Abstract
Objective
Metabolic engineering efforts are guided by identifying gene targets for overexpression and/or deletion. Isobutanol, a biofuel candidate, is biosynthesized using the valine biosynthesis pathway and enzymes of the Ehrlich pathway. Most reported studies for isobutanol production in Escherichia coli employ multicopy plasmids, an approach that suffers from disadvantages such as plasmid instability, increased metabolic burden, and use of antibiotics to maintain selection pressure. Cofactor imbalance is another issue that may limit production of isobutanol, as two enzymes of the pathway utilize NADPH as a cofactor.
Results
To address these issues, we constructed E. coli strains with chromosomally-integrated, codon-optimized isobutanol pathway genes (ilvGM, ilvC, kivd, adh) selected on the basis of their cofactor preferences. Genes involved in diverting pyruvate flux toward fermentation byproducts were deleted. Metabolite analyses of the constructed strains revealed extracellular accumulation of significant amounts of isobutyraldehyde, a pathway intermediate, and the overflow metabolites 2,3-butanediol and acetol.
Conclusions
These results demonstrate that the genetic modifications carried out led to activation of alternative pathways that diverted carbon flux toward formation of unwanted metabolites. The present study highlights how precursor metabolites can be metabolized through enzymatic routes that have not been considered important in previous studies due to the different strategies employed therein. The insights gained from the present study will allow rational genetic modification of host cells for production of metabolites of interest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Isobutanol (2-methyl-1-propanol) is a four-carbon alcohol that is a potential biofuel candidate. It is a superior biofuel compared to ethanol because of its higher energy content, lower vapour pressure, lesser hygroscopicity and greater blend volume (Atsumi and Liao 2008; Tashiro et al. 2015; Yan and Liao 2009). Apart from its use in the transportation and biofuel sector, isobutanol also serves as a precursor for the production of a variety of polymers, paints, plastics and synthetic rubber (Kolodziej and Scheib 2012).
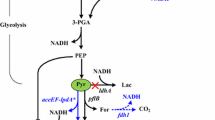
Isobutanol production using engineered Escherichia coli was first reported by Atsumi et al. (2008), who exploited the host’s native branched-chain amino acid (BCAA) biosynthesis pathways for higher alcohol synthesis (Fig. 1). A number of studies have since reported using different approaches to produce isobutanol in E. coli at high yields and titres (Atsumi et al. 2010; Baez et al. 2011; Bastian et al. 2011; Huo et al. 2011; Liu et al. 2016; Shi et al. 2013; Trinh et al. 2011). Most approaches for isobutanol bioproduction utilize plasmids to overexpress genes of the isobutanol biosynthesis pathway. It is advantageous to express genes directly from the chromosome due to drawbacks such as segregational instability and copy-number effects associated with plasmid-based systems (Ajikumar et al. 2010; Lemuth et al. 2011; Sabido et al. 2013). Apart from a few reports (Akita et al. 2015; Trinh et al. 2011), most studies have employed plasmids to overexpress enzymes, which is not feasible for scale up due to the disadvantages associated with using plasmids (Atsumi et al. 2008; Baez et al. 2011; Savrasova et al. 2011).
The isobutanol pathway is cofactor imbalanced under anaerobic conditions as two enzymes of the pathway, acetohydroxy isomeroreductase (AHAIR) and some alcohol dehydrogenases (ADHs), utilize NADPH as cofactor (Fig. 1). A number of studies have focused on addressing this imbalance by increasing cofactor availability either by using enzymes engineered using directed evolution (Bastian et al. 2011) or by employing expression of NADPH-generating pathways (Liu et al. 2016; Shi et al. 2013; Trinh et al. 2011). However, overexpression of membrane-bound transhydrogenases such as PntAB is known to exert metabolic burden on the cell (Chin et al. 2009; Kabus et al. 2007).
The present study sought to address the above concerns by integrating genes of the isobutanol pathway in the E. coli genome and by expressing NADH-utilizing heterologous AHAIR and native ADH isozymes. The plasmid-free strains thus constructed were found to produce isobutyraldehyde, the immediate precursor of isobutanol, as well as metabolites that arise from pathways that branch off at various nodes of the glycolytic and BCAA pathways. Carbon flux through these pathways has not been demonstrated in earlier studies to divert flux from isobutanol formation, illustrating the role of alternative pathways in impacting production of metabolites of interest.
Materials and methods
Strains, plasmids and growth conditions
Bacterial strains and plasmids used in this study are listed in Table 1. Primers used are listed in Table 2. All plasmids were maintained in E. coli DH5α. Genetic manipulations were carried out in E. coli BW25113. E. coli strains were routinely cultivated in LB medium at 37 °C. M9 minimal medium contained 6 g Na2HPO4, 3 g KH2PO4, 1 g NH4Cl, 0.5 g NaCl, 1 ml of 1 M MgSO4 and 0.1 ml of 1 M CaCl2 (per litre). Antibiotics were used at the following final concentrations: ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; chloramphenicol, 25 μg/ml. l-Rhamnose and l-arabinose were used at concentrations of 1 mM and 0.2%, respectively. For screening of integrants in the melAB and mtlADR loci, melibiose and d-mannitol were added to MacConkey agar base at a final concentration of 1%, respectively.
