Abstract
Best management practices for N fertilization should increase yields while reducing negative environmental effects such as losses by ammonia (NH3) volatilization and emission of greenhouse gases, especially nitrous oxide (N2O). We studied the impact on sugarcane of two N sources (UR: urea and CAN: calcium ammonium nitrate) in three N rates (30, 60, and 90 kg N ha−1 in the plant cane cycle and 60, 120, and 180 kg N ha−1 in ratoons) on a sugarcane field grown on a Red Latosol soil in southeastern Brazil. We measured sugarcane yields and N2O, CO2 and CH4 emissions in three crop cycles (plant cane, 2nd and 3rd ratoons), and NH3 in two crop cycles (2nd and 3rd ratoons). The accumulated emission of N2O from UR was significantly higher in comparison with those of CAN in all three crop cycles. The average emission factors for UR were 0.8% (plant cane), 1.1% (2nd ratoon) and 0.8% (3rd ratoon) and the corresponding figures for CAN were 0.4%, 0.7% and 0.5%, respectively. The N2O intensity was higher for UR (20.3 mg N–N2O kg−1 sugarcane stalk) than CAN (16.2 mg N–N2O kg−1 sugarcane stalk). The NH3 volatilization losses for CAN were less than 1% compared to 5–16% of the N applied as UR. Stalk yield increased with N application, but no difference was observed between UR and CAN. We concluded that CAN has the potential to reduce both NH3 and N2O losses compared with urea and is the preferred N source for sugarcane.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sugarcane is cultivated on 10 Mha in Brazil, which uses 17–23% of the N fertilizer employed in Brazilian agriculture (CONAB 2018). Urea (UR), ammonium nitrate (AN) and ammonium sulfate are the main sources of N for this crop (Cantarella et al. 2007).
Urea, the most-used N fertilizer, is subject to high losses of N through ammonia (NH3) volatilization when surface-applied to soils. Under the warm conditions in Brazil, this can reach 60% of the N applied (Pan et al. 2016; Cantarella et al. 2007; Chien et al. 2009), and may reduce sugarcane yields (Vitti et al. 2007; Costa et al. 2003). For this reason, other sources of N fertilizer may be preferable for sugarcane, especially when applied to ratoon cycles, in which a thick mulch of plant residues (10–20 Mg ha−1 dry mass) remains on the soil surface, making it difficult to incorporate urea to prevent NH3 volatilization loss. In this context, calcium ammonium nitrate (CAN 27-00-00) containing 4% Ca and 2% Mg, is a promising N fertilizer for sugarcane in Brazil.
The amount of NH3 lost can vary substantially depending on soil management and climate. For instance, Cantarella et al. (2008) observed volatilization of NH3 from 1 to 25% of the N surface-applied as urea to seven sugarcane fields. Although high amounts of harvest residues remain on the soil surface, fertilization of this crop often occurs in periods with dry and mild temperatures, which may reduce the potential for NH3 losses. Therefore, the information about NH3 loss in sugarcane must be refined for specific situations, such as areas with thick trash mulch, and mitigation strategies must be developed (Cantarella et al. 2018; Otto et al. 2016). In addition, the volatilized NH3 deposited in other places may lead to soil acidification and indirect N2O emission (Behera et al. 2013).
The N2O emissions from N fertilizers and residues range from 0.3 to 2% of the N applied in sugarcane fields in Brazil (Filoso et al. 2015). High N2O emissions are undesirable especially if the crop is used to produce biofuels meant to replace fossil fuels and to decrease greenhouse gases emission; such emissions may even negate the beneficial effect of the CO2 savings from biofuels (Crutzen et al. 2008). N2O may represent up to 40% of the total greenhouse gases emitted in the production of ethanol from sugarcane (Lisboa et al. 2011).
Recent studies show that the N2O emissions in sugarcane fields in Brazil occur mainly due to the nitrification process rather than denitrification (Lourenço et al. 2018a; Soares et al. 2016). For this reason, sources of N containing ammonium may have higher N2O emission than those containing nitrate (Soares et al. 2016; Tenuta and Beauchamp 2003). Snyder et al. (2009) suggested that the emission of N2O with urea is generally higher than that from other N sources, due to the accumulation of nitrite over time in the soil (Venterea et al. 2015), but there is no clear tendency. For instance, Harty et al. (2016) compared N sources in wet temperate grasslands and found higher N2O emissions with AN than with UR. In addition, if an N source results in higher crop yields—i.e., by increasing the efficiency of fertilizer use—the emission of greenhouse gases (GHG) per unit of product may be lowered.
The amount of N applied to crops affects both NH3 loss and N2O emissions. The NH3 volatilization usually shows an exponential effect as a function of the N application rate (Pan et al. 2016; Rochette et al. 2009). In general, this also applies to N2O emissions. For national inventories, the use of a linear relationship is recommended, between the N application rate and N2O emissions (IPCC 2006). However, Snyder et al. (2009) reported a linear response at typical N fertilization rates and an exponential increase when the N rate is substantially higher than the plant demand. Furthermore, in a meta-analysis, Kim et al. (2013) showed that the N2O can be linear or nonlinear depending on the availability of C and the N demand by microorganisms and plants.
Today, in the State of São Paulo, over 95% of the 5 Mha of sugarcane are harvested without burning. Nitrogen fertilizers are applied on top of sugarcane straw, but questions remain whether urea underperforms in such system and at what extent to justify its substitution by other N fertilizers. Best management practices for N fertilization not only influence productivity but also N gaseous losses. Therefore, the objective of the present work was to evaluate the effect of N sources and rates on NH3 volatilization, N2O emissions, and sugarcane yield in Brazil in three crop cycles conducted in a field with mechanical harvest and straw preserved over the soil.
Material and methods
Experiment and treatments
This study was conducted in a traditional area of sugarcane cultivation at the São Paulo Agency of Agribusiness Technology—APTA in Piracicaba, southeastern Brazil (22°41′02″S, 47°38′44″W, 547 m above sea level), with long-term (100 year) average rainfall and air temperature of 1297 mm and 22.5 °C, respectively. The region is classified as Koppen’s CWA humid subtropical climate, with dry winters and hot and wet summers.
