. The recombinant Pichia pastoris SMD1168H–CotA strain (Wang et al. 2015) that can secrete recombinant CotA laccase was used as a positive control strain. Single colonies of SMD1168H–CotA were grown on the LB agar plate containing 50 mg/L zeocin, 0.5 mM ABTS, and 0.2 mM CuSO4 and incubated at 30 °C for 5 days, and methanol (100 μL per 20 mL plate) was supplemented on the plate covers every 24 h.
Laccase activity was determined using syringaldazine as substrate, as previously described (Lu et al. 2012). One unit of enzyme activity was defined as the amount of enzyme required to oxidize 1 μmol substrate per minute. All assays were performed in triplicate.
SDS-PAGE and western blot analysis
Proteins were analyzed by SDS-PAGE (12 % separation gel and 5 % stacking gel); the gel was stained with Coomassie Brilliant Blue R-250 (Solarbio, Beijing, China). For western blot analysis, protein samples were transferred onto PVDF membranes with a Mini Trans-Blot cell (Bio-Rad, Richmond, CA, USA). Immunoblots were blocked in 5 % (w/v) nonfat milk in TBST buffer (20 mM Tris–HCl [pH 7.6], 150 mM NaCl, 0.1 % [v/v] Tween-20) overnight at 4 °C. The membranes were washed with TBST and incubated with anti-His6 mouse IgG (1:1000 dilution) for 1 h. Afterwards, the membranes were washed and incubated with the HRP-labeled goat anti-mouse IgG (H+L) (1:5000 dilution) for 40 min. Immunoblots were developed by SuperEnhanced chemiluminescence detection reagents.
Purification of recombinant CotA laccase
The PSD strain was induced under optimized induction conditions. After induction for 24 h, the culture broth was centrifuged at 8000×g for 15 min at 4 °C. The supernatant was filtered through a 0.45-μm filter to remove any cell debris. Supernatant samples were dialyzed with dialysis tubing (Solarbio, Beijing, China; MWCO 14,000–18,000 kDa). To optimize the purification conditions, aliquots of dialyzed supernatant were loaded onto a 1-mL HisTrap HP affinity column. After column equilibration, target protein was eluted by imidazole at different concentrations according to the protocol (GE Healthcare). The fractions were pooled and analyzed by SDS-PAGE. After the optimal imidazole concentration was selected, purification was scaled up to a 5-mL HisTrap HP affinity column. The elution fractions were desalted by dialysis against 20 mM sodium phosphate (pH 7.4) to remove Ni2+ and NaCl. The purified protein was analyzed by SDS-PAGE and MALDI-TOF MS as described previously (Wang et al. 2015).
Decolorization of single synthetic dyes by recombinant CotA laccase
The decolorization ability of recombinant CotA laccase was tested on seven synthetic dyes, representing four kinds of commercial dyes. Basic information and working concentrations of the synthetic dyes are shown in supplementary Table S2. The reaction system contained 20 U/L purified or crude recombinant CotA laccase from culture supernatant, 0.1 mM acetosyringone (laccase mediator), synthetic dye, and 0.1 M citrate–phosphate buffer (pH 7.0). Each 50-mL shake flask containing 5 mL of reaction solution was incubated at 40 °C, 120 rpm. The samples were withdrawn at different time intervals (1, 2, 4, 6, 8, 10, and 24 h) and analyzed for dye removal. All reactions were carried out in triplicate.
Decolorization of STE by recombinant CotA laccase
The effect of recombinant CotA laccase on STE was also investigated. The composition of STE was devised following the instructions of the manufacturer Bezema AG (Montlingen, Switzerland) for reactive dyes (Mohorčič et al. 2006; Rodríguez-Couto 2012). The STE consisted of 0.5 g/L Reactive Black 5, 0.2 g/L Remazol Brilliant Blue R, 30 g/L NaCl, 5 g/L Na2CO3, and 1.5 ml/L of 32.5 % (w/v) NaOH in deionized water. A 20× stock solution of STE was prepared. A 10-mL reaction including 100 U/L purified or crude recombinant CotA laccase, 500 μL STE stock solution, 0.1 mM acetosyringone, deionized water, or 0.1 M citrate–phosphate buffer (pH 7.0) was incubated at 40 °C, 120 rpm. The decolorization was measured at different time intervals (24 and 48 h). The maximum wavelength of the effluent sample was scanned using UV–Vis Spectrophotometer (Lambda 750, PerkinElmer, Santa Clara, CA, USA). Another experiment was performed to investigate the effect of pH on decolorization rate of STE. A 10-mL reaction included 50 U/L purified or crude recombinant CotA laccase, 500 μL STE stock solution, 0.1 mM acetosyringone, 0.1 M citrate–phosphate buffer (pH 7.0), or 0.1 M Tris–HCl buffer (pH 9.0) was incubated at 40 °C, 120 rpm. The decolorization was measured at different time intervals (1, 2, 4, 6, 8, 10, 12, and 24 h). All reactions were performed in triplicate.