Gene deletion
The frsA, ackA-pta, ldhA, frdBC and adhE genes were sequentially deleted from the E. coli BW25113 genome using the one-step gene inactivation protocol (Datsenko and Wanner 2000) to construct E. coli Δ5. Briefly, the disruption cassette from template plasmid pKD13 (Datsenko and Wanner 2000), comprising of an FRT-flanked kanamycin resistance gene, was amplified using primers that included 50 nt homology extensions upstream and downstream of the genes targeted for deletion. The purified PCR products were electroporated in E. coli BW25113 carrying the pKD46 Red helper plasmid (Datsenko and Wanner 2000). Kanamycin-resistant transformants were tested using PCR to confirm gene deletion, and the FRT-flanked disruption cassette was eliminated by transforming mutants with plasmid pCP20 (Cherepanov and Wackernagel 1995) and incubating at 42 °C for Flp-mediated elimination of the kanamycin resistance gene.
Cloning and genome integration of ilvGM
The gene coding for acetohydroxy acid synthase (AHAS) II, ilvGM, was amplified from the E. coli B genome (GenBank accession CP000819.1) using Pfu DNA polymerase (Thermo Scientific, USA). Splice overlap extension (SOE) PCR was carried out using primers that carried a silent mutation to remove an internal NdeI site from the E. coli B ilvGM gene. The amplified SOE PCR product was cloned in template plasmid pSD112 (Deb et al. 2016) to construct plasmid pSD112-ilvGM. The genome integration cassette from pSD112-ilvGM was amplified using primers for integration within the melAB locus, and the purified PCR product was electroporated in E. coli Δ5 transformed with plasmid pKD46 to construct E. coli Δ51. Transformants were selected on MacConkey agar plates containing kanamycin and melibiose (Albermann et al. 2010), and the FRT-flanked kanamycin resistance gene was eliminated as described above.
To confirm expression of AHAS, E. coli Δ5 (control) and Δ51 were cultivated in LB medium at 37 °C in an orbital shaker (180 rpm). When cultures reached OD600 ∼ 0.6, l-rhamnose was added at a final concentration of 0.2%, and incubation was allowed to continue overnight. The next day, cell-free supernatant was analyzed by the Voges-Proskauer method (Westerfield 1945) to detect formation of acetoin due to overexpression of AHAS.
Replacement of native ilvC with heterologous ilvC homolog
The acetohydroxy acid isoreductomerase (AHAIR) gene from Thermacetogenium phaeum (Brinkmann-Chen et al. 2014) (GenBank accession NC_018870.1) was codon optimized and synthesized by GenScript (Piscataway, NJ, USA) and cloned in template plasmid pSD111 (Deb et al. 2016) to construct plasmid pSD111-CTP. The genome integration cassette (downstream from the T7 promoter) from plasmid pSD111-CTP was amplified using primers for replacement of the native E. coli ilvC gene. The purified PCR product was electroporated in E. coli Δ51 transformed with plasmid pKD46 (Datsenko and Wanner 2000) to construct E. coli Δ52. Transformants were selected on LB agar plates containing kanamycin, and the FRT-flanked kanamycin resistance gene was eliminated.
Replacement of native ilvC with heterologous ilvC was confirmed using PCR. Functional complementation of native ilvC by the heterologous homolog was confirmed by patching E. coli Δ52 on M9 agar plates supplemented with 1% glucose (with no added valine) and observing growth after overnight incubation at 37 °C. E. coli Δ51 was used as control and patched on the same plate.
Genome integration of kivd
The gene coding for ketoisovalerate decarboxylase (KIVD) from Lactococcus lactis (de la Plaza et al. 2004) (GenBank accession NC_013656.1) was codon optimized and synthesized by GenScript (Piscataway, NJ, USA) and cloned in template plasmid pSD112 (Deb et al. 2016) to construct plasmid pSD112-KLL. The genome integration cassette from plasmid pSD112-KLL was amplified using primers for integration within the mltADR locus. The purified PCR product was electroporated in E. coli Δ51 and Δ52 transformed with plasmid pKD46 to construct E. coli Δ54 and Δ55, respectively. Transformants were selected on MacConkey agar plates containing kanamycin and mannitol, and the FRT-flanked kanamycin resistance gene was eliminated.
Promoter replacement of native alcohol dehydrogenase genes
The promoter replacement cassette from template plasmid pSD222 (Deb et al. 2016), comprising of the FRT-flanked chloramphenicol resistance gene and the downstream rhaPBAD promoter, was amplified using primers that included 50 nt homology extensions upstream and downstream of the promoter regions of adhP, yahK, dkgA, fucO and yqhD alcohol dehydrogenase genes. Purified PCR products were individually electroporated in E. coli Δ54 and Δ55 transformed with plasmid pKD46 (Datsenko and Wanner 2000). Transformants were selected on LB agar plates containing chloramphenicol, and promoter replacement was confirmed using PCR.
