Abstract
Glycerol and glucose fermentation redox routes by Escherichia coli and their regulation by oxidizing and reducing reagents were investigated at different pHs. Cell growth was followed by decrease of pH and redox potential (E h ). During glycerol utilization at pH 7.5 ∆pH, the difference between initial and end pH, was lower compared with glucose fermentation. After 8 h growth, during glycerol utilization E h dropped down to negative values (−150 mV) but during glucose fermentation it was positive (+50 mV). In case of glycerol H2 was evolved at the middle log phase while during glucose fermentation H2 was produced during early log phase. Furthermore, upon glycerol utilization, oxidizer potassium ferricyanide (1 mM) inhibited both cell growth and H2 formation. Reducing reagents dl-dithiothreitol (3 mM) and dithionite (1 mM) inhibited growth but stimulated H2 production. The findings point out the importance of reductive conditions for glycerol fermentation and H2 production by E. coli.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glycerol is known as a cheap and abundant carbon source due to its generation as an unavoidable by-product of biodiesel and fuel production [7, 11, 35]. Crude glycerol is attractive feedstock in fermentation processes: glycerol can be used by many bacteria as a carbon source for various valuable and interesting end-products including molecular hydrogen (H2) [7, 11, 35]. Only recently Gonzalez and co-workers [8] have discovered that glycerol dissimilation by Escherichia coli can take place in a fermentative manner. Study of E. coli-based platforms to convert low value raw glycerol to higher value reduced chemicals, fuels, or even to bacterial biomass is very attractive for biotechnology [10, 11, 16]. On the other hand, E. coli is the best-characterized bacterium and promising for glycerol utilization because it is one of the most commonly organisms used for metabolic engineering and industrial applications [10, 11, 16].
Glycerol metabolism in E. coli represents a relatively simple cluster of redox reactions leading to 3-phosphoglyceraldehyde, the entry point to the lower section of glycolytic pathway [5, 22]. These reactions result formation of different organic (acetic, succinic) acids and mostly ethanol, evolution of H2 and CO2. Interestingly, H2 produced at acidic pH has negative impact on cell growth and glycerol fermentation [8, 16]. Depending on medium composition, H2 could be also evolved at alkaline pH [29, 30]. However, the pH dependence of glycerol fermentation and H2 production is not clear.
Besides, bioenergy is frequently stored and released by means of redox reactions. The ability of bacteria to carry out redox reactions depends on the redox state of the environment, or its redox potential (E h ) [32]. The latter itself depends on rate of redox processes. This simple relationship is hard to understand and to apply in biotechnology.
The metabolism of bacteria is known to represents a set of redox processes affecting E h . The drop of E h (down to −550 to 600 mV) during anaerobic growth of bacteria in the absence of external electron acceptors has been well demonstrated [1, 2, 18, 20, 26, 32, 33]. Drop of E h might be a result of the secretion of redox active metabolites into the culture medium, leading to decrease of external pH [1, 2, 19] or might be connected with the processes on (in) the bacterial membranes [12, 15]. On the other hand, E h is suggested to be useful for monitoring changes in the metabolic state of bacterial cultures in biotechnology and for optimizing yield of fermentation products [13, 24]. Moreover, E h has been shown can be applied to discriminate among species of bacteria [6, 26].
In this article, absolutely novel data about oxidative and reductive processes during anaerobic utilization of glycerol by E. coli at a wide range of pH (5.5–7.5) are presented and compared with the fermentation of the other carbon source such as glucose. The kinetics of E h during growth of bacteria upon glycerol fermentation was first studied: significant differences in E h kinetics between glycerol and glucose fermentation by bacteria were observed. The effects of pH on E. coli growth and its link with E h were also established. Moreover, oxidizers and reducers were used for application of positive and negative E h values, respectively. Although bacterial growth was suppressed upon both the oxidative and reductive stresses, the reductive conditions only stimulated H2 formation during glycerol fermentation.