Results
Extracellular production of recombinant CotA laccase in E. coli
After induction for 24 h, the secretion efficiencies of the PSABD strain (with the α-hemolysin secretion system) and the YSD strain (with the YebF secretion system) were 79 and 29 %, respectively (Table 2). The secretion level of YebF was compared before and after fusion with passenger protein CotA laccase. Western blot result (Fig. S4a, Supplementary Material) indicated that the extracellular fusion protein YebF–CotA–His6 secretion by YSD was lower than the YebF–His6 secretion by YD. Notably, 77 % of recombinant CotA laccase expressed by PSD was found in the culture supernatant without the mediation of any secretion element. The extracellular laccase activity of PSD was 157.4 U/L, which was 5- and 78-fold higher than that of PSABD and YSD, respectively. Western blotting indicated that CotA–His6 protein was present in the culture supernatant of the PSD strain (Fig S4b, Supplementary Material). Single colonies of the recombinant strains were cultured on an LB detection plate. Purple reaction zones were seen around the colonies of the PSD strain after 2 days (Fig. 1a), while no reaction zones were seen around the control strain PD (Fig. 1b), which indicated that CotA laccase was released extracellularly by the PSD strain. Normally, the products of ABTS oxidized by laccases are green. The purple by-product was probably related to the particular chemical composition of the detection medium (Solís-Oba et al. 2005). Growth of P. pastoris SMD1168–CotA cells as a control strain on the same detection agar also yielded purple reaction zones (Fig. 1c). The phenomenon has been observed previously (Hu et al. 2007).
Optimization of induction conditions
Five culture factors including induction temperature, copper ion concentration, inducer concentration, induction point, and shake flask volume were selected to test their effects on extracellular production of recombinant CotA laccase by the PSD strain. As the induction process contains two phases (aerobic and microaerobic induction phases), the PSD strain was induced at different aerobic culture temperatures (20, 25, or 30 °C), randomly combined with different microaerobic culture temperatures (15, 20, 25, 30, or 37 °C). Maximum extracellular laccase (167.2 U/L) was detected in the supernatant culture when aerobic culture temperature and microaerobic culture temperature were 25 and 30 °C, respectively (Fig. 2a). Induction temperature >37 or <20 °C caused a reduction in extracellular laccase activity (Fig. 2b).
The level of copper ions had a considerable effect on active laccase expression and extracellular laccase production. No extracellular laccase was detected in the control group (no copper ions added), whereas addition of CuSO4 significantly enhanced the production of extracellular laccase. The extracellular laccase activity peaked at 800 U/L at 2.1 mM CuSO4, but was severely inhibited by higher concentrations (Fig. 2c).
Extracellular production of recombinant CotA laccase evidently increased with cell density when induction was initiated in log phase (OD600 ≤0.8), but gradually slowed down as the strain reached stationary phase (OD600 0.8–1.2). When induction initiated at OD600 = 1.4, production of extracellular laccase was quite low (Fig. 2d). The effect of inducer IPTG or lactose on extracellular laccase production was tested. Extracellular CotA laccase peaked at 256 U/L in the presence of 0.1 mM IPTG (Fig. 2b), but gradually decreased at higher IPTG concentration. When lactose was used as an inducer, the extracellular rCotA laccase reached 557 U/L in the presence of 0.1 % (w/v) lactose (Fig. 2f), which was 2.2-fold higher than the level obtained with 0.1 mM IPTG. However, higher concentrations of lactose caused a reduction of extracellular laccase activity.
Expression was performed in 50-, 150-, 250-, and 500-mL shake flasks, each containing one tenth of the flask volume of LB medium. Extracellular laccase levels increased along with shake flask volume; extracellular laccase activity was 436 U/L with culture in 500-mL shake flasks, which was twice as high as culture in 50-mL shake flasks (Fig. 2e).
