Abstract
Glycerol is an inexpensive and abundant source for biofuel production on a large scale. Escherichia coli is a robust bacterium for producing hydrogen; however, its hydrogen productivity from glycerol is low. In this study, we conducted random transposon mutagenesis to identify uncharacterized genes whose inactivation is beneficial for hydrogen production from glycerol. Through screening, four mutant strains were found that are able to have from 1.3- to 1.6-fold higher hydrogen productivity (μmol H2/mg protein) than that of their parent strain (p < 0.05). These mutations were identified as aroM, gatZ, ycgR, and yfgI. The hydrogen yield (mol H2/mol glycerol consumed) of the aroM, gatZ, ycgR, and yfgI strains was 1.7-, 1.4-, 2.4-, and 2.1-fold higher than that of their parent strain, respectively. Moreover, a single disruption in these genes resulted in a faster cell growth and glycerol consumption under anaerobic conditions. In E. coli, AroM is predicted to be involved in the shikimate pathway, GatZ is tagatose-1,6-bisphosphate aldolase 2 which converts dihydroxyacetone phosphate to 1,6-biphosphate, and YcgR acts as a molecular brake limiting the swimming speed and ATP consumption. So far, the function of YfgI in general and in hydrogen production in particular remains unknown.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fossil fuels such as oil and coal are forecasted to be depleted in the next 50–100 years, while the global thirst for energy will only increase. Biofuels are considered an ideal alternative because they can be easily and renewably produced from available and inexpensive biomass sources such as byproducts or wastes released from agricultural and industrial practices. Among biofuels, hydrogen is attractive because it does not evolve into greenhouse gases or other pollutants after combustion. Furthermore, hydrogen has 2.75-fold higher energy content compared to fossil fuel (Das and Veziroǧlu 2001; Kim and Kim 2011).
As a result of an increasing demand for biodiesel production, the global production of crude glycerol is expected to continue to increase (Anand and Saxena 2012). Meanwhile, the industrial purification of glycerol is likely not economically viable, since the surplus of crude glycerol has negatively impacted the glycerol market in recent years (Dobson et al. 2012; Yang et al. 2012). Moreover, crude glycerol from biodiesel production contains high amounts of salt, methanol, and soap that would be a burden to the environment (Hansen et al. 2009). Therefore, to assure the sustainable development of the biodiesel industry, crude glycerol as well as other byproducts from the biodiesel production should be utilized as a feedstock for other chemicals. In fact, dozens of studies have been recently conducted with the goal of converting crude glycerol into other value-added chemicals and biofuels. Of these, glycerol has been intensively used for hydrogen production via anaerobic fermentation (Blankschien et al. 2010; Clomburg and Gonzalez 2011, 2013; Mazumdar et al. 2013; Murarka et al. 2008; Tokumoto and Tanaka 2012; Tran et al. 2014; Trinh and Srienc 2009).
Escherichia coli is a potential microorganism for hydrogen production at the industrial scale because it can sustain a high growth rate, requires simple technology, and could achieve a high hydrogen yield and production (Maeda et al. 2007, 2008a; Shams Yazdani and Gonzalez 2008; Tran et al. 2014). Although the genome of E. coli is well characterized, only 54 % is experimentally determined, while the rest is either uncharacterized or computationally predicted (Riley et al. 2006). The metabolic flux of E. coli has been gradually elucidated and more new genes have been characterized (Blattner 1997; Gonzalez et al. 2008; Maeda et al. 2007; Sanchez-Torres et al. 2013). More recently, some pseudogenes which are regarded as nonfunctional DNA sequences and uncharacterized genes were determined to play a role in hydrogen production in E. coli (Mohd Yusoff et al. 2013). Hence, there is a gap in the current knowledge of the unknown metabolic pathways or genes related to hydrogen production of E. coli under glycerol metabolism. In addition, anaerobic growth of E. coli in the presence of glycerol is quite low (Hu and Wood 2010; Tran et al. 2014) unlike that of glucose (Maeda et al. 2007, 2008a).
Transposon mutagenesis is considered a powerful genetic tool to create random mutant strains, and this method has been intensively applied to characterize the function of unknown genes in various microorganisms (Ahmed 1985; Hamer et al. 2001; Liu et al. 2012; Nakata 2002; Pannekoek et al. 1980). In fact, random transposon mutagenesis has been used in various hydrogen-producing microorganisms such as Pantoea agglomerans, Rhodobacter, or Rhodovulum sulfidophilum to identify beneficial knockouts for hydrogen production, and this approach has gained momentum (Cai and Wang 2014; Liu et al. 2012; Ma et al. 2012a). Based on the aforementioned issues, this study aims to identify uncharacterized genes that are beneficial for hydrogen production in E. coli under glycerol metabolism by applying random transposon mutagenesis and screening.