The soil in the experimental site has a 4–5% slope and was classified as Red Latosol (Embrapa 2013). Samples of the 0–0.20 m layer were collected yearly from 2013 to 2017 and analyzed for chemical and physical properties (Van Raij et al. 2001) showing the following average values: pH CaCl2: 5.0, OM: 19 g dm−3, CEC: 64 mmolc dm−3, bulk density = 1.4 Mg m−3 clay: 519 g kg−1, silt: 146 g kg−1, sand: 335 g kg−1 (Table 1).
The area has been continuously grown with sugarcane harvested without burning for more than 10 years. The former sugarcane crop was desiccated in November 2012 and the soil was plowed and disked for the new crop. In March 2013, the IAC-5000 variety was planted with pre- sprouted seedlings, in plots containing 5 rows, 15 m in length, spaced by 1.5 m. The plant cane was initially irrigated until 30–45 days after planting and thereafter, including the ratoons, the crop was rain-fed.
The treatments comprised two N sources (UR and CAN) and four N rates. The N rates, termed control, low, medium and high, were 0, 30, 60, and 90 kg ha−1 in the plant cane cycle, and 0, 60, 120, and 180 kg ha−1 in the ratoons, and were defined based on the N response of the sugarcane crop to this nutrient in Brazil (van Raij et al. 1996).
The experimental design was a randomized block with four replicates. The control treatment (without N) was common to both N sources. Granular urea (45% N) and CAN (ammonium nitrate with limestone containing 27% N with 1:1 NH4 to NO3 ratio, 4% Ca, and 2% Mg) were used. Other nutrients were applied at common rates to all treatments: P (65 kg ha−1 in plant cane and 20 kg ha−1 in the ratoons) as single superphosphate, K, (120 kg ha−1) as potassium chloride, Zn (10 kg ha−1) as zinc sulfate, B (1 kg ha−1) as boric acid, and Mo (0.4 kg ha−1) as sodium molybdate.
In the plant cane cycle the fertilizers were applied into the planting furrow and incorporated at a depth of 0.2 m; in the ratoons, the fertilizers were surface-applied over the harvest straw, in bands approximately 0.2 m from the sugarcane rows. The treatments were reapplied in the same plots from 2013 to 2017.
Plants were harvested manually in Aug/Sept of each year (Table 2). The dry leaves and green tops were separated from the stalk and left on the soil as straw. This material was homogeneously distributed over the field. The stalks were weighted with a load cell coupled to a tractor to estimate stalk yields. Samples of straw left on the soil were randomly collected using a 1 by 1 m wooden frame and dried at 60 °C to constant weight in the laboratory. The average dry biomass values of the straw were 13, 14 and 15 Mg ha−1 for the three ratoon cycles (Table 2).
NH3 emission
Ammonia volatilization was measured for 27 days in all UR plots and in one CAN plot (180 kg ha−1) during the two ratoon cycles. Ammonia was not measured in the plant cane cycle since the fertilizers were incorporated into the soil, a condition in which significant losses are not expected.
The N losses by NH3 volatilization were measured using a semi open chamber made of PVC cylindrical tubes 0.40 m in height × 0.20 m in diameter, placed over PVC bases 0.10 m height and 0.19 m in diameter and following the procedure described by Cantarella et al. (2003, 2008). The chamber bases (10 per plot) were inserted 0.05 m into the soil, and 0.20 m from the sugarcane rows, maintaining the straw inside each chamber. The amounts of N for each treatment were previously weighed in the laboratory using an analytical balance. The chambers were fitted with two polyethylene foam discs (0.002 m in height × 0.2 m diameter), previously soaked with phosphoric acid and glycerin solution (Cantarella et al. 2003). The first disc was placed in the lower part of the chamber at a height of 0.15 m from the ground to capture the volatilized NH3 from the soil and fertilizer treatments. The other disc, placed in the upper part of the chamber 0.25 m from the ground, captured the NH3 from the external environment and avoided the contamination of the lower disc. A protective cap was placed on the top of the chamber to prevent rain from reaching the foam discs.
The NH3 trapping discs were collected 1, 3, 5, 7, 9, 11, 15, 17, 21, and 27 days after N application. After each sampling, the chamber was moved to an adjacent base where the fertilizers had been exposed to the weather as the remainder of the plot to ensure that the measurements reflect the multifactorial conditions that affect NH3 losses (rain, wind, and temperature) and decrease the interferences of the chamber.
The NH3 trapped in the foams was extracted with successive portions of KCl solution (1 mol L−1), transferred to a volumetric flask, and the volume was made to 500 mL. A 25 mL aliquot was transferred to a 200 mL distillation flask and NaOH (10 M) was added to raise the pH to > 7.5. The distillate was collected in 10 mL of a boric acid solution containing a pH indicator and then titrated with 0.0025 mol L−1 sulfuric acid (Cantarella et al. 2003). Daily (mg day−1) and cumulative NH3 losses (kg ha−1) were calculated to express the percentage loss in relation to the N applied.
N2O emission
The static chambers consisted of PVC cylinders 0.20 m in height and 0.30 m in diameter installed in the fertilizer band as described by Carmo et al. (2013) and Soares et al. (2015).
The fluxes of greenhouse gases (GHG) were measured in the plant cane and in the 2nd and 3rd ratoon cycles starting 1 day after N fertilization. To decrease the GHG data variation, the amount of N fertilizer was weighed separately for each chamber.
Gas samples were collected daily for 7 days following N application. After that, the samples were collected three times a week during the first 2 months, twice a week in the third month, once a week in the fourth month, and subsequently every 15 or 30 days. In addition, samples were also collected 1 day after rain events.
For the GHG sampling, the chambers were closed for 30 min. The cover lids are fitted with two valves, one for collecting gases and the other to equalize the internal and external pressures. The gas samples were collected using nylon syringes (60 mL) in three time intervals after the closing of the chambers, i.e. 0, 15, and 30 min according to Mosier et al. (2006) and stored in 12-mL Exetainer® vials (Labco Limited, United Kingdom) for no more than 2 weeks before analysis.