After optimizing the culture condition, the extracellular yield of recombinant CotA laccase was improved from an initial level of 157.4 to 1598.7 U/L in laboratory LB medium. With industrial LB medium (0.5 % [w/v] industrial yeast extract, 1 % [w/v] industrial peptone, and 1 % [w/v] crude salt), extracellular laccase activity reached 2401.3 U/L after induction for 24 h.
Purification and characterization of recombinant CotA laccase
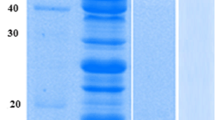
The concentration of imidazole for elution was optimized to get highly purified protein. A concentration of 100 mM imidazole was chosen for large-scale CotA purification (Fig. S5 in the Supplementary Material). After dialysis, affinity purification, and desalting, 64.41 % of target protein was recovered from 1.2 L culture supernatant, with a 6.25-fold increase in purity (Table 3). SDS-PAGE analysis proved that CotA laccase was purified to homogeneity (Fig. 3). The molecular weight of the fusion protein on SDS-PAGE was ~63 kDa. The MALDI-TOF MS result proved the purified protein to be B. subtilis CotA laccase (Table S3, Supplementary Material). The biochemical properties of recombinant CotA laccase were characterized and found to be similar (data not shown) to those of wild-type CotA laccase from B. subtilis LS02 (Du 2011).
Synthetic dye decolorization by recombinant CotA laccase
Purified CotA laccase had a high capacity for decolorization of seven synthetic dyes. After incubation for 1 h, the decolorization efficiency of Indigo Carmine, Orange G, Malachite Green, and Alizarin Red reached 99.1, 96.0, 93.0, and 80.8 %, respectively (Fig. 4a). However, the decolorization efficiency of Congo Red, Crystal Violet, and Remazol Brilliant Blue R was only 51.2, 56.1, and 34.5 % after 1 h. The low decolorization rate for these three dyes was probably because of their complex structures (Table S2, Supplementary Material) that inhibit penetration into the active site of laccase (Riva 2006). The decolorization efficiency for the three dyes increased with time and reached 83.1, 91.6, and 84.0 % after 8 h. The performance of crude enzyme in decolorization of synthetic dyes was similar to that of purified enzyme (Fig. 4b).
STE decolorization by recombinant CotA laccase
The decolorization of a STE by purified or crude recombinant CotA laccase was studied. The STE had maximum absorbance peaks at 602, 586, or 586 nm when dissolved with deionized water, 0.1 M citrate–phosphate buffer (pH 7.0), or 0.1 M Tris–HCl buffer (pH 9.0), respectively. When purified or crude CotA laccase was used for STE decolorization, the decolorization efficiency of STE was 68.1 or 77.0 % after 48 h (Fig. 5a). The color of the reaction system changed from dark blue to red-brown, indicating the production of new metabolites. The decolorization rates of purified and crude CotA laccase were much higher when STE was buffered to pH 7.0, both plateauing at 77 % by 24 h of incubation (Fig. 5a). The effect of pH on decolorization rate was investigated. The decolorization rates of purified and crude laccase were both higher with STE buffered to pH 7.0 than to pH 9.0. Besides, the crude laccase showed higher rate of decolorization than purified laccase. After a 2-h reaction, the decolorization efficiency of crude laccase rapidly reached 72.6 % at pH 7.0, which was three times higher than that (24.6 %) of purified laccase under the same conditions (Fig. 5b).
Discussion
In this study, recombinant E. coli strains were all induced under microaerobic conditions, as the strains expressed barely detectable levels of active laccase under traditional aerobic induction condition (data not shown). Microaerobic induction has been reported to promote the expression of active recombinant laccase (Durão et al. 2008; Ihssen et al. 2015; Mohammadian et al. 2010; Sherif et al. 2013) by enhancing the copper incorporation into the enzyme. After microaerobic induction, recombinant CotA laccase was exported into the culture medium by either the α-hemolysin secretion system or the YebF secretion system. The secretion efficiency of YSD was quite low, presumably because the large size of the CotA sequence that was added to YebF in the fusion context imposed additional constraints on the translocation machinery. The fusion protein linker between the YebF and the CotA sequences might also affect the efficiency of protein expression (Chen et al. 2013).