Materials and methods
Random transposon mutagenesis
A single colony of E. coli BW25113, which is obtained from Yale Coli Genetic Stock Center (New Heaven, USA) (Table 1), was inoculated in Luria-Bertani (LB) medium without sodium chloride at 37 °C, 120 rpm overnight. The cell pellets were harvested by centrifugation at 7000 rpm for 10 min. Transposon mutagenesis was performed as described previously with some slight modifications (Liu et al. 2012; Nakata 2002). A Tnp Transposome™ Kit EZ-TN5 <KAN-2> Tnp Transposome™ Kit (Epicentre, Madison, WI, USA) was used to introduce random insertions into the host cell. The transformation was performed by electroporation at the parameters 1.2 kV and 200 Ω with a 0.1-cm electroporation gap cuvette. The electroporated cells were immediately transferred into 1 mL of SOC medium (2 % tryptone, 0.5 % yeast extract, 10 mM NaCl, 2.5 mM KCl, 10 mM MgCl2, 10 mM MgSO4, and 20 mM glucose) and incubated at 37 °C for 1 h to facilitate cell outgrowth. The number of transformants was counted by spreading 100 μL of electroporated cells on a LB plate with kanamycin (final conc 50 μg/mL). The transformation efficiency was calculated as the number of colony forming unit (cfu) over the amount of transposon used (Nakata 2002).
Preliminary screening of hydrogen production
The hydrogen assay was conducted anaerobically. A sealed crimp top vial (68 mL) which was sparged with nitrogen gas for 5 min was used for the fermentation with the rationale being that the fastest growing cells on glycerol would be selected. About 100 μL of electroporated cells were added into five separated sealed crimp top vials containing 20 mL of glycerol minimal medium (Murarka et al. 2008) supplemented with 50 μg/mL of kanamycin and incubated at 37 °C, 120 rpm for 2 days. The fermentation batches were successively cultured for five passages for mutant enrichment. In the final step, the fermentation broths were stocked and kept at −80 °C for screening purposes.
Single colonies were obtained by streaking the adapted culture stock onto LB plates containing kanamycin and were incubated overnight at 37 °C. To prevent missing any beneficial mutants for hydrogen production, the Taro Yamane formula was applied to calculate the population sample or the numbers of single mutant strain needed for investigation with the level of significance of 0.1 (Yamane 1967). A single E. coli strain was randomly selected for screening hydrogen production (below).
Identification of insertion site
The candidate mutant strains were cultivated overnight in LB medium containing kanamycin (50 μg/mL). Cell pellets were harvested and then the chromosomal DNA was extracted by UltraClean™ Microbial DNA Isolation Kit (Mo Bio Laboratories, Inc., Carlsbad, CA, USA). A two-round arbitrary PCR with some modifications was consecutively conducted to amplify the transposon flanking site (Das et al. 2005; Knobloch et al. 2003; Ma et al. 2012b). The arbitrary, internal and external primers used are listed in Table 1. First round arbitrary PCR (PCR1) was conducted using the arbitrary primer 1 together with the internal primer under the following conditions: 94 °C for 5 min; 6 cycles of 94 °C for 30 s, 30 °C for 30 s, for 72 °C 1.5 min; 30 cycles of 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 1.5 min; and 72 °C for 5 min. Samples were held at 4 °C. The PCR1 product was purified by QIAquick PCR Purification Kit (Qiagen, Hilden, Germany). About 100 ng of purified PCR1 was used as the DNA template for the second round PCR (PCR2), where the arbitrary primer 2 and the external primer were used with the following conditions: 95 °C for 1 min; 30 cycles of 95 °C for 30 s, 65 °C for 30 s, and 72 °C for 1.5 min; and 72 °C for 4 min.
The PCR2 products were run on 0.8 % agarose gel at 100 V for 25 min. A single band with a target size from 700 to 1200 base pairs was subjected for gel cutting. QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany) was used for gel extraction. The extracted DNA fragment was then run on 0.8 % agarose gel to assure that only a single band was attained prior to the DNA sequencing. A basic local alignment search tool (BLAST) from NCBI (http://www.ncbi.nlm.nih.gov/) (Wheeler and Bhagwat 2007) and Ecogene (http://www.ecogene.org) (Zhou and Rudd 2013) were mutually used for the alignment between the chromosomal DNA sequence of the amplified fragment and the genome database of E. coli strain, respectively. Only alignments with a high score value (above 98 % of similarity) were considered for the hydrogen confirmation and other analysis.