Materials and Methods
Bacterial Strain and Growth, pH Determination
The E. coli BW25113 (lacl q rrnB T14 ∆lacZ W116 hsdR514 ∆araBAD AH33 ∆rha BAD LD78 ) was supplied by Prof. T.K. Wood (Department of Chemical Engineering, Texas A & M University College Station, TX, USA; presently Department of Biotechnology, Pennsylvania State University, University Park, PA, USA). Bacteria were grown in batch culture under anaerobic conditions at 37 °C in peptone medium (20 g/l peptone, 2 g/l K2HPO4, 5 g/l NaCl) with glycerol or glucose of different concentrations from 1 to 15 g/l at different pHs. The pH was adjusted by 0.1 M NaOH and 0.1 N HCl and measured using a pH meter with selective pH electrode (HJ1131B, HANNA Instruments, Portugal). Optical density (OD) was measured by a spectrophotometer at a wavelength of 600 nm.
E h Measurements and H2 Production Assays
E h was measured by use of redox platinum (Pt) (EPB-1, Measuring Instruments State Enterprise (MISE), Gomel, Belarus, or PT42BNC, HANNA Instruments, Portugal) and titanium–silicate (Ti–Si) electrodes (EO-02, MISE, Gomel, Belarus). Ag/AgCl (saturated by KCl) electrode was as reference electrode. In contrast to Pt electrode sensitive to H2 and oxygen, Ti–Si electrode measures the overall E h and is not affected by the presence of H2 (or oxygen); this is allowing H2 detection under anaerobic conditions [22, 30]. H2 production rate (\( V_{{{\text{H}}_{ 2} }} \)) is expressed as difference between Pt and Ti–Si electrodes readings in mV in time per unit of OD.
Before assays, E h of two electrodes were checked in the control solution which was comprised by the mixture of 0.049 M potassium ferricyanide (K3[Fe(CN)6]) and 0.05 M potassium ferrocyanide (K4[Fe(CN)6]·3H2O) (pH 6.86). E h of both electrodes at 25 °C was of 245 ± 10 mV. Note that there are no significant differences between Pt and Ti–Si electrodes readings were detected during H2 assays in bacterial suspension without carbon source added; bacterial count alteration in the suspension by ~eightfold to tenfold had no marked effect on E h value [30]. Moreover, the determination used is closed to the method with Clark-type electrode employed by Noguchi et al. [17]. These authors had shown a strong correlation between E h and H2 production in liquid media.
The H2 yield is calculated by the decrease of E h to low negative values as described by Piskarev et al. [21]; it is expressed in mol L−1. Note that the E h decrease by H2 evolution did not depend on salt content in water solution; pH was not affected by H2 supplemented [21]. Moreover, H2 yield of 0.73 mol L−1 (at pH 6.5) (Table 1) was close to that of 0.90 mol L−1 determined by using different method—gas chromatography [16].
Others, Reagents, and Data Processing
All assays were done at 37 °C. dl-dithiothreitol (DTT), sodium dithionite, meat peptone, potassium ferry and ferro cyanide were from Sigma (USA), glycerol was from Unichem (China); other reagents used were of analytical grade. Data were averaged from duplicate- or triplicate-independent measurements, for which the standard errors do not exceed 3 % (if not indicated).
Results and Discussion
The Effects of Growth Medium pH on E. coli Growth During Glycerol and Glucose Fermentation and E h Drop
In this study, the lowering of medium pH and the drop of E h was observed with increase of bacterial count (OD) in E. coli BW25113 batch culture at different pHs (Figs. 1, 2, 3). Interestingly, in contrast to glycerol upon increasing glucose concentration (1–15 g/l) bacterial yield was increased; maximal growth was observed for glucose concentration range of 5–15 g/l, especially at pH 7.5 (Fig. 1b). The results can be explained due to low concentration of protons in the external medium at a high pH when fermentation of more substrate is favored. Another explanation of this effect can be that the membrane bound transport proteins and enzymes such as proton and potassium ion translocating F 0 F 1-ATPase and TrkA system, respectively, have optimal activity at pH 7.5 [27, 28] and play an essential role during glycerol and glucose fermentation [4]. This might be in favor with operation mode of F 0 F 1 in association with TrkA having evidenced thermodynamic and biological consequences [27].