GC–MS analysis for metabolite detection
For detection of metabolites, E. coli strains were cultivated in 10 ml LB broth supplemented with 0.5% glucose and 0.5% l-rhamnose at 30 °C in an orbital shaker (180 rpm). After 24 h of cultivation (OD600 = 1.8), cells were collected by centrifugation and resuspended in 1 ml fresh LB broth supplemented with 1% glucose in a screw capped 15 ml tube and incubated at 30 °C in an orbital shaker (180 rpm). After 4 h, when glucose in the medium was exhausted, cells were separated from the medium by centrifugation at 9000×g for 10 min at 4 °C, and the supernatant extracted with 1 ml ethyl acetate. 5 μl of the ethyl acetate layer was injected in an Agilent Technologies (USA) 7890A gas chromatograph (GC) system used with an Agilent 5975C mass selective detector equipped with an Agilent DB-Wax column (30 m × 320 μm × 0.50 μm). Helium was used as carrier gas (1 ml/min flow rate). The GC oven temperature was held at 40 °C for 2 min, initially ramped to 110 °C at 8 °C/min and held for 2 min, and then increased to 220 °C at 20 °C/min with a run time of 18.25 min. For detection of isobutyraldehyde, an Agilent 19091 J-413 HP-5 column (30 m × 320 μm × 0.25 μm) was used. The GC oven temperature was held at 40 °C for 2 min, ramped to 230 °C at 10 °C/min and held for 2 min with a run time of 23 min.
Sample peaks were identified by comparing with authentic standards, and by using the NIST Mass Spectral Search Program (version 2.0 f)
Results and discussion
Deletion of non-essential fermentative pathways in Escherichia coli BW25113
To conserve pyruvate and reduce carbon flux through fermentative pathways, the gene coding for FrsA was deleted from the E. coli BW25113 genome. FrsA binds to the unphosphorylated state of IIAGlc and acts as a fermentation-respiration switch that regulates the switch between fermentation and respiration pathways (Koo et al. 2004). Deletion of this gene leads to reduced formation of fermentation by-products, whereas overexpression leads to decreased rate of cellular respiration (Koo et al. 2004).
To further increase availability of pyruvate for diversion toward isobutanol synthesis, genes coding for production of acetate, lactate, succinate and ethanol (ackA-pta, ldhA, frdBC and adhE, respectively) were sequentially deleted to construct E. coli Δ5. Formation of lactate, formate and succinate was eliminated in E. coli Δ5, while acetate production was reduced (Fig. 2).
Expression of enzymes of the valine biosynthesis pathway
Acetohydroxy acid synthase (AHAS; EC 2.2.1.6) catalyzes the first step in the valine biosynthesis pathway and is the rate limiting step (Park et al. 2007). Of the three AHAS isozymes present in E. coli, AHAS II (coded by ilvGM) is resistant to feedback inhibition by l-valine (Gedi and Yoon 2012; Hill et al. 1997). As AHAS II is inactive in E. coli K-12 strains due to a frameshift mutation (Lawther et al. 1981), AHAS II from E. coli B was chosen for overexpression. ilvGM from E. coli strain B was integrated in E. coli Δ5 under control of the l-rhamnose inducible rhaPBAD promoter, the resultant integrant being designated E. coli Δ51. Functionality of the integrated gene was confirmed by performing enzyme assay for AHAS, which is based on the formation of acetoin through 2-acetolactate and further detection of acetoin using the Voges-Proskauer method. The formation of a cherry red color confirmed the functionality of AHAS (Fig. 3).
The rhaPBAD promoter is an inducible, strong and tunable promoter with low basal transcriptional activity that provides a varied range of expression levels depending on the concentration of inducer used (Brautaset et al. 2009; Egan and Schleif 1993; Giacalone et al. 2006; Haldimann et al. 1998). The advantage of using the rhaPBAD promoter for the isobutanol pathway is that it provides a possibility to tune gene expression levels by varying the inducer concentrations. This is an important parameter to consider since isobutanol is toxic to E. coli at concentrations > 8 g/l (Brynildsen and Liao 2009). Moreover, as l-rhamnose is not metabolized by the host strain E. coli BW25113 due to deletion of the chromosomal araBAD genes, the inducer concentration remains constant in the medium. Therefore, this promoter was chosen for overexpression of ilvGM and other downstream genes of the pathway.
The second step of the valine biosynthesis pathway is catalyzed by the enzyme acetohydroxy acid isomeroreductase (AHAIR; EC 1.1.1.86), encoded by the ilvC gene. Native AHAIR from E. coli is NADPH-dependant (Chunduru et al. 1989). Based on its low Km values and cofactor preference toward NADH (Brinkmann-Chen et al. 2014), we chose to express AHAIR from Thermacetogenium phaem.
2-Acetolactate, the product of AHAS II, is a substrate for AHAIR. A 100-fold increase in binding of RNA polymerase to the ilvC promoter is mediated by the positive activator protein IlvY in the presence of 2-acetolactate (Opel and Hatfield 2001; Rhee et al. 1998, 1999; Wek and Hatfield 1988). Therefore, expression of heterologous NADH-dependant ilvC gene from the native ilvC promoter may be sufficient to drive flux through the rest of the pathway. Based on this reasoning, ilvC from T. phaem was integrated in strain E. coli Δ51 under control of the native promoter of ilvC with the simultaneous replacement of the native ilvC gene to construct E. coli Δ52.