The samples were analyzed with a GC-2014 Shimadzu gas chromatograph (Shimadzu Corp., Japan) with simultaneous determination of N2O using an EC detector operating at 325 °C (Hutchinson and Mosier 1981) and of CH4 and CO2 using an FID operating at 250 °C. The gas concentrations were corrected for the atmospheric pressure and temperature measured at the time of sampling. The fluxes were calculated as described by Denmead et al. (2010). The cumulative nitrous oxide emission was calculated by linear interpolation between adjacent sampling dates. The emission factor (EF) was calculated using Eq. (1) as follows:
The N2O intensity was calculated considering the emission of N2O from the treatment and control plots in relation to the stalk yield in each plot, according to Eq. (2):
The EF was also represented in relation to the net emission for each treatment, discounting the N loss by NH3 volatilization as follows:
Soil sampling
Soil samples at a depth of 0–0.10 m were collected weekly in the fertilization band and close to the chambers on the same day as the GHG samples. Three subsamples per plot were collected to measure soil moisture, NO3− and NH4+ (mineral N). The mineral N concentration was determined by steam distillation after extraction with KCl (1 M), according to Van Raij et al. (2001). Soil moisture was determined after drying the samples at 100 °C to constant weight. The water-filled pore space (WFPS) was estimated as follows:
where GWC is the gravimetric water content (Mg Mg−1), BD is the bulk density (1.4 Mg m−3), and PS is the soil porosity (0.48 m3 m−3). BD and SP were determined in four replicates per block, once a year.
Statistical analysis
Data for stalk yield and the cumulative emissions of NH3, N2O, CO2, and CH4 were checked for a normal distribution of residues and stability of the variance (Rawlings et al. 1998), submitted to analysis of variance (ANOVA) and were compared by orthogonal contrasts (P ≤ 0.05). The softwares used were SAS, version 9.2, and Sigma Plot, version 12.5 (Systat Software, Inc.).
Results
NH3 emissions
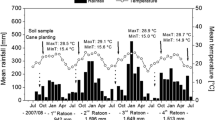
NH3 volatilization losses were measured in the 2nd and 3rd ratoon crops, immediately following the surface application of N fertilizers, which was done in the middle of spring, 3 and 2 months after the sugarcane of the previous cycles had been harvested without burning, respectively. Approximately 14–15 Mg ha−1 of straw was left on the soil (Table 2). At that time, the rainy season was starting, and temperatures were already high. In both years, daily maximum temperatures were above 30 °C (results not shown).
Losses from the urea treatments varied from 6.4 to 28.8 kg ha−1 N in the 2nd ratoon and 7.7–15.0 kg ha−1 NH3-N in the 3rd ratoon for rates of application ranging from 60 to 180 kg N ha−1 (Fig. 1). As a proportion of the N application rates, the NH3 losses varied from 10.8 to 16.0% in the 2nd ratoon and from 6.9 to 10.8% in the 3rd ratoon (Fig. 2).
Ammonia volatilization losses during the 2nd ratoon 2015/2016 (a) and 3rd ratoon 2016/2017 (b) as affected by N sources and application rates. Control (without N), UR: urea; CAN: calcium ammonium nitrate. The values 60, 120, and 180 are N application rates, in kg ha−1. Vertical gray bars in the chart represent daily rainfall. Vertical bars inside each graphic indicate minimum significant difference—“MSD” (Tukey, P ≤ 0.05). The results for each treatment were adjusted with the model [\({\text{y}} = {\text{a}}\left( {1 - {\text{e}}^{{ - {\text{bx}}}} } \right)\)]
Ammonia volatilization losses as a percentage of the N applied over the straw blanket as affected by sources of N and application rates in two sugarcane ratoon cycles. UR: urea; CAN: calcium ammonium nitrate; 60, 120, and 180 are N application rates (kg ha−1). Uppercase and lowercase letters compare the means (Tukey, P ≤ 0.05) for the 2nd and 3rd ratoons, respectively
In both years, the NH3 losses observed in the treatments with the highest N application rate (180 kg ha−1) as urea were higher than those obtained with 60 and 120 kg ha−1, but these did not differ significantly (Tukey P ≤ 0.1) (Fig. 1). However, NH3 losses expressed as a percentage of the applied N varied little in both ratoons (Fig. 2): the large amounts of straw on the soil caused the experimental error to be high, especially in the 2nd ratoon (CV = 62%). Nonetheless, the increasing NH3 losses as the urea rates increased were as expected.
In the plots with CAN, NH3 losses were very small: 0.7% and 0.3% of the applied N at the rate of 180 kg ha−1, not significantly different from the control and much lower than those observed for the urea treatments (Fig. 1). The lower volatilization with CAN is due to the lower amount of NH4+-N compared to hydrolyzed urea. Besides, part of the granules of CAN that fall through the straw end up on the acidic soil (pH in CaCl2: 4.9–5.0) (Table 1), which does not lead to relevant NH3 losses from CAN.
N2O emissions
Fertilization was performed in November, 2 months (3rd ratoon) and in October, 3 months (2nd ratoon) after the previous crop harvest (Table 2), when plants were approximately 0.5 m high. At that time, the raining season was starting and rain events were frequent (Fig. 3). Most of the peaks of N2O emissions occurred in the first 30 to 40 days after the N fertilizer application during the three seasons when these measurements were performed (Fig. 3). The main driver of N2O emissions was the combination of rain events (Fig. 3), which determined the time when the peaks occurred and the availability of inorganic NH4+ and NO3− in the soil. After 40 days the N2O emissions declined following the decline of the concentration of soil inorganic N, since soil moisture (rainfall) was not limiting. Emissions tended to be higher in plots fertilized with urea than CAN and responded to the N application rates (Fig. 3). Data of soil inorganic N, soil moisture and the daily fluxes of N2O are in the Supplementary Material.