E. coli BW25113 growth yields during fermentation of different concentrations of glycerol (a) or glucose (b) at different pHs. OD600 after 24 h bacterial growth was presented. Bacteria were grown as described in “Materials and methods”
pH change during E. coli BW25113 growth upon glycerol and glucose fermentation at different pHs. ∆pH was the difference between initial pH and after 24 h pH value. 10 g/l glucose or glycerol was added into the growth medium. For others, see legends to Fig. 1
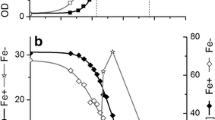
The kinetics of E h by E. coli BW25113 during glycerol and glucose fermentation at different pHs. E h , measured by platinum (a) and titanium–silicate (b) electrodes were expressed in mV (vs Ag/AgCl (saturated by KCl)). Experiments were performed in triplicate, average data are shown. For others, see legends to Fig. 2
During glucose fermentation E. coli exponential growth was usually observed for period of ~5–6 h, starting when the culture was ~2 h and ending at ~7–8 h that was non-depending on pH, whereas in glycerol fermented culture exponential growth was prolonged: it was monitored for period of ~10 h, starting when the culture was of ~2 h and ending ~12 h (not shown). This seems to contradict to data that during glycerol fermentation exponential growth is more prolonged: it lasts ~24 h [9]. This contradiction might be due to differences of growth medium composition. However, maximal yield with glycerol fermented cells was observed at pH 6.5 or 7.5 with all concentrations of glycerol, especially with the concentration of 10 g/l glycerol OD was 0.95 (Fig. 1a). In spite of differences, our findings are consistent with the results obtained by different authors [8, 35] that glycerol was metabolized optimally at pH 6.3.
Effects of pH on E. coli growth on glycerol (see Fig. 1) can be explained by many factors: for instance, pH may affect on the activity of glycerol dehydrogenase, which is the main enzyme of glycerol metabolism [9]. The enzyme represents more oxidizing properties at pH 7.5, in contrast to pH 6.5, when it has more reductive properties. On the other hand, alkaline conditions might increase the toxicity of methylglyoxal [9]. Another factor which could contribute to the impairment of glycerol fermentation is a limited generation of CO2, which is required for growth of bacteria and glycerol metabolism [23].
∆pH, which is the difference between initial pH and pH value after 24 h, was 4.2- and 3.8-fold lower during glycerol utilization at pH 6.5 and 7.5, respectively, compared with glucose fermentation (Fig. 2). Besides, during bacterial glycerol fermentation at pH 5.5 was not observed medium acidification and, moreover, growth medium pH was increased compared with both glycerol and glucose fermenting cells. This probably points out differences in fermentation end-products depending on carbon sources and medium pH [8, 14, 16, 22]. Indeed, lower amounts of acetic and less lactic acids could be produced during glycerol fermentation compared with glucose one as shown by Murarka et al. [16]. Another point is that as we have shown recently [4] H+ extrusion through F 0 F 1 and via other ways is lower compared with that under glucose fermentation.
It is worth to mention about interesting observation when bacteria were grown upon glycerol utilization at pH 5.5: in contrast to glucose fermentation during log growth phase E. coli has tendency to alkalize medium pH from 5.5 to 6.2 (data not shown). Probably, bacteria become well suited to acidic environment in a manner different from glucose fermented cells or involve other acid resistance mechanisms. In this respect, the intracellular pH might be different between glycerol and glucose fermentation; future determination of intracellular pH is required.
E h Drop and H2 Production
E h dependence on pH in two-based system is determined by the equation
(E 0 is a standard ORP, R, T, and F are known parameters, [ox] and [red] are activities of oxidized and reduced compounds), following E h decrease with increasing pH [32]. This was checked and confirmed for the assay mixture without bacteria (not shown). Though, for bacterial suspension, E h decrease could not be described by the equation above because of complexity of processes during glucose or glycerol fermentation.
In case of glycerol fermentation by E. coli, E h (measured by Pt electrode) dropped down to −400 ± 12 and −315 ± 10 mV when the culture was of 8 h growth only at pH 7.5 and 6.5, respectively (Fig. 3a). Consequently, H2 production was observed at the middle log growth phase. In glucose fermenting cells E h dropped to low negative values down to −600 ± 10 mV (Fig. 3b). H2 production was observed at the beginning of log growth phase, starting when the culture was of ~2–3 h, and at the stationary phase at different pHs. However, \( V_{{{\text{H}}_{ 2} }} \) was higher at acidic pH during glucose fermentation and it was decreased upon increasing pH (Fig. 4; Table 1). Interestingly, \( V_{{{\text{H}}_{ 2} }} \) was stimulated, even higher at pH 6.5 during glycerol fermentation than it was in glucose fermented culture (Fig. 4; Table 1). These results are expected due to many reasons. Among these are formate hydrogenlyase, which is responsible for H2 production and has optimal activity at acidic pH [25, 30, 31]. On the other hand, glycerol might be metabolized more rapidly, because of its shorter metabolic pathway compared with glucose [5, 8, 22, 35]. Moreover, maximal H2 yield was shown to occur at acidic pH [14]. It should be noted that E h drop for 24 h of growth was less during glycerol fermentation compared with glucose one (data not shown).