Functionality of heterologous ilvC was confirmed by growing the transformants containing the heterologous ilvC gene in minimal medium lacking l-valine. E. coli Δ52 showed similar growth as compared to the control strain E. coli Δ51, suggesting that the heterologous ilvC was able to functionally complement the replaced native E. coli ilvC gene (Fig. 4).
Expression of enzymes for isobutanol production
2-Ketoisovalerate is the penultimate metabolite in valine biosynthesis. This intermediate can be diverted toward isobutanol production by overexpression of genes of the Ehrlich pathway (Atsumi et al. 2008). The first step of the non-native isobutanol pathway is catalyzed by a 2-keto acid decarboxylase (KIVD; EC 4.1.1.72) encoded by the gene kivd from L. lactis, which leads to the formation of isobutyraldehyde by decarboxylation of 2- ketoisovalerate (de la Plaza et al. 2004).
kivd from L. lactis was integrated at the mtlADR locus in the E. coli Δ51 and E. coli Δ52 strains, the resultant integrants being designated E. coli Δ54 and E. coli Δ55, respectively. GC–MS analysis was carried out to detect isobutyraldehyde formation in E. coli Δ54 and E. coli Δ55 (data not shown).
The last step of the isobutanol pathway involves the conversion of isobutyraldehyde to isobutanol by alcohol dehydrogenases. Both native and non-native alcohol dehydrogenases have been assessed for isobutanol production (Atsumi et al. 2010). Overexpression of heterologous enzymes may sometimes lead to protein misfolding and formation of inclusion bodies (Atsumi et al. 2010). Overexpression of native enzymes may avoid issues of incompatible codon usage, misfolding and protein aggregation.
The isobutanol pathway is a NADPH- or a NADH-dependant pathway depending on the cofactor preferences of the AHAIR and ADH isozymes expressed. NADH-dependant enzymes are preferred since the intracellular levels of NADH are higher than those of NADPH (Bastian et al. 2011; Chin et al. 2009). Both NADPH- and NADH-dependant alcohol dehydrogenases have been used for isobutanol production (Atsumi et al. 2008, 2010; Bastian et al. 2011; Shi et al. 2013; Savrasova et al. 2011).
To assess the effect of different alcohol dehydrogenases on isobutanol production, five native E. coli alcohol dehydrogenases, based on their cofactor dependence, kinetic parameters and substrate preference, were chosen as candidates for overexpression.
YqhD is a NADPH-dependant aldehyde reductase that is active toward a variety of substrates, including isobutyraldehyde, butyraldehyde, methylglyoxal, furfural and acetol (Jarboe 2011). YqhD has been shown to be responsible for isobutanol production in the absence of overexpression of heterologous ADH, indicating its ability to convert isobutyraldehyde to isobutanol (Atsumi et al. 2010). AdhP is a NADH-dependant medium chain dehydrogenase/reductase that is active toward isobutyraldehyde (Rodriguez and Atsumi 2012; Shafqat et al. 1999). YahK is a NADPH-dependant medium chain dehydrogenase active toward a multitude of substrates including isobutyraldehyde, butyraldehyde and other medium chain aldehydes, with highest activity for isobutyraldehyde (Pick et al. 2013; Rodriguez and Atsumi 2014). DkgA (previously known as YqhE) is a NADPH-dependant oxidoreductase that is involved in ketogluconate metabolism (Habrych et al. 2002; Yum et al. 1999). DkgA is known to be active toward medium chain substrates including isobutyraldehyde and has no activity toward acetaldehyde (Rodriguez and Atsumi 2014). FucO is a well-characterized NADH-dependant lactaldehyde:propanediol oxidoreductase with activity toward isobutyraldehyde (Boronat and Aguilar 1979; Rodriguez and Atsumi 2014).
YqhD, AdhP, YahK, DkgA and FucO were screened for isobutanol production by individually expressing each of these genes by replacing their native promoters with the strong rhaPBAD promoter in E. coli Δ54 and E. coli Δ55.
Analysis of metabolite production in constructed strains
E. coli Δ54 and E. coli Δ55 individually overexpressing YqhD, AdhP, YahK, DkgA and FucO were grown in LB broth supplemented with glucose, and extracellular metabolites produced were analyzed using GC–MS. Isobutyraldehyde, the penultimate metabolite in the isobutanol biosynthesis pathway, was detected (Table 3), along with isovaleraldehyde, which is the penultimate metabolite in the pathway leading to 3-methyl-1-butanol (Fig. 5). However, isobutanol was not detected. E. coli Δ54.P, expressing NADPH-dependent native AHAIR and NADH-dependent AdhP, produced the highest amount of isobutyraldehyde, which represents ~ 6% of theoretical maximum (0.4 g/g glucose). This indicates a low flux through the isobutanol pathway; isobutanol formed from such low amounts of isobutyraldehyde may be difficult to detect, and would require special equipment (Rodriguez and Atsumi 2012) to trap and measure (for e.g., a gas stripping apparatus).