Hydrogen assay, growth condition, and confirmation
Based on the nucleotide BLAST (blastn) results, the candidate strains were obtained from the Keio library (National Institute of Genetics, Japan) as listed in the Table 1. These Keio strains were used to investigate their hydrogen production from glycerol. Minimal glycerol medium at pH 7.5 was used for both screening and confirmation of hydrogen production. Details of the hydrogen assay, growth condition, and medium were previously reported (Tran et al. 2014). Due to the requirement of a large sample population for parametric data analysis, hydrogen production of each strain was conducted for 10 independent batches. A test of analysis of variance (ANOVA) and Tukey post hoc with the significance level of 0.05 were conducted using a Statistical Package for the Social Sciences (SPSS) software (IBM, USA) to determine whether there is any difference among the strains regarding hydrogen production.
Quantification of organic acids, glycerol, and ethanol
Prior to the quantifications, the fermentation broth was filtered by a 0.2-μm membrane (Sartorious, Göttingen, Germany) to remove cells. A high-performance liquid chromatograph (8 mm × 300 mm, Shimadzu Co., Tokyo, Japan) using a Shim-Pack SCR-102H column and a CDD-6A electric conductivity detector was used to quantify organic acids; 5 mM p-toluenesulfonic acid monohydrate with a flow rate of 0.8 mL/min was used as the mobile phase (Maeda et al. 2009; Tran et al. 2014). To determine the glycerol consumption and yield, glycerol was quantified after 24, 48, 72, and 96 h of fermentation by the Free Glycerol Reagent Kit (Sigma, St. Louis, MO, USA). Ethanol was measured by GC 2025 gas chromatograph using CHP20-M25-025 capillary column (Shimadzu, Tokyo, Japan) (Sanchez-Torres et al. 2013; Tran et al. 2014). Data were collected from and represented as the mean of at least three biological replicate samples in each experiment.
Results
Random mutagenesis, preliminary screening, DNA sequencing, and BLAST analysis
The transposon Tn5 was successfully introduced into the genome of the strain E. coli BW25113. After 16 h of incubation at 37 °C, the numbers of cfu were roughly 3200 cfu/mL. The transformation efficiency was 1.6 × 105 cfu/μg. By applying the Taro Yamane formula with the significance level of 0.1, the population sample needed for the screening was 97. In other words, 97 individual colonies were required for the screening of hydrogen production. In fact, over 100 different colonies were randomly selected for screening of hydrogen production from glycerol. In the preliminary screening of hydrogen production, this study found over 30 single colonies that likely produced higher hydrogen than their parent strain. Besides, we also found some mutant strains whose hydrogen productions were significantly lower than that of strain BW25113 (data not published). Such strains were subjected for identification of the insertion site via arbitrary PCR and DNA sequencing. After two rounds of arbitrary PCR and gel extraction, single amplified DNA fragments containing kanamycin flanking sequence were obtained and ready for the DNA sequencing. Empirically, DNA template samples were successfully sequenced when the size ranges from 700 to 1200 base pairs.
Finally, excluding failed samples of DNA sequencing, low alignment score, and similar insertion sites, this study found nine different insertions by which each of the nine mutant strains produced 1.1–1.6-fold higher hydrogen production than the wild-type strain. These insertion sites were individually determined as aroM, garR, gatY, gatZ, pitA, ycgR, yfgI, yfhK, and yjhU (Table 2).
Confirmation of hydrogen production, glycerol consumption, and cell growth
The nine candidate strains selected from the preliminary screening were attained from the Keio library (Japan) and their hydrogen production ability was confirmed. Due to a small difference between the mutant strains and the wild-type strain in terms of hydrogen production, about 10 independent fermentation batches were conducted for each strain. Prior to the data analysis, to assure the consistency of the data, the outliers were removed. Additionally, the Kolmogorov-Smirnov test was conducted which indicated that the data were normally distributed and ready for parametric data analyses (p > 0.05).
A test of ANOVA and Tukey post hoc analysis were conducted to judge whether these differences were statistically significant or not. The statistical analysis showed that the hydrogen productivity of four out of the nine candidate strains, namely aroM, gatZ, ycgR, and yfgI, were statistically significantly different from that of the wild-type strain (p < 0.05) (Table 2). Although they had higher hydrogen production than the wild-type strain, strains garR, gatY, pitA, yfhK, and yjhU were not statistically different from BW25113 (p > 0.05). In other words, a single disruption in garR, gatY, pitA, yfhK, and yjhU was not beneficial for hydrogen production in E. coli from glycerol.