Unexpectedly, we found that the PSD strain was able to release laccase into the culture medium without the introduction of any secretion system. Moreover, the extracellular laccase activity of the PSD strain was 5- and 78-fold higher than that of the PSABD strain and the YSD strain. Previous reports have also described finding cytoplasmic proteins in the extracellular medium without the assistance of an additional secretion element. Su et al. (2013) found Thermobifida fusca cutinase synthesized in the cytoplasm of E. coli being “secreted” into the culture medium due to enhanced cell leakage caused by the phospholipid hydrolysis property of recombinant cutinase. Teng et al. (2011) and Zhang et al. (2013) found cytoplasmic recombinant proteins to be released into the culture medium at a high level with unknown reasons. Generally, secretion of recombinant cytoplasmic proteins from E. coli was achieved by variation of induction conditions (Rinas and Hoffmann 2004) or by chemical or enzymatic cell permeabilization (Falconer et al. 1999; Fu et al. 2005; Liu et al. 2012). Till now, various secretion systems have been utilized to export recombinant protein out of E. coli. However, the secretion level may be limited by shortage of transport machinery (Low et al. 2010). Also, some secretion element fused with the recombinant protein cannot be removed after expression (Low et al. 2011), which may interfere with protein activity and structure. In this study, the secretion pattern of the PSD strain efficiently circumvents these two limitations, which could be beneficial to large-scale production of laccase for downstream applications. The mechanism underlying the extracellular expression pattern is currently unknown and will be investigated in our future studies.
The effects of induction parameters on extracellular laccase production were assessed. Our results demonstrated that the induction temperature played an important role in laccase release from PSD. The secretion level of the PSD strain was highest between 25 and 30 °C. An induction temperature >30 or <25 °C led to reduced laccase secretion, probably because of accumulation of inclusion bodies or a low metabolic level. Laccase is a copper-dependent enzyme; the production of enzymatically active laccase is closely related to the concentration of copper in the culture medium (Durão et al. 2008). During PSD induction, no extracellular laccase was detected in the supernatant when no CuSO4 was added into the culture medium. The extracellular laccase activity reached a maximum at 2.1 mM CuSO4 but fell rapidly when the concentration of copper ions was higher than 2.1 mM, possibly because the copper tolerance of E. coli had been exceeded. Two inducers IPTG and lactose were used for PSD induction. Low levels of laccase were secreted into the medium in the absence of inducer due to basal expression of the T7 promoter-based expression system (Studier 2005). Maximum extracellular laccase was detected following induction with 0.1 mM IPTG or 0.1 % lactose. The extracellular laccase production was higher when the PSD strain was induced with lactose than with IPTG. Lactose is a nontoxic and cost-saving inducer, which is especially suitable for large-scale laccase production. The secretion level of recombinant laccase was severely inhibited when the lactose concentration was above 2 % (data not shown). This high concentration might have imposed a metabolic burden on PSD cells, causing overaccumulation of target proteins and the formation of inclusion bodies (Zou et al. 2014). Alternatively, metabolism of lactose might cause a fall in pH in the culture (Studier 2005), which affected the stability of extracellular laccase.
After optimizing the induction parameters, extracellular laccase production was improved to 2401.3 U/L, using industrial LB as the culture medium. In our previous study, 1647.2 U/L extracellular laccase was achieved using P. pastoris as expression host after a 20-day fed-batch induction (Wang et al. 2015). We have effectively shortened the induction time to 1 day and increased extracellular laccase production, demonstrating that the E. coli expression system is an effective host for industrial expression of extracellular laccase.
Industrial textile dye effluent usually contains a high concentration of reactive dyes, most of which are highly toxic and nondegradable by conventional treatment processes (Murugesan et al. 2009). Moreover, these effluents have high levels of pH and salinity because of the presence of dyeing auxiliaries, alkalis, and salt (Allègre et al. 2006). Most biocatalysts cannot tolerate these harsh treatment conditions. Therefore, treatment of textile dye effluents is a complicated environmental problem. Several reports have demonstrated efficient decolorization of STE by laccase-producing fungi (Rodríguez-Couto 2012; Zhuo et al. 2011) or fungal laccase (Osma et al. 2010). However, these studies required buffering of STE to acidic pH before reaction, because of the pH dependence of fungal laccase. The pretreatment is inconvenient and costly especially for large-scale treatment.
The ability of recombinant CotA laccase for STE decolorization was assessed. The STE showed a pH of 11.6 and a high salinity (3.5 %) and gave an absorbance of 10.42 at the maximum wavelength. Recombinant CotA laccase had excellent decolorization capacity in this tough environment. Buffering STE to pH 7.0 or 9.0 did not increase the extent of color removal but helped to reduce the time required for decolorization. The STE decolorization rate by crude laccase was higher than that by purified laccase. No decolorization of STE was observed after a 48-h reaction with crude supernatant from the PD strain. Proteins in the crude culture supernatants might act as an enzyme-protective agent that helped to maintain the catalytic activity and stability of CotA laccase in the adverse reaction environment. Overall, the results demonstrate that crude recombinant CotA laccase can be used directly in dye effluent treatment, thereby reducing the production cost.