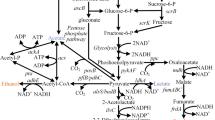
Pathway intermediates (in red boxes) and overflow metabolites (in blue boxes) observed in culture supernatants of E. coli Δ54 and E. coli Δ55 individually overexpressing YqhD, AdhP, YahK, DkgA and FucO. Enzyme activities reported in literature are indicated. Genes overexpressed in this study are underlined
The l-leucine biosynthesis pathway produces 2-ketoisocaproate, which is the precursor for l-leucine. 2-ketoisocaproate is a substrate for KIVD and can be further converted to 3-methyl-1-butanol by the action of various alcohol dehydrogenases (Atsumi et al. 2008). Detection of isovaleraldehyde in cell culture supernatants indicates diversion of carbon from 2-ketoisovalerate, which is the precursor for isobutanol synthesis, toward l-leucine biosynthesis (Fig. 5). Deletion of genes of the l-leucine pathway may prevent diversion of 2-ketoisovalerate into the l-leucine pathway. However, this was not attempted as deletion of the leuABCD operon (encoding the first three steps of the l-leucine pathway) would lead to l-leucine auxtrophy and therefore necessitate supplementation of growth media with l-leucine.
Overflow metabolites originating at two branch points, dihydroxyacetone phosphate (DHAP) and 2-acetolactate, were also observed in the culture supernatants of E. coli Δ54 and E. coli Δ55 expressing YqhD, AdhP, YahK, DkgA and FucO (Fig. 5). Detection of acetol and 1,2-propanediol (originating from DHAP) and diacetyl, acetoin and 2,3-butanediol (originating from 2-acetolactate) suggest that alternative pathways were activated due to the genetic modifications made in the constructed strains. The observation of overflow metabolites originating at the DHAP and 2-acetolactate nodes is in agreement with the results reported by Milne et al. (2016) in Saccharomyces cerevisiae engineered to produce isobutanol.
Overflow metabolites observed at dihydroxyacetone phosphate branch point
There are two routes for production of 1,2-propanediol (1,2-PDO): one route occurs via the glycolytic intermediate DHAP, and the other route involves the 6-deoxysugars fucose and rhamnose (Bennett and San 2001).
The 1,2-PDO pathway via DHAP involves the latter’s conversion to methylglyoxal by methylglyoxal synthase encoded by mgsA (Cooper 1984). An increased rate of carbon influx leads to elevated amounts of methylglyoxal (Tötemeyer et al. 1998). Methylgloxal is converted to 1,2-PDO (Fig. 5) via a l-lactaldehyde or an acetol intermediate (Cameron et al. 1998; Ko et al. 2005).
The alcohol dehydrogenase enzymes (YqhD, AdhP, YahK, DkgA and FucO) overexpressed in this study exhibit broad substrate specificities and can act on a range of substrates, including methylglyoxal, acetol and l-lactaldehyde (Jarboe 2011; Ko et al. 2005; Altaras and Cameron 1999, 2000; Lee et al. 2010). For instance, YqhD is a NADPH-dependant E. coli aldehyde reductase that has been used for production of 1,2-PDO, and is active toward both isobutyraldehyde and methylglyoxal (Atsumi et al. 2010; Jarboe 2011; Lee et al. 2010). The K isobutyraldehyde m and K methylglyoxal m values of YqhD are 2 mM and 2.6 mM, respectively, indicating that it can use either of the two substrates to form either isobutanol or acetol. However, YqhD has a higher kcat value for methylglyoxal (4.7 s−1) than isobutyraldehyde (1 s−1), implying a higher catalytic efficiency with the former. In addition, the specificity constant is kcat/km of YqhD toward methylglyoxal is 3.6-fold higher than that toward isobutyraldehyde (Atsumi et al. 2010; Jarboe 2011; Lee et al. 2010).
Similarly, YahK, DkgA and FucO are broad range aldehyde reductases that are active toward a broad range of substrates such as l-lactaldehdye, methylglyoxal and acetol, which are precursors of 1,2-PDO (Pick et al. 2013; Rodriguez and Atsumi 2014; Boronat and Aguilar 1979; Chen and Lin 1984; Niu and Guo 2015; Zhu and Lin 1989). l-Rhamnose has been reported to induce synthesis of FucO (Boronat and Aguilar 1979; Chen and Lin 1984); as l-rhamnose has been used to induce transcription of the integrated genes, FucO expression may be an artefact of the strategy employed in this study. Interestingly, the mgsA locus, coding for methylglyoxal synthase, has been used to integrate and overexpress ilvC for isobutanol production, which would have prevented formation of acetol and 1,2-PDO (Shi et al. 2013).
Therefore, the detection of acetol and 1,2-PDO in cell culture supernatants can be attributed to the broad substrate range of the alcohol dehydrogenases that have been employed in this study for the conversion of isobutyraldehyde to isobutanol in the engineered E. coli strain.
Overflow metabolites observed at 2-acetolactate branch point
GC–MS analysis of cell culture supernatants revealed presence of metabolites of the 2,3-butanediol pathway (diacetyl and acetoin) and 2,3-butanediol (2,3-BDO). This indicated diversion of carbon flux at the 2-acetolactate branch point toward 2,3-BDO synthesis, which may have occurred due to overexpression of native alcohol dehydrogenases exhibiting broad substrate ranges.