Among the mutant strains whose hydrogen productivity was significantly different from their parent strain, aroM has the highest hydrogen productivity—1.6-fold higher than that of the wild-type strain. Interestingly, all mutant strains grew anaerobically from 1.2- to 1.3-fold faster than their parent strain (Table 2). Hence, the respective disruptions were not only beneficial for hydrogen production, but also for biomass synthesis, which is an advantage for overcoming low cell growth during glycerol metabolism (Gonzalez et al. 2008; Murarka et al. 2008; Poladyan et al. 2013).
The kinetics of hydrogen production shows that strains aroM, gatZ, ycgR, and yfgI gradually increased their production over time and almost reached the maximum level by 96 h of fermentation. While strain aroM has the highest hydrogen production during the early log phase (after 48 h), strain gatZ was likely most active during the middle log phage or after 72 h (Fig. 1).
Under anaerobic conditions, strains aroM, gatZ, ycgR, and yfgI grew steadily and likely reached the log phase after 72 h. Unlike the parent strain, all the mutant strains reached the stationary phase after 72 h of fermentation. Additionally, four mutant strains with higher hydrogen productivity also consumed glycerol in a faster manner compared to the strain BW25113. After 96 h of fermentation, about 60 % of glycerol was consumed (Fig. 2).
Hydrogen yield
All strains whose hydrogen productivities were statistically significantly higher than those of the wild-type strain were analyzed for glycerol consumption. Hence, glycerol quantification was conducted for the four mutant strains: aroM, gatZ, ycgR, and yfgI (Table 3). Consistent with previous studies, the hydrogen yield of the wild-type strain was about 0.2 mol H2 produced per mol of glycerol consumed (Tran et al. 2014). Among the four strains, the ycgR mutant had the highest hydrogen yield—0.49 mol H2/mol glycerol consumed (Table 3). Even though the hydrogen yield of the four mutant strains were far lower than the theoretical maximum of 1 mol H2 formed per 1 mol glycerol consumed, these results are a considerable improvement in E. coli under glycerol fermentation; however, this yields less than for the best engineered strain, BW25113 ∆frdC ldhA fdnG ppc narG mgsA hycA which produces 0.67 mol H2/mol glycerol after 24 h under the same condition (Tran et al. 2014).
Organic acids and ethanol production
Together with hydrogen, ethanol, formate, and acetate were the main products during the glycerol metabolism. In fact, formate is the precursor of hydrogen production in that it is converted into hydrogen and carbon dioxide by the formate hydrogen lyase complex system (FHL) and the formate dehydrogenase (encoded by fdhF) (Axley et al. 1990; Bagramyan et al. 2002; Enoch and Lester 1975). Results from the organic acid measurements showed that a higher level of formate was detected in the fermentation broth of the mutant strains compared with that of the parent strain (Table 4, Fig. 3a). The results suggest that all four mutant strains, aroM, gatZ, ycgR, and yfgI, produced more formate than the wild-type strain during the fermentation.
Similar to previous studies, the wild-type strain produced more ethanol and somewhat slightly higher acetate than the mutant strains (Table 4, Fig. 3b) (Tran et al. 2014). This result is understandable since the intermediate products in the mutant strains were directed toward hydrogen production; thus, a reduced amount was converted into other end products such as ethanol and acetate.
Discussion
Generally, the metabolism of E. coli is rather complicated and the metabolites vary with each carbon source. Regarding hydrogen production, some knockouts are beneficial under glucose metabolism but they are not necessarily beneficial for glycerol or other carbon sources (Maeda et al. 2008a; Tran et al. 2014). With persistent effort, the metabolic pathway of E. coli for producing hydrogen has been gradually elucidated. Recently, hydrogen production in E. coli has been improved by applying metabolic engineering and by optimizing the fermentation conditions (Kim et al. 2006, 2009; Kim and Kim 2011; Maeda et al. 2007; Shams Yazdani and Gonzalez 2008; Tran et al. 2014). For example, in our previous study, we were able to create an engineered E. coli strain, BW25113 ∆frdC ldhA fdnG ppc narG mgsA hycA, which produced 5-fold higher hydrogen yield than the parent strain, and this strain could reach the theoretical maximum of 1 mol H2 formed per 1 mol of glycerol consumed after 48 h of fermentation (Tran et al. 2014). However, the hydrogen production of E. coli from glycerol is not yet industrially viable due to a slow cell growth and glycerol consumption. Therefore, additional effort to enhance hydrogen production in E. coli is necessary to overcome the emerging issues of overproduced glycerol and an increasing demand for renewable and clean energy.
In other aspects, controlling the metabolism of E. coli remains a challenge since only half of the genome of this species has been empirically characterized (Riley et al. 2006). Thus, there is potential to find out unknown genes or metabolic routes that are beneficial for hydrogen production. In addition to the well-characterized genes that play a critical role in hydrogen production, the current research aimed to find uncharacterized genes via random transposon mutagenesis.