Although CotA laccases have been shown to be potential catalysts for industrial applications, the substrate spectrum and catalytic efficiency of CotA laccases are limited by their low redox potential (Mate and Alcalde 2015). Redox mediators act as electron shuttles between laccase and substrates (Sharma et al. 2007) and can effectively expand the redox potential of laccase to include recalcitrant substrates. The application of artificial mediators is limited by their toxicity and high cost (Cañas and Camarero 2010). By contrast, the natural mediator acetosyringone which is readily available in nature (Cañas and Camarero 2010) causes no harm to the environment and thus is beneficial for the reuse of decolorized textile effluents. In addition to the usage of mediators, protein engineering is also an important tool to overcome the shortcoming of CotA laccase as biocatalysts (Mate and Alcalde 2015). Our future research will be directed toward attempts to refine the properties of CotA laccase to make this enzyme a more effective catalyst for industrial applications.
References
Allègre C, Moulin P, Maisseu M, Charbit F (2006) Treatment and reuse of reactive dyeing effluents. J Membrane Sci 269(1–2):15–34. doi:10.1016/j.memsci.2005.06.014
Ausec L, Črnigoj M, Šnajder M, Ulrih N, Mandic-Mulec I (2015) Characterization of a novel high-pH-tolerant laccase-like multicopper oxidase and its sequence diversity in Thioalkalivibrio sp. Appl Microbiol Biotechnol 99(23):9987–9999. doi:10.1007/s00253-015-6843-3
Beneyton T, Wijaya IP, Salem CB, Griffiths AD, Taly V (2013) Membraneless glucose/O2 microfluidic biofuel cells using covalently bound enzymes. Chem Commun (Camb) 49(11):1094–1096. doi:10.1039/c2cc37906f
Beneyton T, Coldren F, Baret JC, Griffiths AD, Taly V (2014) CotA laccase: high-throughput manipulation and analysis of recombinant enzyme libraries expressed in E. coli using droplet-based microfluidics. Analyst 139(13):3314–3323. doi:10.1039/c4an00228h
Brander S, Mikkelsen JD, Kepp KP (2014) Characterization of an alkali- and halide-resistant laccase expressed in E. coli: CotA from Bacillus clausii. PLoS One 9(6):e99402. doi:10.1371/journal.pone.0099402
Brissos V, Pereira L, Munteanu FD, Cavaco-Paulo A, Martins LO (2009) Expression system of CotA-laccase for directed evolution and high-throughput screenings for the oxidation of high-redox potential dyes. Biotechnol J 4(4):558–563. doi:10.1002/biot.200800248
Cañas AI, Camarero S (2010) Laccases and their natural mediators: biotechnological tools for sustainable eco-friendly processes. Biotechnol Adv 28(6):694–705. doi:10.1016/j.biotechadv.2010.05.002
Chang YC, Choi D, Takamizawa K, Kikuchi S (2014) Isolation of Bacillus sp. strains capable of decomposing alkali lignin and their application in combination with lactic acid bacteria for enhancing cellulase performance. Bioresour Technol 152:429–436. doi:10.1016/j.biortech.2013.11.032
Chen XY, Zaro JL, Shen WC (2013) Fusion protein linkers: property, design and functionality. Adv Drug Deliver Rev 65(10):1357–1369. doi:10.1016/j.addr.2012.09.039
Du MH (2011) Isolation of laccase-producing Bacillus subtilis strain and characterization and dye decolorization capacity of its laccase. Master thesis, Northeast Forestry University, Harbin, China
Durão P, Chen Z, Fernandes AT, Hildebrandt P, Murgida DH, Todorovic S, Pereira MM, Melo EP, Martins LO (2008) Copper incorporation into recombinant CotA laccase from Bacillus subtilis: characterization of fully copper loaded enzymes. J Biol Inorg Chem 13(2):183–193. doi:10.1007/s00775-007-0312-0
Enguita FJ, Marcal D, Martins LO, Grenha R, Henriques AO, Lindley PF, Carrondo MA (2004) Substrate and dioxygen binding to the endospore coat laccase from Bacillus subtilis. J Biol Chem 279(22):23472–23476. doi:10.1074/jbc.M314000200