Under aerobic conditions, 2-acetolactate spontaneously decarboxylates to form diacetyl, which is further converted to acetoin by the action of acetolactate decarboxylase (Nielsen et al. 2010). In the last step, butanediol dehydrogenase catalyzes the conversion of acetoin to 2,3-BDO. The presence of 2,3-BDO pathway intermediates suggests that native E. coli enzymes may be responsible for catalyzing these reactions. Interestingly, Silber et al. (1974) reported purification of a NADPH-dependant reductase capable of reducing diacetyl to acetoin, the gene for which has not been identified.
Native and heterologous AHAIR, which catalyzes the second step in the valine biosynthesis pathway, were expressed from the native ilvC promoter. It is possible that expression of ilvC from its native promoter was not sufficient to pull carbon flux toward the isobutanol pathway and resulted in 2-acetolactate being diverted toward 2,3-BDO synthesis. Increased expression of ilvC from a stronger promoter may prevent carbon overflow from the valine biosynthesis pathway. Additionally, overexpression of ilvD encoding for DHAD may further drive flux through the isobutanol pathway and prevent formation of overflow metabolites at the 2-acetolactate branch point.
Conclusion
In the present study, E. coli BW25113 was rationally engineered to construct plasmid-free isobutanol producing strains. Three major strategies were adopted: conserving pyruvate, channelling flux toward valine production, and balancing cofactor usage. Analysis of performance of the constructed strains revealed diversion of carbon to alternative pathways, leading to formation of unwanted metabolites. This may be attributed to a low carbon flux through the valine biosynthesis pathway due to the use of the native promoter to express AHAIR, and the broad substrate ranges of the ADH isozymes overexpressed.
The low concentration of isobutyraldehyde observed in this study warrants using strong promoters to overexpress the valine biosynthesis pathway. Savrasova et al. (2011) reported using a valine-producing E. coli strain expressing four copies of genome-integrated ilvGMED operon from the strong λ phage PR promoter to produce isobutanol, while Akita et al. (2015) expressed genome-integrated valine and isobutanol biosynthesis genes from the strong T7 promoter. This allows the isobutanol-producing pathway to divert the high levels of 2-ketoisovalerate toward isobutanol formation.
The choice of ADH expressed to catalyze the last step of the isobutanol pathway influences the metabolites formed. Most studies have utilized the native YqhD alcohol dehydrogenase, which has been reported to have a high affinity for isobutyraldehyde (Atsumi et al. 2010). However, YqhD utilizes NADPH as a cofactor; in our attempt to use NADH-utilizing ADHs, we overexpressed the native E. coli enzymes AdhP and FucO. The results obtained suggest that in conjunction with our approach to increase flux through the valine pathway, this strategy led to the activation of alternative pathways, an issue not encountered by other researchers in earlier studies. This observation underscores the importance of alternative pathways in diverting carbon flux from molecules sought to be overproduced.
The insights gained from the present study will allow rational genetic modification of E. coli for production of metabolites of interest.
References
Ajikumar PK, Xiao WH, Tyo KEJ, Wang Y, Simeon F, Leonard E, Mucha O, Phon TH, Pfeifer B, Stephanopoulos G (2010) Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science 330:70–74
Akita H, Nakashima N, Hoshino T (2015) Bacterial production of isobutanol without expensive reagents. Appl Microbiol Biotechnol 99:991–999
Albermann C, Trachtmann N, Sprenger GA (2010) A simple and reliable method to conduct and monitor expression cassette integration into the Escherichia coli chromosome. Biotechnol J 5:32–38
Altaras NE, Cameron DC (1999) Metabolic engineering of a 1,2-propanediol pathway in Escherichia coli. Appl Environ Microbiol 65:1180–1185
Altaras NE, Cameron DC (2000) Enhanced production of (R)-1,2-propanediol by metabolically engineered Escherichia coli. Biotechnol Prog 16:940–946
Atsumi S, Liao JC (2008) Metabolic engineering for advanced biofuels production from Escherichia coli. Curr Opin Biotechnol 19:414–419
Atsumi S, Hanai T, Liao JC (2008) Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451:86–89
Atsumi S, Wu TY, Eckl EM, Hawkins SD, Buelter T, Liao JC (2010) Engineering the isobutanol biosynthetic pathway in Escherichia coli by comparison of three aldehyde reductase/alcohol dehydrogenase genes. Appl Microbiol Biotechnol 85:651–657
Baez A, Cho KM, Liao JC (2011) High-flux isobutanol production using engineered Escherichia coli: a bioreactor study with in situ product removal. Appl Microbiol Biotechnol 90:1681–1690