Cumulative emissions of N2O-N as affected by sources of N and application rates. UR: urea; CAN: calcium ammonium nitrate; 30, 60, and 90 are N rates in kg ha−1 applied to the plant cane and 60, 120, and 180 kg ha−1 are N rates applied to ratoons. Plant cane 2013/2014 (a), 2nd ratoon 2015/2016 (b) and 3rd ratoon 2016/2017 (c) cycles. Vertical black bars represent daily rainfall for the early sampling period. LSD is the least significant difference from the t test (P ≤ 0.05). The results for each treatment were adjusted to the model [\({\text{y}} = {\text{a}}\left( {1 - {\text{e}}^{{ - {\text{bx}}}} } \right)\)]
The cumulative N2O emission of the control treatment (without N) was much lower (P ≤ 0.001) than those of the fertilized plots in both the plant cane and the ratoon measurements. The cumulative amounts of N2O emitted in the plant cane (552 days), 2nd ratoon (250 days), and 3rd ratoon (269 days) were 168, 53, and 29 mg N2O-N m−2 year−1, respectively (Table 3). As there was at time span of at least 6 months after the previous sugarcane crop and the cane plant cycle of the present study, the higher emission in the control treatment in plant cane than in ratoons was probably caused by mineralization of soil organic N stimulated by the plowing and disking operations before planting. The soil remained undisturbed in the ratoon cycles.
The values of N2O emission reported refer to measurements performed in the fertilizer band, which represents approximately 20% of the field area. The mean cumulative N2O emission in the three cycles was significantly lower in the plots with CAN than in those with urea (Table 3). The average N2O-N emission for the three N application rates applied as CAN was 315 mg m−2 year−1, 675 mg m−2 year−1, and 427 mg m−2 year−1, for plant cane and 2nd and 3rd ratoons, respectively. The corresponding values for the urea treatments were 528 mg m−2 year−1, 884 mg m−2 year−1 and 668 mg m−2 year−1 (Table 3).
Considering the average of all three N application rates, the fertilizer N2O emission factor (EF), expressed as a percentage of the applied N, varied from 0.76 to 1.13% for urea and from 0.38 to 0.68% for CAN, that is, the EF for CAN was on average 42% lower than that for urea (Table 3). The EF for urea was also calculated considering the amount of N lost as NH3. In this case, the NH3-N lost was subtracted from the N amount applied as urea. The EF corrected for NH3 losses were, for the 2nd and 3rd ratoons respectively, 1.31% and 0.85%, whereas those calculated from the full N application rate were 1.13% and 0.76%. The fertilizer N2O EF, in general, increased with increasing N rates except for the UR treatments in the 2nd ratoon, which showed relatively high EF at lower N fertilizer rates (Fig. 4).
Emission factor N2O-N emission factor (EF) during the plant cane (a), 2nd ratoon (b), and 3rd ratoon (c) cycles as affected by N sources and application rates. UR: urea; CAN: calcium ammonium nitrate; 30, 60, and 90 are N rates in kg ha−1 applied to the plant cane and 60, 120, and 180 kg ha−1 are N rates applied to ratoons. Bars indicate the mean standard error (n = 4)
Sugarcane productivity and N2O intensity
Except for the plant cane cycle, when there was no yield response to N fertilization, N application significantly increased stalk yields in the 2nd and 3rd ratoons, as well as in the sum of the three cycles (Table 4). The main difference in stalk yield was between the unfertilized control and the fertilized plots but the response to N application rates (60, 120, and 180 kg N ha−1 in the ratoon cycles) was of little significance. In addition, there was no effect of the N source on yields in any of the cycles evaluated: the cumulative stalk yields obtained with UR and CAN were 269.3 and 269.8 t ha−1 (Table 4). The results of the 1st ratoon are not reported because in that cycle NH3 and GHG were not measured. However, the yields followed the same trend, that is, there was a significant difference between unfertilized (82.5 t ha−1) and fertilized plots (average yields of all N application rates: UR, 94.7 t ha−1; CAN, 97.1 t ha−1).
Yields of the 3rd ratoon were unexpectedly low: the stalk yield of the control treatment was only 49.9 t ha−1 whereas those for UR and CAN (average of the three N application rates) were 75.1 t ha−1 and 73.4 t ha−1, respectively. The reason for this was an infestation of spittle bug (Mahanarva fimbriolata), probably induced by the high amounts of straw (approximately 14 Mg ha−1 of dry matter) close to the plants, that was perceived and treated only in the mid-season when substantial damage had already occurred.
The fertilizer treatments did not affect the sugar concentration in stalks in any season; therefore, the sugar yields (not presented) were proportional to the stalk yields reported in Table 4.
The N2O intensity, expressed in mg N2O-N per kg of sugarcane stalk produced, was significantly affected by both the N fertilizer application rate and the source of N used in all cycles evaluated (Table 4). In the average of three harvests, 11.4 mg N-N2O was emitted for every kilogram of stalk in the unfertilized control; the corresponding figures for plots fertilized with UR and CAN were 19.9 mg kg−1 and 16.3 mg kg−1, respectively (Table 4). Increasing the N application rates for both sources of N caused a significant increase in the N2O intensity (Table 4).
CO2 and CH4 emissions
The accumulated CO2 emission varied from 780 to 1180 g m−2 in the three seasons and, except for the plant cane cycle, it was not affected by the treatments. In the plant cane cycle, the application of N increased CO2 emission although this seems to be due to an outlier observed in plots fertilized with 90 kg N ha−1 as urea. There was little variation in the average CO2 emission in the three cycles.
In general, the soil was a sink rather than an emitter of CH4. Except for an increase in CH4 consumption with increasing rates of N as urea in the plant cane and the 3rd ratoon cycle, the effects of the source of N fertilizer and application rates were not statistically significant. Data of CO2 and CH4 emissions are in the supplementary material (Table S3 and S4).