Falconer RJ, O'Neill BK, Middelberg APJ (1999) Chemical treatment of Escherichia coli: 3. Selective extraction of a recombinant protein from cytoplasmic inclusion bodies in intact cells. Biotechnol Bioeng 62(4):455–460. doi:10.1002/(SICI)1097-0290(19990220)62:4<455::AID-BIT8>3.0.CO;2-2
Fang Z, Zhou P, Chang F, Yin Q, Fang W, Yuan J, Zhang X, Xiao Y (2014) Structure-based rational design to enhance the solubility and thermostability of a bacterial laccase Lac15. PLoS One 9(7):e102423. doi:10.1371/journal.pone.0102423
Fu XY, Tong WY, Wei DZ (2005) Extracellular production of human parathyroid hormone as a thioredoxin fusion form in Escherichia coli by chemical permeabilization combined with heat treatment. Biotechnol Prog 21(5):1429–1435. doi:10.1021/Bp050137z
Furtado GP, Ribeiro LF, Lourenzoni MR, Ward RJ (2013) A designed bifunctional laccase/β-1,3-1,4-glucanase enzyme shows synergistic sugar release from milled sugarcane bagasse. Protein Eng Des Sel 26(1):15–23. doi:10.1093/protein/gzs057
Guan ZB, Song CM, Zhang N, Zhou W, Xu CW, Zhou LX, Zhao H, Cai YJ, Liao XR (2014) Overexpression, characterization, and dye-decolorizing ability of a thermostable, pH-stable, and organic solvent-tolerant laccase from Bacillus pumilus W3. J Mol Catal B Enzym 101:1–6. doi:10.1016/j.molcatb.2013.11.009
Hildén K, Hakala TK, Lundell T (2009) Thermotolerant and thermostable laccases. Biotechnol Lett 31(8):1117–1128. doi:10.1007/s10529-009-9998-0
Hu MR, Chao YP, Zhang GQ, Yang XQ, Xue ZQ, Qian SJ (2007) Molecular evolution of Fome lignosus laccase by ethyl methane sulfonate-based random mutagenesis in vitro. Biomol Eng 24(6):619–624. doi:10.1016/j.bioeng.2007.08.020
Ihssen J, Reiss R, Luchsinger R, Thöny-Meyer L, Richter M (2015) Biochemical properties and yields of diverse bacterial laccase-like multicopper oxidases expressed in Escherichia coli. Sci Rep 5:10465. doi:10.1038/srep10465
Kanonenberg K, Schwarz CK, Schmitt L (2013) Type I secretion systems—a story of appendices. Res Microbiol 164(6):596–604. doi:10.1016/j.resmic.2013.03.011
Kudanga T, Prasetyo EN, Sipilä J, Eberl A, Nyanhongo GS, Guebitz GM (2009) Coupling of aromatic amines onto syringylglycerol β-guaiacylether using Bacillus SF spore laccase: a model for functionalization of lignin-based materials. J Mol Catal B Enzym 61(3–4):143–149. doi:10.1016/j.molcatb.2009.06.003
Liu SL, Du K, Chen WZ, Liu G, Xing M (2012) Effective approach to greatly enhancing selective secretion and expression of three cytoplasmic enzymes in Escherichia coli through synergistic effect of EDTA and lysozyme. J Ind Microbiol Biotechnol 39(9):1301–1307. doi:10.1007/s10295-012-1136-7
Lončara N, Božićb N, Lopez-Santinc J, Vujčić Z (2013) Bacillus amyloliquefaciens laccase--from soil bacteria to recombinant enzyme for wastewater decolorization. Bioresour Technol 147:177–183. doi:10.1016/j.biortech.2013.08.056
Low KO, Mahadi NM, Abdul Rahim R, Rabu A, Abu Bakar FD, Abdul Murad AM, Illias RM (2010) Enhanced secretory production of hemolysin-mediated cyclodextrin glucanotransferase in Escherichia coli by random mutagenesis of the ABC transporter system. J Biotechnol 150(4):453–459. doi:10.1016/j.jbiotec.2010.10.001
Low KO, Mahadi NM, Rahim RA, Rabu A, Abu Bakar FD, Murad AM, Illias RM (2011) An effective extracellular protein secretion by an ABC transporter system in Escherichia coli: statistical modeling and optimization of cyclodextrin glucanotransferase secretory production. J Ind Microbiol Biotechnol 38(9):1587–1597. doi:10.1007/s10295-011-0949-0
Lu L, Zhao M, Wang TN, Zhao LY, Du MH, Li TL, Li DB (2012) Characterization and dye decolorization ability of an alkaline resistant and organic solvents tolerant laccase from Bacillus licheniformis LS04. Bioresour Technol 115:35–40. doi:10.1016/j.biortech.2011.07.111