A higher accumulation of formate together with a higher hydrogen production of the four mutant strains suggests that the deletions in aroM, gatZ, ycgR, and yfgI lead to an increase in the conversion of glycerol to formate. Therefore, the mutant strains were able to produce more hydrogen than the wild type. Consistent with previous studies, formate accounted for the highest amount of detected organic acids. It is unknown why formate accumulation occurs in glycerol metabolism but not in glucose metabolism (Sanchez-Torres et al. 2013; Tran et al. 2014; Zhang et al. 2010). Perhaps in glycerol metabolism, formate is highly excreted from the intracellular to the extracellular by the formate transporter (encoded by focA). In turn, a high level of formate was detected.
To our knowledge, aroM is cotranscribed with aroL which is required for the synthesis of shikimate kinase II. In general, the phosphoenol pyruvate is converted into shikimate that is a precursor of aromatic compounds (Ely and Pittard 1979; Herrmann 1995) (Fig. 4). Thus, blocking the shikimate pathway would result in an increase of phosphoenol pyruvate, and logically, more intermediates are available for the synthesis of formate. A higher level of accumulated formate detected in the fermentation broth of aroM mutant strain is evidence for this argument (Table 4, Fig. 3a). However, so far, the function of aroM in the metabolic pathway is not experimentally characterized (DeFeyter and Pittard 1986).
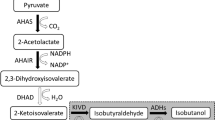
The metabolic pathway of E. coli under glycerol metabolism (Altaras and Cameron 1999; Bongaerts et al. 2001; Chao et al. 1993; Cooper 1984; Gonzalez et al. 2008; Herrmann 1995; Hu and Wood 2010; Saikusa et al. 1987; Tran et al. 2014; Truniger and Boos 1994). DHA dihydroxylacetone, DHAP dihydroxylacetone phosphate, G3P glycerol-3-phosphate, G3AP glyceraldehyde-3-phosphate, PEP phosphoenol pyruvate, T-1,6-diP tagarose-1,6-diphosphate
gatYZ encodes for the tagatose-1,6-bisphosphate aldolase, which catalyzes D-tagatofuranose 1,6-bisphosphate into dihydroxyacetone phosphate and D-glyceraldehyde 3-phosphate (Brinkkötter et al. 2002; Nobelmann and Lengeler 1995, 1996; Reizer et al. 1996). Blocking the pathway to the synthesis of tagatose-1,6-bisphosphate aldolase 2 (encoded by gatYZ) was shown to enhance hydrogen in E. coli (Hu and Wood 2010). However, in this study, a disruption in gatY did not make any significant difference in hydrogen production. In general, gatY requires gatZ for full activity and stability of adolases (Brinkkötter et al. 2002). Moreover, due to a considerable higher hydrogen production and growth rate, gatZ likely plays a bigger role than gatY does in the glycerol metabolism.
YcgR controls flagella motility in enterobacteria (Ko and Park 2000). It is predicted that the interaction between the YcgR-c-di-GMP complex and the flagella motor results in an impairment to the motility. Therefore, inactivation of ycgR enhances motility in the hns-deficient cell (Girgis et al. 2007; Ko and Park 2000). In other words, the c-di-GMP-binding protein YcgR acts as a backstop brake in enterobacter (Armitage and Berry 2010; Boehm et al. 2010; Paul et al. 2010). In this study, it is not clear why a disruption in ycgR led to an increase in hydrogen production. YfgI is an uncharacterized protein and the yfgI mutant is sensitive to the nalidixic acid, which is a DNA-damaging agent (Škunca et al. 2013). Hence, our knowledge about the yfgI-encoding protein and its role remains insufficient and it should be determined in future studies.
Hydrogen production of E. coli can be further improved by various methods such as controlling the pH and atmospheric condition and metabolic and protein engineering (Durnin et al. 2009; Kim et al. 2006; Maeda et al. 2008a, b; Sanchez-Torres et al. 2009; Tran et al. 2014; Trchounian et al. 2011). Undoubtedly, the findings of four new genes in this study could significantly contribute to the enhancement of hydrogen production in E. coli.