Discussion
NH3 volatilization
Sugarcane straw mulch tends to enhance NH3 losses because it acts as a barrier to incorporation of urea into the soil and because part of the losses may come directly from the urea that stays in the mulch (Pinheiro et al. 2018). These authors found, in two studies, that NH3 losses increased by 1.5% and 2.6% of the applied N per metric ton of straw left on the field. In addition, the magnitude of NH3 losses when urea is surface-applied to sugarcane fields is highly dependent on the weather and therefore on specific site conditions. The straw blanket may also retain moisture and increase the urease activity. Freney et al. (1992) reported maximum losses of 32–39% of the applied urea–N in Australia. In Brazil (Costa et al. 2003) observed losses of 36% of the urea-N (100 kg ha−1) applied over the straw. For this same rate of urea-N applied over sugarcane straw, NH3 losses of 20% were reported by Otto et al. (2017) and from 14 to 33% in five sites by Mira et al. (2017), within the range of values found in the present study.
Higher application rates of N or localized (band vs broadcast) application usually increases NH3 losses expressed as a percentage of the applied N because of the concentration effect of urea hydrolysis on the soil pH in the vicinity of the fertilization zone (Cantarella et al. 2003; Vitti et al. 2007). However, if excess urea saturates the active sites of urease in the soil or in straw, the effect of increasing N application rates may not hold. Mariano et al. (2012) reported NH3 losses of 16% of the urea-N applied at 50 kg ha−1 over sugarcane straw but the losses were in the range of 24% as N application rates increased from 100 kg ha−1 up to 200 kg ha−1. In the present study, NH3 losses increased from 11 to 16% as the N application rates increased from 60 to 180 kg ha−1 in one year but did not vary in the following year. Uneven fertilizer distribution may also explain such results. However, all these studies indicate that significant amounts of N may be lost as urea is applied to sugarcane fields with preserved straw.
The differences in the amounts and pattern of NH3 volatilization in the 2 years of observation are explained by the climate conditions, especially the rain regime at the time of fertilizer application. In the 2nd ratoon experiment, NH3 volatilization from the urea treatments started soon after fertilization: in the third and fourth days, respectively, 9.2 and 16.8 kg ha−1 NH3–N had been lost from the urea applied at 180 kg ha−1 N (Fig. 1). In that experiment rain totaling 40 mm occurred in the 4 days that preceded the fertilizer application. The moist soil combined with maximum daily temperatures above 30 °C drives NH3 losses up. Interestingly, the 28.6 mm precipitation that occurred on the day of the fertilizer application was not enough to stop NH3 volatilization (Fig. 1). The losses continued up to approximately the 9th day when they leveled off, probably because of cumulative rain events.
Usually, it is assumed that 10–20 mm of rain is enough to incorporate urea into the soil, thereby reducing or even controlling NH3 volatilization losses (Holcomb et al. 2011). However, the nature of the sugarcane straw (large particles), its amounts and arrangement over the soil may affect how rain acts to reduce losses. Rain may go through preferential channels in the straw and may not effectively wash the urea to the soil (Freney et al. 1994). This may explain why 28.6 mm of rain did not stop NH3 losses in our study as well as other cases of rain greater than 20 mm that had only a small effect on reducing losses from urea applied to sugarcane blankets (Cantarella et al. 2007).
Nonetheless, soil moisture and rain play an important role in determining NH3 losses from urea used in sugarcane fields. Cantarella et al. (2008) reported NH3 losses varying from 1 to 25% of the applied N in seven field studies with sugarcane; most of the variation could be explained by the weather conditions. Urea applied to dry soil—common when sugarcane fertilization is done in the winter—resulted in very little NH3 losses as the urea remained unhydrolyzed on the soil/straw for approximately 20 days (Cantarella et al. 2008; Mira et al. 2017). We can derive associations between the weather and NH3 losses observed in our study. In the 3rd ratoon experiment, the soil was dry when the fertilizer treatments were applied; no rain had been registered in 5 days. In the 4 days that followed fertilization practically no rain occurred. Consequently, NH3 losses were very small in the measurements done on the 1st and the 3rd days after fertilization. However, NH3 volatilization increased sharply after the 5th day (Fig. 1), which coincided with rain events that supplied the water necessary to cause urea hydrolysis. As in the previous year, NH3 volatilization tended to decrease after 9–10 days due to several consecutive rain events.
Urea incorporation into the soil is an effective way to reduce NH3 volatilization losses (Rochette et al. 2013), including on sugarcane blanketed soils (Cantarella et al. 1999) but this operation is time-consuming and requires high-powered machines in fields with large amounts of straw; therefore, mechanical incorporation of urea is seldom done by farmers in Brazil.
Acidic soils such as those of our study are not prone to NH3 volatilization losses from nitrogen fertilizer compounds that contain N as ammonium or nitrate, such as CAN. Indeed, the NH3 losses found in our study were 0.3% and 0.7% of the N applied as CAN (Fig. 2). Because of the negligible NH3 volatilization losses, ammonium nitrate is the fertilizer of choice of the sugarcane sector when surface-applied. However, there are increasing legal restrictions to ammonium nitrate trade and storage because of its use in the manufacture of explosives; for this reason, a major fertilizer company operating in Brazil stopped selling this product recently. CAN has fewer restrictions because it contains limestone, which reduces its potential as an explosive material, but its price is higher than that of urea.
Despite its drawback as a N source when surface-applied, urea is unlikely to be totally displaced in sugarcane because urea is the less expensive N fertilizer and comprises approximately 60% of the N in the Brazilian and the international market. In addition to management practices such as soil incorporation, options to reduce NH3 losses include the use of urease inhibitors (Cantarella et al. 2018). The use of NBPT (N-(n-butyl) thiophosphoric triamide), a urease inhibitor, reduced NH3 losses by 35% as an average of five experiments in sugarcane fields with straw-covered soils (Cantarella et al. 2008). Controlled-release fertilizers can also reduce losses and increase N use efficiency but currently they are not price-competitive for sugarcane (Verburg et al. 2016). Moreover, the results of the use of controlled-release fertilizer in sugarcane are variable, but increasing testing and use may turn them more reliable and economic (Verburg et al. 2016).