Martins LO, Soares CM, Pereira MM, Teixeira M, Costa T, Jones GH, Henriques AO (2002) Molecular and biochemical characterization of a highly stable bacterial laccase that occurs as a structural component of the Bacillus subtilis endospore coat. J Biol Chem 277(21):18849–18859. doi:10.1074/jbc.M200827200
Mate DM, Alcalde M (2015) Laccase engineering: from rational design to directed evolution. Biotechnol Adv 33(1):25–40. doi:10.1016/j.biotechadv.2014.12.007
Mergulhão FJ, Summers DK, Monteiro GA (2005) Recombinant protein secretion in Escherichia coli. Biotechnol Adv 23(3):177–202. doi:10.1016/j.biotechadv.2004.11.003
Mohammadian M, Fathi-Roudsari M, Mollania N, Badoei-Dalfard A, Khajeh K (2010) Enhanced expression of a recombinant bacterial laccase at low temperature and microaerobic conditions: purification and biochemical characterization. J Ind Microbiol Biotechnol 37(8):863–869. doi:10.1007/s10295-010-0734-5
Mohorčič M, Teodorovič S, Golob V, Friedrich J (2006) Fungal and enzymatic decolourisation of artificial textile dye baths. Chemosphere 63(10):1709–1717. doi:10.1016/j.chemosphere.2005.09.063
Mollania N, Khajeh K, Ranjbar B, Rashno F, Akbari N, Fathi-Roudsari M (2013) An efficient in vitro refolding of recombinant bacterial laccase in Escherichia coli. Enzym Microb Technol 52(6–7):325–330. doi:10.1016/j.enzmictec.2013.03.006
Murugesan K, Kim YM, Jeon JR, Chang YS (2009) Effect of metal ions on reactive dye decolorization by laccase from Ganoderma lucidum. J Hazard Mater 168(1):523–529. doi:10.1016/j.jhazmat.2009.02.075
Ni Y, Chen R (2009) Extracellular recombinant protein production from Escherichia coli. Biotechnol Lett 31(11):1661–1670. doi:10.1007/s10529-009-0077-3
Osma JF, Toca-Herrera JL, Rodríguez-Couto S (2010) Biodegradation of a simulated textile effluent by immobilised-coated laccase in laboratory-scale reactors. Appl Catal, A 373(1–2):147–153. doi:10.1016/j.apcata.2009.11.009
Pereira L, Coelho AV, Viegas CA, Santos MM, Robalo MP, Martins LO (2009) Enzymatic biotransformation of the azo dye Sudan Orange G with bacterial CotA-laccase. J Biotechnol 139(1):68–77. doi:10.1016/j.jbiotec.2008.09.001
Pezzella C, Guarino L, Piscitelli A (2015) How to enjoy laccases. Cell Mol Life Sci 72(5):923–940. doi:10.1007/s00018-014-1823-9
Prehna G, Zhang G, Gong X, Duszyk M, Okon M, McIntosh LP, Weiner JH, Strynadka NC (2012) A protein export pathway involving Escherichia coli porins. Structure 20(7):1154–1166. doi:10.1016/j.str.2012.04.014
Rinas U, Hoffmann F (2004) Selective leakage of host-cell proteins during high-cell-density cultivation of recombinant and non-recombinant Escherichia coli. Biotechnol Prog 20(3):679–687. doi:10.1021/bp034348k
Riva S (2006) Laccases: blue enzymes for green chemistry. Trends Biotechnol 24(5):219–226. doi:10.1016/j.tibtech.2006.03.006
Rodríguez-Couto S (2012) A promising inert support for laccase production and decolouration of textile wastewater by the white-rot fungus Trametes pubescens. J Hazard Mater 233-234:158–162. doi:10.1016/j.jhazmat.2012.07.003
Rosano GL, Ceccarelli EA (2014) Recombinant protein expression in Escherichia coli: advances and challenges. Front Microbiol 5:172. doi:10.3389/fmicb.2014.00172
Sharma P, Goel R, Capalash N (2007) Bacterial laccases. World J Microbiol Biotechnol 23(6):823–832. doi:10.1007/s11274-006-9305-3
Sherif M, Waung D, Korbeci B, Mavisakalyan V, Flick R, Brown G, Abou-Zaid M, Yakunin AF, Master ER (2013) Biochemical studies of the multicopper oxidase (small laccase) from Streptomyces coelicolor using bioactive phytochemicals and site-directed mutagenesis. Microb Biotechnol 6(5):588–597. doi:10.1111/1751-7915.12068