References
Ahmed A (1985) A rapid procedure for DNA sequencing using transposon-promoted deletions in Escherichia coli. Gene 39(2–3):305–310
Altaras NE, Cameron DC (1999) Metabolic engineering of a 1,2-propanediol pathway in Escherichia coli. Appl Environ Microbiol 65(3):1180–1185
Anand P, Saxena RK (2012) A comparative study of solvent-assisted pretreatment of biodiesel derived crude glycerol on growth and 1,3-propanediol production from Citrobacter freundii. N Biotechnol 29(2):199–205
Armitage JP, Berry RM (2010) Time for bacteria to slow down. Cell 141(1):24–26
Axley MJ, Grahame DA, Stadtman TC (1990) Escherichia coli formate-hydrogen lyase. Purification and properties of the selenium-dependent formate dehydrogenase component. J Biol Chem 265(30):18213–18218
Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006 0008
Bagramyan K, Mnatsakanyan N, Poladian A, Vassilian A, Trchounian A (2002) The roles of hydrogenases 3 and 4, and the F0F1-ATPase, in H2 production by Escherichia coli at alkaline and acidic pH. FEBS Lett 516(1–3):172–178
Blankschien MD, Clomburg JM, Gonzalez R (2010) Metabolic engineering of Escherichia coli for the production of succinate from glycerol. Metab Eng 12(5):409–419
Blattner FR (1997) The complete genome sequence of Escherichia coli K-12. Science 277(5331):1453–1462
Boehm A, Kaiser M, Li H, Spangler C, Kasper CA, Ackermann M, Kaever V, Sourjik V, Roth V, Jenal U (2010) Second messenger-mediated adjustment of bacterial swimming velocity. Cell 141(1):107–116
Bongaerts J, Krämer M, Müller U, Raeven L, Wubbolts M (2001) Metabolic engineering for microbial production of aromatic amino acids and derived compounds. Metab Eng 3(4):289–300
Brinkkötter A, Shakeri-Garakani A, Lengeler JW (2002) Two class II D-tagatose-bisphosphate aldolases from enteric bacteria. Arch Microbiol 177(5):410–419
Cai J, Wang G (2014) Photo-biological hydrogen production by an acid tolerant mutant of Rhodovulum sulfidophilum P5 generated by transposon mutagenesis. Bioresour Technol 154:254–259
Chao Y, Patnaik R, Roof WD, Young RF, Liao JC (1993) Control of gluconeogenic growth by pps and pck in Escherichia coli. J Bacteriol 175(21):6939–6944
Clomburg JM, Gonzalez R (2011) Metabolic engineering of Escherichia coli for the production of 1,2-propanediol from glycerol. Biotechnol Bioeng 108(4):867–879
Clomburg JM, Gonzalez R (2013) Anaerobic fermentation of glycerol: a platform for renewable fuels and chemicals. Trends Biotechnol 31(1):20–28
Cooper RA (1984) Metabolism of methylglyoxal in microorganisms. Annu Rev Microbiol 38(0066–4227):49–68
Das D, Veziroǧlu TN (2001) Hydrogen production by biological processes: a survey of literature. Int J Hydrog Energy 26(1):13–28
Das S, Noe JC, Paik S, Kitten T (2005) An improved arbitrary primed PCR method for rapid characterization of transposon insertion sites. J Microbiol Methods 63(1):89–94
DeFeyter RC, Pittard J (1986) Genetic and molecular analysis of aroL, the gene for shikimate kinase II in Escherichia coli K-12. J Bacteriol 165(1):226–232
Dobson R, Gray V, Rumbold K (2012) Microbial utilization of crude glycerol for the production of value-added products. J Ind Microbiol Biotechnol 39(2):217–226
Durnin G, Clomburg J, Yeates Z, Alvarez PJ, Zygourakis K, Campbell P, Gonzalez R (2009) Understanding and harnessing the microaerobic metabolism of glycerol in Escherichia coli. Biotechnol Bioeng 103(1):148–161
Ely B, Pittard J (1979) Aromatic amino acid biosynthesis: regulation of shikimate kinase in Escherichia coli K-12. J Bacteriol 138(3):933–943
Enoch HG, Lester RL (1975) The purification and properties of formate dehydrogenase and nitrate reductase from Escherichia coli. J Biol Chem 250(17):6693–6705
Girgis HS, Liu Y, Ryu WS, Tavazoie S (2007) A comprehensive genetic characterization of bacterial motility. PLoS Genet 3(9):1644–1660
Gonzalez R, Murarka A, Dharmadi Y, Yazdani SS (2008) A new model for the anaerobic fermentation of glycerol in enteric bacteria: trunk and auxiliary pathways in Escherichia coli. Metab Eng 10(5):234–245