Sugarcane yield
Despite the loss of NH3 ranging between 7% and 16% of the N applied as UR and the insignificant losses observed with CAN (0.3–0.7%) (Fig. 2), the source of N did not affect stalk yield in either the 2nd or 3rd ratoon (Table 4). The same occurred in the 1st ratoon (results not shown). However, it is risky to conclude that UR and CAN have the same efficiency in supplying N to sugarcane grown in soils covered with straw. Sugarcane has a long cycle (in our study 18 months in plant cane and 12 months in the ratoons); the contribution of N fertilizer to the overall N supply to sugarcane tends to be low. Therefore, the amounts of N lost as NH3 may have a limited effect on crop nutrition on the short term.
Vitti et al. (2011) reported that 30% of the N applied as urea was recovered by sugarcane plants at the end of the cycle. Similar results were reported by Ng Kee Kwong and Deville (1987) in Mauritius. Usually, the amount of fertilizer-derived N is higher in the early stages of sugarcane growth but declines as the plant matures. Franco et al. (2011) found that in young sugarcane plants 40% and 70% of the plant N was derived from fertilizer in plant cane and ratoon, respectively. However, these proportions decreased to 10% and 30% in mature plants, that is, most of the N at the end of the cycle had come from other sources, more likely the soil. In a recent review of the sugarcane literature in Brazil, Otto et al. (2016) reported that on average only 26% of the N taken up by sugarcane plants comes from fertilizer but 32% of the N applied as fertilizer ends up immobilized in the soil organic fractions. Thus, part of the N that is not lost as NH3 probably will accumulate in the soil and form the stock of soil organic N that will mineralize and supply the crop in subsequent years (Meier et al. 2006; Ng Kee Kwong et al. 1987) even though the short-term yield response may not be clear. The N lost as NH3 may also affect the yield, despite the low fertilizer-N recovery by this crop, as reported by Vitti et al. (2007). In their study, plots fertilized with ammonium sulfate (not subject to NH3 losses in acidic soils) yielded 73 t ha−1 of stalks, significantly higher than the yields obtained with UR (60 t ha−1). However, the yield with ammonium nitrate was 64 t ha−1, similar to that of urea.
The fact that the soil supplies part or even most of the crop N tends to mask the effect of losses. A review about urease inhibitors (Cantarella et al. 2018) showed that NBPT added to urea reduced NH3 volatilization losses by 52% in an average of 35 studies with different crops but yields increased by only 6%; in the 11 studies with sugarcane, the effect of the urease inhibitor on sugarcane yield was null.
When N was applied in October or November (Table 2) the plants were approximately 0.5 m tall and growing rapidly, that is, intensively taking up N. A tall plant canopy may also absorb part of the NH3 that volatilizes. Between 3 and 15% of the NH3 volatilized from the soil-applied urea was taken up by corn plants, the highest proportion being when the plants were fertilized at a later growth stage, presenting 10 expanded leaves (Schoninger et al. 2018). However, it is difficult to establish how foliar uptake of volatilized NH3 can affect the urea-N budget in sugarcane.
The fertilizers were applied on top of the straw, which should lead to a higher efficiency of CAN because of the insignificant NH3 losses. However, a recent study showed that incorporation of ammonium nitrate under the straw layer increased sugarcane yields by approximately 12% compared to band application over the straw (de Castro et al. 2017), as done in our study, suggesting that part of the fertilizer N may be immobilized by the high C:N ratio in the sugarcane harvest residue, which might reduce the advantage of this N source over UR, although both fertilizers may be subject to the same immobilization process.
GHG emission
The N2O emission observed for UR was higher than for CAN (P ≤ 0.01) and increased with increasing N application rates for both N fertilizers (Table 3). The average N2O-N emission factor (EF) for UR was 0.90% (ranging from 0.41 to 1.39%) and for CAN was 0.52% (ranging from 0.21 to 1.06%). The effect of N rate on EF was less clear but, in general, EF values increased with increasing N rates (Fig. 4). The climate conditions, especially rain near the time of fertilizer application, play an important role in determining the magnitude of the N2O losses. However, the average EF values for both sources of N were lower than the IPCC default value (1%). This has been reported in most studies of sugarcane done in Brazil (Borges et al. 2019; Carmo et al. 2013; da Silva et al. 2017; Gonzaga et al. 2018; Lourenço et al. 2019; Paredes et al. 2014; Pitombo et al. 2017; Soares et al. 2015). The relatively low EF values have been attributed, among other reasons, to the good drainage of Brazilian Oxisols (Jantalia et al. 2008). However, straw mulch may increase the N2O emissions from N fertilizers (Carmo et al. 2013; Gonzaga et al. 2018), as was the case in the present study.
In our study, the N2O emissions from CAN were lower than those of UR but the effect of the fertilizer source is probably site- and climate-dependent and there is no clear difference in the various studies reported in the literature (Snyder et al. 2009). For instance, Roche et al. (2016), working with barley fertilization, did not find a significant difference between the EF for UR and CAN. Martins et al. (2015) in a study with maize in an Oxisol similar to the soil used in our study also reported similar emissions for urea and a nitrate-based fertilizer (calcium nitrate), whereas Harty et al. (2016) reported much higher EF values for CAN (1.49%, ranging from 0.58 to 3.81%) than for UR (0.25%, ranging from 0.10 to 0.49%). Their study was conducted in pastures in Ireland, a wetter region than Central-South Brazil and presumably on a very different soil type. One possible reason for the lower emissions with CAN in our study is the prevalence of nitrification over denitrification as the main process driving N2O emissions in sugarcane in our region (Lourenço et al. 2018a, b; Soares et al. 2016). In fact, Soares et al. (2016) found that N2O emissions in plots of sugarcane fertilized with calcium nitrate were as low as those of the unfertilized control, as opposed to the plots with UR, where the EF was 1.68%. Siqueira Neto et al. (2016) also reported lower N2O emissions in sugarcane with ammonium nitrate than with urea.