Solís-Oba M, Ugalde-Saldívar VM, González I, Viniegra-González G (2005) An electrochemical–spectrophotometrical study of the oxidized forms of the mediator 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) produced by immobilized laccase. J Electroanal Chem 579(1):59–66. doi:10.1016/j.jelechem.2005.01.025
Sondhi S, Sharma P, Saini S, Puri N, Gupta N (2014) Purification and characterization of an extracellular, thermo-alkali-stable, metal tolerant laccase from Bacillus tequilensis SN4. PLoS One 9(5):e96951. doi:10.1371/journal.pone.0096951
Studier FW (2005) Protein production by auto-induction in high-density shaking cultures. Protein Expr Purif 41(1):207–234. doi:10.1016/j.pep.2005.01.016
Su L, Woodard RW, Chen J, Wu J (2013) Extracellular location of Thermobifida fusca cutinase expressed in Escherichia coli BL21(DE3) without mediation of a signal peptide. Appl Environ Microbiol 79(14):4192–4198. doi:10.1128/AEM.00239-13
Teng C, Jia H, Yan Q, Zhou P, Jiang Z (2011) High-level expression of extracellular secretion of a β-xylosidase gene from Paecilomyces thermophila in Escherichia coli. Bioresour Technol 102(2):1822–1830. doi:10.1016/j.biortech.2010.09.055
Ulčnik A, Cigić IK, Pohleven F (2013) Degradation of lindane and endosulfan by fungi, fungal and bacterial laccases. World J Microbiol Biotechnol 29(12):2239–2247. doi:10.1007/s11274-013-1389-y
Uthandi S, Prunetti L, De Vera IM, Fanucci GE, Angerhofer A, Maupin-Furlow JA (2012) Enhanced archaeal laccase production in recombinant Escherichia coli by modification of N-terminal propeptide and twin arginine translocation motifs. J Ind Microbiol Biotechnol 39(10):1523–1532. doi:10.1007/s10295-012-1152-7
Wang TN, Lu L, Wang JY, Xu TF, Li J, Zhao M (2015) Enhanced expression of an industry applicable CotA laccase from Bacillus subtilis in Pichia pastoris by non-repressing carbon sources together with pH adjustment: recombinant enzyme characterization and dye decolorization. Process Biochem 50(1):97–103. doi:10.1016/j.procbio.2014.10.009
Zhang G, Brokx S, Weiner JH (2006) Extracellular accumulation of recombinant proteins fused to the carrier protein YebF in Escherichia coli. Nat Biotechnol 24(1):100–104. doi:10.1038/nbt1174
Zhang Y, Liu YH, Li Y, Liu XG, Lu FP (2013) Extracellular expression of pullulanase from Bacillus naganoensis in Escherichia coli. Ann Microbiol 63(1):289–294. doi:10.1007/s13213-012-0472-1
Zhuo R, Ma L, Fan F, Gong Y, Wan X, Jiang M, Zhang X, Yang Y (2011) Decolorization of different dyes by a newly isolated white-rot fungi strain Ganoderma sp. En3 and cloning and functional analysis of its laccase gene. J Hazard Mater 192(2):855–873. doi:10.1016/j.jhazmat.2011.05.106
Zou C, Duan X, Wu J (2014) Enhanced extracellular production of recombinant Bacillus deramificans pullulanase in Escherichia coli through induction mode optimization and a glycine feeding strategy. Bioresour Technol 172:174–179. doi:10.1016/j.biortech.2014.09.035
Acknowledgments
We sincerely thank Professor Joel H. Weiner for kindly providing the pYebFH6/T7 plasmid.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
This study was funded by the financial support of the “948” project of the National Forestry Bureau (No. 2012-4-03), the National Key Basic Research Program of China (2014FY210400), and the Fundamental Research Funds for the Central Universities (2572014AA05).
Conflict of interest
All authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 640 kb)
Rights and permissions
About this article
Cite this article
Wang, TN., Zhao, M. A simple strategy for extracellular production of CotA laccase in Escherichia coli and decolorization of simulated textile effluent by recombinant laccase. Appl Microbiol Biotechnol 101, 685–696 (2017). https://doi.org/10.1007/s00253-016-7897-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7897-6