H2 production by E. coli BW25113 during glycerol and glucose fermentation at different pHs. H2 production rate (\( V_{{{\text{H}}_{ 2} }} \)) was expressed as difference between Pt and Ti–Si electrodes readings per h (for 8 h of growth) and OD. For others, see legends to Fig. 2
Investigating the kinetics of E h , additional remarkable differences were observed at different pHs: E h (measured by Ti–Si electrode) with glycerol culture was negative, whereas in glucose culture at the 3 h growth E h started to increase and after 8 h it became positive (Fig. 3b). But the E h values for both cases depended on pH. These data confirm the effects of pH on E h kinetics which was shown with E. coli and other bacteria: when growth medium pH was higher, E h represented more negative values [18, 26, 32, 34].
The effect of substrate on E h kinetics can be regarded by the properties of glycerol having highly reduced carbon and a higher production of reducing equivalents (NADH/NADPH/FADH2) during its fermentation compared with glucose [8, 35]. The reducing equivalents should have a profound effect on the whole metabolic network, when the higher NADH availability significantly changes the end-products pattern under anaerobic conditions [3, 35].
Effects of Reducing and Oxidizing Reagents on E. coli Growth, E h and H2 Production During Glycerol Fermentation
E h determinant role in E. coli growth in anaerobic conditions [1, 2, 32] means that various oxidizers and reducers affecting E h can mediate growth of bacteria. An impermeable oxidizer K3[Fe(CN)6] (1 mM) and reducers DTT (3 mM) and dithionite (1 mM) were used for application of E h initial positive (+235 ± 10 mV) and negative (−250 ± 10 mV) values to bacteria, respectively. In the presence of K3[Fe(CN)6] E. coli growth at different pHs was inhibited (Fig. 5) resulting in decrease of E h only to 130 ± 5 mV at the log growth phase. E h positive or less negative values following the presence of K3[Fe(CN)6] may oxidize thiol groups on bacterial surface and affect on membrane transport and enzymes like F 0 F 1 or TrkA under anaerobic conditions, leading to low activity of key enzymes in bacterial fermentation metabolism [2].
The effects of ferricyanide and DTT on E. coli BW25113 growth during fermentation of glycerol at different pHs. Ferricyanide (1 mM) or DTT (3 mM) was added into the growth medium. OD600 was determined after 24 h bacterial growth. For others, see legends to Fig. 2
In order to carry out the metabolic processes efficiently, it is very important to maintain the intracellular environment in the reducing state. However, membrane-permeating reducing reagents DTT (Fig. 5) and dithionite (data not shown), lowering E h down to enough negative values (−280 ± 12 mV), suppressed the growth of E. coli during glycerol fermentation in a concentration-dependent manner (not shown). These results are obviously expected. When reducing conditions prevail to the extreme, they may lead to instability in the metabolism. On the other hand, it was obtained, that during glycerol fermentation DTT at different pHs and dithionite but not oxidizer at pH 7.5 stimulated ~twofold to threefold H2 production in the log growth phase (Table 1). Similar effect with DTT was observed previously during glucose fermentation in E. coli [12]. This effect may be regarded to the reduced environment which might lead the increase of formate concentration [24] which then can be oxidized to H2 and CO2 [5, 22, 25, 31].
Note that the effect of dithionite on bacteria was investigated only at pH 7.5 because of instability of this reagent in acidic environment.
Conclusions
In this study, concentration-dependent glycerol and glucose fermentation by E. coli has been investigated. During bacterial growth the decrease of E h was observed with the acidification of the medium. The kinetics of E h by E. coli was first established during glycerol fermentation. The differences between E. coli growth on glycerol and glucose and their oxidative and reductive routes at different pHs were revealed.
The results with E h drop indicate the strengthening of reduction processes during glycerol fermentation and suggest that appropriate concentrations of carbon sources and reductive conditions are essential for E. coli growth and H2 production. The latter was observed at middle and early log growth phase during glycerol and glucose fermentation, respectively.