Bastian S, Liu X, Meyerowitz JT, Snow CD, Chen MM, Arnold FH (2011) Engineered ketol-acid reductoisomerase and alcohol dehydrogenase enable anaerobic 2-methylpropan-1-ol production at theoretical yield in Escherichia coli. Metab Eng 13:345–352
Bennett GN, San KY (2001) Microbial formation, biotechnological production and applications of 1,2-propanediol. Appl Microbiol Biotechnol 55:1–9
Boronat A, Aguilar J (1979) Rhamnose-induced propanediol oxidoreductase in Escherichia coli: purification, properties, and comparison with the fucose-induced enzyme. J Bacteriol 140:320–326
Brautaset T, Lale R, Valla S (2009) Positively regulated bacterial expression systems. Microb Biotechnol 2:15–30
Brinkmann-Chen S, Cahn JK, Arnold FH (2014) Uncovering rare NADH-preferring ketol-acid reductoisomerases. Metab Eng 26:17–22
Brynildsen MP, Liao JC (2009) An integrated network approach identifies the isobutanol response network of Escherichia coli. Mol Syst Biol 5:277
Cameron DC, Altaras NE, Hoffman ML, Shaw AJ (1998) Metabolic engineering of propanediol pathways. Biotechnol Prog 14:116–125
Chen YM, Lin EC (1984) Dual control of a common L-1,2-propanediol oxidoreductase by L-fucose and L-rhamnose in Escherichia coli. J Bacteriol 157:828–832
Cherepanov PP, Wackernagel W (1995) Gene disruption in Escherichia coli: tcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9–14
Chin JW, Khankal R, Monroe CA, Maranas CD, Cirino PC (2009) Analysis of NADPH supply during xylitol production by engineered Escherichia coli. Biotechnol Bioeng 102:209–220
Chunduru SK, Mrachko GT, Calvo KC (1989) Mechanism of ketol acid reductoisomerase–steady-state analysis and metal ion requirement. Biochemistry 28:486–493
Cooper RA (1984) Metabolism of methylglyoxal in microorganisms. Annu Rev Microbiol 38:49–68
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97:6640–6645
de la Plaza M, Fernández de Palencia P, Peláez C, Requena T (2004) Biochemical and molecular characterization of alpha-ketoisovalerate decarboxylase, an enzyme involved in the formation of aldehydes from amino acids by Lactococcus lactis. FEMS Microbiol Lett 238:367–374
Deb SS, Reshamwala SMS, Lali AM (2016) A series of template plasmids for Escherichia coli genome engineering. J Microbiol Methods 125:49–57
Egan SM, Schleif RF (1993) A regulatory cascade in the induction of rhaBAD. J Mol Biol 234:87–98
Gedi V, Yoon MY (2012) Bacterial acetohydroxyacid synthase and its inhibitors–a summary of their structure, biological activity and current status. FEBS J 279:946–963
Giacalone MJ, Gentile AM, Lovitt BT, Berkley NL, Gunderson CW, Surber MW (2006) Toxic protein expression in Escherichia coli using a rhamnose-based tightly regulated and tunable promoter system. Biotechniques 40:355–364
Habrych M, Rodriguez S, Stewart JD (2002) Purification and identification of an Escherichia coli beta-keto ester reductase as 2,5-diketo-d-gluconate reductase YqhE. Biotechnol Prog 18:257–261
Haldimann A, Daniels LL, Wanner BL (1998) Use of new methods for construction of tightly regulated arabinose and rhamnose promoter fusions in studies of the Escherichia coli phosphate regulon. J Bacteriol 180:1277–1286
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580
Hill CM, Pang SS, Duggleby RG (1997) Purification of Escherichia coli acetohydroxyacid synthase isoenzyme II and reconstitution of active enzyme from its individual pure subunits. Biochem J 327:891–898
Huo YX, Cho KM, Rivera JG, Monte E, Shen CR, Yan Y, Liao JC (2011) Conversion of proteins into biofuels by engineering nitrogen flux. Nat Biotechnol 29:346–351
Jarboe LR (2011) YqhD: a broad-substrate range aldehyde reductase with various applications in production of biorenewable fuels and chemicals. Appl Microbiol Biotechnol 89:249–257
Kabus A, Georgi T, Wendisch VF, Bott M (2007) Expression of the Escherichia coli pntAB genes encoding a membrane-bound transhydrogenase in Corynebacterium glutamicum improves l-lysine formation. Appl Microbiol Biotechnol 75:47–53
Ko J, Kim I, Yoo S, Min B, Kim K, Park C (2005) Conversion of methylglyoxal to acetol by Escherichia coli aldo-keto reductases. J Bacteriol 187:5782–5789
Kolodziej R, Scheib J (2012) Bio-isobutanol: the next-generation biofuel. hydrocarbon processing. http://www.hydrocarbonprocessing.com/magazine/2012/september-2012/special-report-refining-developments/bio-isobutanol-the-next-generation-biofuel. Accessed on 9 Dec 2018
Koo BM, Yoon MJ, Lee CR, Nam TW, Choe YJ, Jaffe H, Peterkofsky A, Seok YJ (2004) A novel fermentation/respiration switch protein regulated by enzyme IIAGlc in Escherichia coli. J Biol Chem 279:31613–31621
Lawther RP, Calhoun DH, Adams CW, Hauser CA, Gray J, Hatfield GW (1981) Molecular basis of valine resistance in Escherichia coli K-12. Proc Natl Acad Sci USA 78:922–925