The effect of N fertilizer on biomass production may offset the GHG emissions if expressed per unit of crop produced (Snyder et al. 2014). However, in our study, the N2O emission per unit of sugarcane stalk was significantly higher (P ≤ 0.01) in the fertilized plots than in the unfertilized control, and higher (P ≤ 0.01) for UR than for CAN: the N2O intensity for the unfertilized control was, on the average of three cycles, 11.4 mg N2O–N kg−1 of sugarcane stalk, whereas the corresponding values for UR and CAN were 19.9 and 16.3 mg N2O–N kg−1 stalk (Table 4). Therefore, CAN had a lower carbon footprint than UR but, because the relatively small yield gains of the fertilized plots and lower N2O emissions (Table 4) the lowest emissions per unit of stalk produced were still obtained in the unfertilized plots. Similar results were reported by Borges et al. (2019) and by Gonzaga et al. (2018) in studies with sugarcane fertilized with 120 kg N ha−1 conducted in the same region.
Our data corroborate others cited above, indicating that in most cases N2O emissions in sugarcane are lower than 1%. These results will be useful to support future estimates of the Brazilian GHG inventory, currently based on IPPC’s default values (IPCC 2013).
Although net cumulative CH4 emissions were observed in the control and the low fertilizer N treatments in the 2nd and 3rd ratoon cycles, they were small and, in most treatments, the soil was a sink of CH4 (Table 4S). Except under conditions of poor drainage (Denmead et al. 2010), sugarcane soils act mostly as sinks for this gas (Bento et al. 2018; Borges et al. 2019; Carmo et al. 2013; Lourenço et al. 2019). Therefore, CH4 has little contribution to the net GHG emissions of sugarcane.
Conclusion
Losses of NH3 by volatilization and N2O emissions were higher when UR was surface-applied over sugarcane straw harvest residue, as compared with CAN. However, stalk yields were not affected by the source of N and were little affected by application rates above 60 kg N ha−1. At least from an environmental point of view CAN should be preferred as a source of N for sugarcane grown under conditions similar to those of the present study because N dynamics in soils are site-specific.
References
Behera SN, Sharma M, Aneja VP, Balasubramanian R (2013) Ammonia in the atmosphere: a review on emission sources, atmospheric chemistry and deposition on terrestrial bodies. Environ Sci Pollut Res 20:8092–8131. https://doi.org/10.1007/s11356-013-2051-9
Bento CB, Filoso S, Pitombo LM, Cantarella H, Rossetto R, Martinelli LA, Carmo JB (2018) Impacts of sugarcane agriculture expansion over low-intensity cattle ranch pasture in Brazil on greenhouse gases. J Environ Manage 206:980–988. https://doi.org/10.1016/j.jenvman.2017.11.085
Borges CD, Carvalho JLN, Kölln OT, Sanches GM, Silva MJ, Castro SGQ, Castro SAQ, Sousa LL, Oliveira JVC, Cantarella H, Vargas VP, Tsai SM, Franco HCJ (2019) Can alternative N-fertilization methods influence GHG emissions and biomass production in sugarcane fields? Biomass Bioenergy 120:21–27. https://doi.org/10.1016/j.biombioe.2018.10.017
Cantarella H, Rossetto R, Barbosa W, Penna MJ, Resende LCL (1999) Nitrogen losses as NH3 volatilization and sugarcane response to N fertilization in sugarcane harvested without straw burning (In Portuguese, with English abstract) In: Proceedings of the Brazilian Society of Sugar and Alcohol Technicians (STAB), vol 7, pp 782–787. Londrina, Brazil
Cantarella H, Mattos JRD, Quaggio JA, Rigolin AT (2003) Fruit yield of Valencia sweet orange fertilized with different N sources and the loss of applied N. Nutr Cycl Agroecosys 67:215–223. https://doi.org/10.1023/B:FRES.0000003600.20499.76
Cantarella H, Trivelin PCO, Vitti AC (2007) Nitrogen and sulfur in sugarcane: Nitrogen and Sulfur in Brazilian Agriculture. Yamada T, Abdalla SRS, Vitti GC (eds.), IPNI: International Plant Nutrition Institute Piracicaba, Brazil, pp 355–412 (In Portuguese)
Cantarella H, Trivelin PCO, Contin TLM, Dias FLF, Rossetto R, Marcelino R, Coimbra RB, Quaggio JA (2008) Ammonia volatilisation from urease inhibitor-treated urea applied to sugarcane trash blankets. Sci Agric 65:397–401. https://doi.org/10.1590/S0103-90162008000400011
Cantarella H, Otto R, Soares JR, Silva AGB (2018) Agronomic efficiency of NBPT as a urease inhibitor: a review. J Adv Res 13:19–27. https://doi.org/10.1016/j.jare.2018.05.008
Carmo JB, Filoso S, Zotelli LC, Souza-Neto ER, Pitombo LM, Duarte-Neto PJ, Vargas VP, Andrade CA, Gava GJC, Rossetto R, Cantarella H, Elias-Neto A, Martinelli LA (2013) Infield greenhouse gas emissions from sugarcane soils in Brazil: effects from synthetic and organic fertilizer application and crop trash accumulation. Global Change Biol Bioenergy 5:267–280. https://doi.org/10.1111/j.1757-1707.2012.01199.x
Chien SH, Prochnow LI, Cantarella H (2009) Recent developments of fertilizer production and use to improve nutrient efficiency and minimize environmental impacts. Adv Agron 102:267–322. https://doi.org/10.1016/S0065-2113(09)01008-6
CONAB (2018) Survey of the sugarcane production: Brazilian Sugarcane Production. Technical Report 2017–2018. http://www.conab.gov.br. Accessed May 2018. (In Portuguese)