Hamer L, DeZwaan TM, Montenegro-Chamorro MV, Frank SA, Hamer JE (2001) Recent advances in large-scale transposon mutagenesis. Curr Opin Chem Biol 5(1):67–73
Hansen CF, Hernandez A, Mullan BP, Moore K, Trezona-Murray M, King RH, Pluske JR (2009) A chemical analysis of samples of crude glycerol from the production of biodiesel in Australia, and the effects of feeding crude glycerol to growing-finishing pigs on performance, plasma metabolites and meat quality at slaughter. Anim Prod Sci 49(2):154–161
Herrmann KM (1995) The shikimate pathway as an entry to aromatic secondary metabolism. Plant Physiol 107(1):7–12
Hu H, Wood TK (2010) An evolved Escherichia coli strain for producing hydrogen and ethanol from glycerol. Biochem Biophys Res Commun 391(1):1033–1038
Kim D, Han S, Kim S, Shin H (2006) Effect of gas sparging on continuous fermentative hydrogen production. Int J Hydrog Energy 31(15):2158–2169
Kim DH, Kim MS (2011) Hydrogenases for biological hydrogen production. Bioresour Technol 102(18):8423–8431
Kim S, Seol E, Oh Y-K, Wang GY, Park S (2009) Hydrogen production and metabolic flux analysis of metabolically engineered Escherichia coli strains. Int J Hydrog Energy 34(17):7417–7427
Knobloch JKM, Nedelmann M, Kiel K, Bartscht K, Horstkotte MA, Dobinsky S, Rohde H, Mack D (2003) Establishment of an arbitrary PCR for rapid identification of Tn917 insertion sites in Staphylococcus epidermidis: characterization of biofilm-negative and nonmucoid mutants. Appl Environ Microbiol 69(10):5812–5818
Ko M, Park C (2000) Two novel flagellar components and H-NS are involved in the motor function of Escherichia coli. J Mol Biol 303(3):371–382
Liu H, Wang G, Zhu D, Pan G (2012) Improvement of hydrogen production by transposon-mutagenized strain of Pantoea agglomerans BH18. Int J Hydrog Energy 37(10):8282–8287
Ma C, Guo L, Yang H (2012a) Improved photo—hydrogen production by transposon mutant of Rhodobacter capsulatus with reduced pigment. Int J Hydrog Energy 37(17):12229–12233
Ma Q, Fonseca A, Liu W, Fields AT, Pimsler ML, Spindola AF, Tarone AM, Crippen TL, Tomberlin JK, Wood TK (2012b) Proteus mirabilis interkingdom swarming signals attract blow flies. ISME J 6(7):1356–1366
Maeda T, Sanchez-Torres V, Wood TK (2007) Enhanced hydrogen production from glucose by metabolically engineered Escherichia coli. Appl Microbiol Biotechnol 77:879–890
Maeda T, Sanchez-Torres V, Wood TK (2008a) Metabolic engineering to enhance bacterial hydrogen production. Microb Biotechnol 1(1):30–39
Maeda T, Sanchez-Torres V, Wood TK (2008b) Protein engineering of hydrogenase 3 to enhance hydrogen production. Appl Microbiol Biotechnol 79(1):77–86
Maeda T, Yoshimura T, Shimazu T, Shirai Y, Ogawa HI (2009) Enhanced production of lactic acid with reducing excess sludge by lactate fermentation. J Hazard Mater 168(2–3):656–663
Mazumdar S, Blankschien DM, Clomburg MJ, Gonzalez R (2013) Efficient synthesis of L-lactic acid from glycerol by metabolically engineered Escherichia coli. Microb Cell Factories 12:7
Mohd Yusoff MZ, Hashiguchi Y, Maeda T, Wood TK (2013) Four products from Escherichia coli pseudogenes increase hydrogen production. Biochem Biophys Res Commun 439(4):576–579
Murarka A, Dharmadi Y, Yazdani SS, Gonzalez R (2008) Fermentative utilization of glycerol by Escherichia coli and its implications for the production of fuels and chemicals. Appl Environ Microbiol 74(4):1124–1135
Nakata PA (2002) The generation of a transposon-mutagenized Burkeholderia glumae library to isolate novel mutants. Plant Sci 162(2):267–271
Nobelmann B, Lengeler JW (1995) Sequence of the gat operon for galactitol utilization from a wild-type strain EC3132 of Escherichia coli. Biochim Biophys Acta 1262(1):69–72
Nobelmann B, Lengeler JW (1996) Molecular analysis of the gat genes from Escherichia coli and of their roles in galactitol transport and metabolism. J Bacteriol 178(23):6790–6795
Pannekoek H, Hille J, Noordermeer I (1980) Relief of polarity caused by transposon Tn5: application in mapping a cloned region of the Escherichia coli uvrB locus essential for UV resistance. Gene 12(1–2):51–61