Lee C, Kim I, Lee J, Lee KL, Min B, Park C (2010) Transcriptional activation of the aldehyde reductase YqhD by YqhC and its implication in glyoxal metabolism of Escherichia coli K-12. J Bacteriol 192:4205–4214
Lemuth K, Steuer K, Albermann C (2011) Engineering of a plasmid-free Escherichia coli strain for improved in vivo biosynthesis of astaxanthin. Microb Cell Fact 10:29
Liu Z, Liu P, Xiao D, Zhang X (2016) Improving isobutanol production in metabolically engineered Escherichia coli by co-producing ethanol and modulation of pentose phosphate pathway. J Ind Microbiol Biotechnol 43:851–860
Milne N, Wahl SA, van Maris AJA, Pronk JT, Daran JM (2016) Excessive by-product formation: a key contributor to low isobutanol yields of engineered Saccharomyces cerevisiae strains. Metab Eng Commun 3:39–51
Nielsen DR, Yoon SH, Yuan CJ, Prather KL (2010) Metabolic engineering of acetoin and meso-2, 3-butanediol biosynthesis in E. coli. Biotechnol J 5:274–284
Niu W, Guo J (2015) Stereospecific microbial conversion of lactic acid into 1,2-propanediol. ACS Synth Biol 4:378–382
Opel ML, Hatfield GW (2001) DNA supercoiling-dependent transcriptional coupling between the divergently transcribed promoters of the ilvYC operon of Escherichia coli is proportional to promoter strengths and transcript lengths. Mol Microbiol 39:191–198
Park JH, Lee KH, Kim TY, Lee SY (2007) Metabolic engineering of Escherichia coli for the production of l-valine based on transcriptome analysis and in silico gene knockout simulation. Proc Natl Acad Sci USA 104:7797–7802
Pick A, Rühmann B, Schmid J, Sieber V (2013) Novel CAD-like enzymes from Escherichia coli K-12 as additional tools in chemical production. Appl Microbiol Biotechnol 97:5815–5824
Rhee KY, Senear DF, Hatfield GW (1998) Activation of gene expression by a ligand-induced conformational change of a protein-DNA complex. J Biol Chem 273:11257–11266
Rhee KY, Opel M, Ito E, Hung SP, Arfin SM, Hatfield GW (1999) Transcriptional coupling between the divergent promoters of a prototypic LysR-type regulatory system, the ilvYC operon of Escherichia coli. Proc Natl Acad Sci USA 96:14294–14299
Rodriguez GM, Atsumi S (2012) Isobutyraldehyde production from Escherichia coli by removing aldehyde reductase activity. Microb Cell Fact 11:90
Rodriguez GM, Atsumi S (2014) Toward aldehyde and alkane production by removing aldehyde reductase activity in Escherichia coli. Metab Eng 25:227–237
Sabido A, Martínez LM, de Anda R, Martínez A, Bolívar F, Gosset G (2013) A novel plasmid vector designed for chromosomal gene integration and expression: use for developing a genetically stable Escherichia coli melanin production strain. Plasmid 69:16–23
Savrasova EA, Kivero AD, Shakulov RS, Stoynova NV (2011) Use of the valine biosynthetic pathway to convert glucose into isobutanol. J Ind Microbiol Biotechnol 38:1287–1294
Shafqat J, Höög JO, Hjelmqvist L, Oppermann UC, Ibáñez C, Jörnvall H (1999) An ethanol-inducible MDR ethanol dehydrogenase/acetaldehyde reductase in Escherichia coli: structural and enzymatic relationships to the eukaryotic protein forms. Eur J Biochem 263:305–311
Shi A, Zhu X, Lu J, Zhang X, Ma Y (2013) Activating transhydrogenase and NAD kinase in combination for improving isobutanol production. Metab Eng 16:1–10
Silber P, Chung H, Gargiulo P, Schulz H (1974) Purification and properties of a diacetyl reductase from Escherichia coli. J Bacteriol 118:919–927
Tashiro Y, Rodriguez GM, Atsumi S (2015) 2-Keto acids based biosynthesis pathways for renewable fuels and chemicals. J Ind Microbiol Biotechnol 42:361–373
Tötemeyer S, Booth NA, Nichols WW, Dunbar B, Booth IR (1998) From famine to feast: the role of methylglyoxal production in Escherichia coli. Mol Microbiol 27:553–562
Trinh CT, Li J, Blanch HW, Clark DS (2011) Redesigning Escherichia coli metabolism for anaerobic production of isobutanol. Appl Environ Microbiol 77:4894–4904
Wek RC, Hatfield GW (1988) Transcriptional activation at adjacent operators in the divergent-overlapping ilvY and ilvC promoters of Escherichia coli. J Mol Biol 203:643–663
Westerfield WW (1945) A colorimetric determination of blood acetoin. J Biol Chem 161:495–502
Yan Y, Liao JC (2009) Engineering metabolic systems for production of advanced fuels. J Ind Microbiol Biotechnol 36:471–479
Yum DY, Lee BY, Pan JG (1999) Identification of the yqhE and yafB genes encoding two 2,5-diketo-D-gluconate reductases in Escherichia coli. Appl Environ Microbiol 65:3341–3346
Zhu Y, Lin EC (1989) L-1,2-propanediol exits more rapidly than L-lactaldehyde from Escherichia coli. J Bacteriol 171:862–867
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deb, S.S., Reshamwala, S.M.S. & Lali, A.M. Activation of alternative metabolic pathways diverts carbon flux away from isobutanol formation in an engineered Escherichia coli strain. Biotechnol Lett 41, 823–836 (2019). https://doi.org/10.1007/s10529-019-02683-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-019-02683-5