Moreover, reducing reagents stimulate H2 formation during log growth phase upon glycerol fermentation. Probably, (i) this effect was regarded to properties of carbon sources, (ii) this was due to different fermentation end-products secreted to the growth medium or, at last, (iii) this might be related with modification of bacterial membrane structure, particularly of accessible thiol groups number and composition.
All this is important for bacterial cell growth and physiology.
E h might be an additional physicochemical parameter to take into account for optimizing fermentation processes and developing H2 production biotechnology. The coupling of H2 production to utilization of waste materials containing high concentrations of glycerol may simultaneously provide economic and environmental benefits.
References
Bagramyan K, Trchounian A (1997) Decrease of redox potential in the anaerobic growing Escherichia coli suspension and proton-potassium exchange. Bioelecrochem Bioenerg 43:129–134
Bagramyan K, Galstyan A, Trchounian A (2000) Redox potential is a determinant in the Escherichia coli anaerobic fermentative growth and survival: effects of impermeable oxidant. Bioelectrochemistry 51:151–156
Berrios-Rivera SJ, Sanchez AM, Bennett GN, San KY (2004) Effect of different levels of NADH availability on metabolite distribution in Escherichia coli fermentation in minimal and complex media. Appl Microbiol Biotechnol 65:26–432
Blbulyan S, Avagyan A, Poladyan A, Trchouinian A (2011) Role of Escherichia coli different hydrogenases in H+ efflux and the FOF1-ATPase activity during glycerol fermentation at different pH. Biosci Rep 31:179–184
Booth IR (2005) Module 3.4.3. Glycerol and methylglyoxal metabolism. In: Neidhardt FG (Ed.-in-Chief). Escherichia coli and Salmonella: cellular and molecular biology. Online ed. ASM Press, Washington, DC. http://www.ecosal.org
Brasca M, Morandi R, Lodi R, Tamburini A (2006) Redox potential to discriminate among species of lactic acid bacteria. J Appl Microbiol 103:1516–1524
da Silva GP, Mack M, Contiero J (2009) Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol Adv 27:30–39
Dharmadi Y, Murarka A, Gonzalez R (2006) Anaerobic fermentation of glycerol by Escherichia coli: a new platform for metabolic engineering. Biotechnol Bioeng 94:821–828
Gonzalez R, Murarka A, Dharmadi Y, Yazdani S (2008) A new model for the anaerobic fermentation of glycerol in enteric bacteria: trunk and auxiliary pathways in Escherichia coli. Metab Eng 10:234–245
Hu H, Wood TK (2010) An evolved Escherichia coli strain for producing hydrogen and ethanol from glycerol. Biochem Biophys Res Commun 391:1033–1038
Khanna S, Goyal A, Moholkar VS (2011) Microbial conversion of glycerol: present status and future prospects. Crit Rev Biotechnol. doi:10.3109/07388551.2011.604839
Kirakosyan G, Bagramyan K, Trchounian A (2004) Redox sensing by Escherichia coli: effects of dithiothreitol, a redox reagent reducing disulphides, on bacterial growth. Biochem Biophys Res Commun 325:803–806
Kwong SCW, Rao G (1992) Effect of reducing agents in anaerobic amino acid fermentation. Biotechnol Bioeng 40:851–857
Lee H, Salerno MB, Rittmann BE (2008) Thermodynamic evaluation on H2 production in glucose fermentation. Environ Sci Technol 42:2401–2407
Michelon D, Abraham S, Ebel B, De Coninck J, Husson F, Feron G, Gervais P (2010) Contribution of exofacial thiol groups in the reducing activity of Lactococcus lactis. FEBS J 277:2282–2290
Murarka A, Dharmadi Y, Yazmandi S, Gonzalez R (2008) Fermentative utilization of glycerol by Escherichia coli and its implications for the production of fuels and chemicals. Appl Environ Microbiol 74:1124–1135
Noguchi K, Riggins DP, Eldahan KG, Kitko RD, Slonczewski JL (2010) Hydrogenase-3 contributes to anaerobic acid resistance of Escherichia coli. PLoS ONE 5:e10132
Oblinger JL, Kraft AA (1973) Oxidation–reduction potential and growth of Salmonella and Pseudomonas fluorescens. J Food Sci 38:1108–1112