Paul K, Nieto V, Carlquist WC, Blair DF, Harshey RM (2010) The c-di-GMP binding protein YcgR controls flagellar motor direction and speed to affect chemotaxis by a “backstop brake” mechanism. Mol Cell 38(1):128–139
Poladyan A, Avagyan A, Vassilian A, Trchounian A (2013) Oxidative and reductive routes of glycerol and glucose fermentation by Escherichia coli batch cultures and their regulation by oxidizing and reducing reagents at different pHs. Curr Microbiol 66(1):49–55
Reizer J, Ramseier TM, Reizer A, Charbit A, Saier MH Jr (1996) Novel phosphotransferase genes revealed by bacterial genome sequencing: a gene cluster encoding a putative N-acetylgalactosamine metabolic pathway in Escherichia coli. Microbiology 142(Pt 2):231–250
Riley M, Abe T, Arnaud MB, Berlyn MK, Blattner FR, Chaudhuri RR, Glasner JD, Horiuchi T, Keseler IM, Kosuge T, Mori H, Perna NT, Plunkett G 3rd, Rudd KE, Serres MH, Thomas GH, Thomson NR, Wishart D, Wanner BL (2006) Escherichia coli K-12: a cooperatively developed annotation snapshot-2005. Nucleic Acids Res 34(1):1–9
Saikusa T, Rhee H-I, Watanabe K, Murata K, Kimura A (1987) Metabolism of 2-oxoaldehydes in bacteria: purification and characterization of methylglyoxal reductase from Escherichia coli. Agric Biol Chem 51(7):1893–1899
Sanchez-Torres V, Maeda T, Wood TK (2009) Protein engineering of the transcriptional activator FhlA to enhance hydrogen production in Escherichia coli. Appl Environ Microbiol 75(17):5639–5646
Sanchez-Torres V, Mohd Yusoff MZ, Nakano C, Maeda T, Ogawa HI, Wood TK (2013) Influence of Escherichia coli hydrogenases on hydrogen fermentation from glycerol. Int J Hydrog Energy 38(10):3905–3912
Shams Yazdani S, Gonzalez R (2008) Engineering Escherichia coli for the efficient conversion of glycerol to ethanol and co-products. Metab Eng 10(6):340–351
Škunca N, Bošnjak M, Kriško A, Panov P, Džeroski S, Šmuc T, Supek F (2013) Phyletic profiling with cliques of orthologs is enhanced by signatures of paralogy relationships. PLoS Comput Biol 9(1):e1002852
Tokumoto H, Tanaka M (2012) Novel anaerobic digestion induced by bacterial components for value-added byproducts from high-loading glycerol. Bioresour Technol 107:327–332
Tran KT, Maeda T, Wood TK (2014) Metabolic engineering of Escherichia coli to enhance hydrogen production from glycerol. Appl Microbiol Biotechnol 98(10):4757–4770
Trchounian K, Sanchez-Torres V, Wood TK, Trchounian A (2011) Escherichia coli hydrogenase activity and H2 production under glycerol fermentation at a low pH. Int J Hydrog Energy 36(7):4323–4331
Trinh CT, Srienc F (2009) Metabolic engineering of Escherichia coli for efficient conversion of glycerol to ethanol. Appl Environ Microbiol 75(21):6696–6705
Truniger V, Boos W (1994) Mapping and cloning of gldA, the structural gene of the Escherichia coli glycerol dehydrogenase. J Bacteriol 176:1796–1800
Wheeler D, Bhagwat M (2007) BLAST QuickStart: example-driven web-based BLAST tutorial. In: Bergman NH (ed) Comparative genomics: volumes 1 and 2. Humana Press Inc, Totowa
Yamane T (1967) Statistics, an introductory analysis. Harper and Row, New York
Yang F, Hanna MA, Sun R (2012) Value-added uses for crude glycerol-a byproduct of biodiesel production. Biotechnol Biofuels 5:13
Zhang X, Shanmugam KT, Ingram LO (2010) Fermentation of glycerol to succinate by metabolically engineered strains of Escherichia coli. Appl Environ Microbiol 76(8):2397–2401
Zhou J, Rudd KE (2013) EcoGene 3.0. Nucleic Acids Res 41(Database issue):D613–D624
Acknowledgments
The authors would like to thank the Japan Student Services Organization for providing the scholarship of K. T. Tran during this study. This research was supported by the JGC-S Scholarship Foundation. Many thanks to the NBRP-E. coli at the National Institute of Genetics (Japan) for providing Keio mutant strains.
Conflict of interest
We declare that we do not have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tran, K.T., Maeda, T., Sanchez-Torres, V. et al. Beneficial knockouts in Escherichia coli for producing hydrogen from glycerol. Appl Microbiol Biotechnol 99, 2573–2581 (2015). https://doi.org/10.1007/s00253-014-6338-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6338-7