Oktyabrsky ON, Smirnova GV (1988) Changes in the redox potential and the number of accessible sulfhydryl groups in Escherichia coli and Bacillus subtilis cultures during transient processes. Biokhimia 53:2042–2050 (in Russian)
Ouvry A, Wache Y, Tourdot-Marechal R, Divies C, Cachon R (2002) Effects of oxidoreduction potential combined with acetic acid, NaCl and temperature on growth, acidification, and membrane properties of Lactobacillus plantarum. FEMS Microbiol Lett 214:257–261
Piskarev M, Ushkanov VA, Aristova NA, Likhachev PP, Myslivets TS (2010) Establishment of the redox potential of water saturated with hydrogen. Biophysics 55:19–24
Poladyan A, Trchounian A (2009) Production of molecular hydrogen by mixed-acid fermentation in bacteria and its energetic. In: Trchounian A (ed) Bacterial membranes ultrastructure, bioelectrochemistry, bioenergetics and biophysics. Research Signpost, Trivandrum, pp 197–231
Repaske R, Clayton MA (1978) Control of Escherichia coli growth by CO2. J Bacteriol 135:1162–1164
Riondet C, Cachon R, Waché Y, Alcaraz G, Divies C (2000) Extracellular oxidoreduction potential modifies carbon and electron flow in Escherichia coli. J Bacteriol 182:620–624
Sawers RG (2005) Formate and its role in hydrogen production in Escherichia coli. Biochem Soc Trans 33:42–46
Soghomonyan D, Akopyan K, Trchounian A (2011) pH and oxidation–reduction potential change of environment during growth of lactic acid bacteria: effect of oxidizers and reducers. Appl Biochem Microbiol 47:33–38
Trchounian A (2004) Escherichia coli proton-translocating F 0 F 1-ATP synthase and its association with solute secondary transpopters and/or enzymes of anaerobic oxidation–reduction under fermentation. Biochem Biophys Res Commun 315:1051–1057
Trchounian A, Kobayashi H (1999) Kup is the major K+ uptake system in Escherichia coli upon hyper-osmotic stress at a low pH. FEBS Lett 447:144–148
Trchounian K, Trchounian A (2009) Hydrogenase 2 is most and hydrogenase 1 is less responsible for H2 production by Escherichia coli under glycerol fermentation at neutral and slightly alkaline pH. Int J Hydrogen Energy 34:8839–8845
Trchounian K, Sanchez-Torres V, Wood TK, Trchounian A (2011) Escherichia coli hydrogenase activity and H2 production under glycerol fermentation at low pH. Int J Hydrogen Energy 36:4323–4331
Trchounian K, Poladyan A, Vassilian A, Trchounian A (2012) Multiple and reversible hydrogenases for hydrogen production by Escherichia coli: dependence on fermentation substrate, pH and FOF1-ATPase. Crit Rev Biochem Mol Biol 47(3):236–249
Vassilian A, Trchounian A (2009) Environment oxidation–reduction potential and redox sensing by bacteria. In: Trchounian A (ed) Bacterial membranes ultrastructure, bioelectrochemistry, bioenergetics and biophysics. Research Signpost, Trivandrum, pp 163–195
Waché Y, Riondet C, Diviès C, Cachon R (2002) Effect of reducing agents on the acidification capacity and the proton motive force of Lactococcus lactis ssp. cremoris resting cells. Bioelectrochemistry 57:113–118
Warden WC, Hentgen DJ (1975) Differential effects of oxygen and oxidation–reduction potential on the multiplication of three species of anaerobic intestinal bacteria. J Appl Microbiol 30:781–785
Yazdani S, Gonzalez R (2007) Anaerobic fermentation of glycerol, a path to economic viability for the biofuels industry. Curr Opin Biotechnol 18:213–221
Acknowledgments
This study was done in frame of Research Grant to AT (#11-F-202) from Ministry of Education and Sciences of the Republic of Armenia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poladyan, A., Avagyan, A., Vassilian, A. et al. Oxidative and Reductive Routes of Glycerol and Glucose Fermentation by Escherichia coli Batch Cultures and Their Regulation by Oxidizing and Reducing Reagents at Different pHs. Curr Microbiol 66, 49–55 (2013). https://doi.org/10.1007/s00284-012-0240-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-012-0240-2