Costa MCG, Vitti GC, Cantarella H (2003) Ammonia volatilization from N fertilizers in sugarcane harvested without straw burning (In Portuguese, with English abstract). R Bras Cien Solo 27:631–637. https://doi.org/10.1590/S0100-06832003000400007
Crutzen PJ, Mosier AR, Smith KA, Winiwarter W (2008) N2O release from agro biofuel production negates global warming reduction by replacing fossil fuels. Atmos Chem Phys 8:389–395. https://doi.org/10.5194/acp-8-389-2008
da Silva JF, de Carvalho AM, Rein TA, Coser TR, Ribeiro Júnior WQ, Vieira DL, Coomes DA (2017) Nitrous oxide emissions from sugarcane fields in the Brazilian Cerrado. Agric Ecosyst Environ 246:55–65. https://doi.org/10.1016/j.agee.2017.05.019
de Castro SGQ, Decaro ST, Franco HCJ, Graziano Magalhães PS, Garside A, Mutton MA (2017) Best practices of nitrogen fertilization management for sugarcane under green cane trash blanket in Brazil. Sugar Tech 19:51–56. https://doi.org/10.1007/s12355-016-0443-0
Denmead OT, Macdonald BCT, Bryant G, Naylor T, Wilson S, Griffith DWT, Wang WJ, Salter B, White I, Moody PW (2010) Emissions of methane and nitrous oxide from Australian sugarcane soils. Agr Forest Meteorol 150:748–756. https://doi.org/10.1016/j.agrformet.2009.06.018
Embrapa-Empresa Brasileira de Pesquisa Agropecuária (2013) Brazilian System of Soil Classification 3rd ed Rio de Janeiro: Embrapa Solos (In Portuguese)
Filoso S, Carmo JB, Mardegan SF, Lins SRM, Gomes TF, Martinelli LA (2015) Reassessing the environmental impacts of sugarcane ethanol production in Brazil to help meet sustainability goals. Renew Sust Energ Rev 52:1847–1856. https://doi.org/10.1016/j.rser.2015.08.012
Franco AA, Otto R, Faroni CE, Vitti AC, Oliveira ECA, Trivelin PCO (2011) Nitrogen in sugarcane derived from fertilizer under Brazilian field conditions. Field Crop Res 121:29–41. https://doi.org/10.1016/j.fcr.2010.11.011
Freney JR, Denmead OT, Wood AW, Saffigna PG, Chapman LS, Ham GJ, Hurney AP, Stewart RL (1992) Factors controlling ammonia loss from trash covered sugarcane fields fertilized with urea. Fert Res 31:341–349. https://doi.org/10.1007/BF01051285
Freney JR, Denmead OT, Wood AW, Saffigna PG (1994) Ammonia loss following urea addition to sugar cane trash blankets. In: Egan BT (ed.), Proceedings of the conference of the Australian Soc. Sugar Cane Technologists. Townsville: Qld pp 114–121
Gonzaga LC, Carvalho JLN, Oliveira BG, Soares JR, Cantarella H (2018) Crop residue removal and nitrification inhibitor application as strategies to mitigate N2O emissions in sugarcane fields. Biomass Bioenergy 119:206–216. https://doi.org/10.1016/j.biombioe.2018.09.015
Harty MA, Forrestal PJ, Watson CJ, McGeough KL, Carolan R, Elliot C, Krol D, Laughlin RJ, Richards KG, Lanigan GJ (2016) Reducing nitrous oxide emissions by changing N fertiliser use from calcium ammonium nitrate (CAN) to urea based formulations. Sci Total Environ 563–564:576–586. https://doi.org/10.1016/j.scitotenv.2016.04.120
Holcomb JC, Sullivan DM, Horneck DA, Clough GH (2011) Effect of irrigation rate on ammonia volatilization. Soil Sci Soc Am J 75:2341–2347. https://doi.org/10.2136/sssaj2010.0446
Hutchinson GL, Mosier AR (1981) Improved soil cover method for field measurement of nitrous oxide fluxes. Soil Sci Soc Am J 45:311–316. https://doi.org/10.2136/sssaj1981.03615995004500020017x
IPCC Intergovernamental Panel on Climate Change (2006) Guidelines for national greenhouse gas inventories. Greenhouse gas inventory reference manual, v.4. 2006. http://www.ipcc-nggip.iges.or.jp/public/2006gl/vol4.html
IPCC Summary for Policymakers In: Climate Change (2013) The physical science basis. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds.), Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
Jantalia CP, dos Santos HP, Urquiaga S, Boddey RM, Alves BJR (2008) Fluxes of nitrous oxide from soil under different crop rotations and tillage systems in the South of Brazil. Nutr Cycl Agroecosys 82:161–173. https://doi.org/10.1007/s10705-008-9178-y
Kim DG, Ramirez GH, Giltrap D (2013) Linear and nonlinear dependency of direct nitrous oxide emissions on fertilizer nitrogen input: a meta-analysis. Agric Ecosyst Environ 168:53–65. https://doi.org/10.1016/j.agee.2012.02.021
Lisboa CC, Butterbach-Bahl K, Mauder M, Kiese R (2011) Bioethanol production from sugarcane and emissions of greenhouse gases—known and unknowns. Global Change Biol Bioenergy 3:277–292. https://doi.org/10.1111/j.1757-1707.2011.01095.x
Lourenço KS, Cassman NA, Pijl AS, van Veen JA, Cantarella H, Kuramae EE (2018a) Nitrosospira sp. govern nitrous oxide emissions in a tropical soil amended with residues of bioenergy crop. Front Microbiol 9:674. https://doi.org/10.3389/fmicb.2018.00674
Lourenço KS, Dimitrov MR, Pijl A, Soares JR, Carmo JB, van Veen JA, Cantarella H, Kuramae EE (2018b) Dominance of bacterial ammonium oxidizers and fungal denitrifiers in the complex nitrogen cycle pathways related to nitrous oxide emission. Global Change Biol Bioenergy 10:645–660. https://doi.org/10.1111/gcbb.12519
Lourenço KS, Rossetto R, Vitti AC, Montezano ZF, Soares JR, Sousa RM, do Carmo JB, Kuramae EE, Cantarella H (2019) Strategies to mitigate the nitrous oxide emissions from nitrogen fertilizer applied with organic fertilizers in sugarcane. Sci Total Environ 650:1476–1486. https://doi.org/10.1016/j.scitotenv.2018.09.037
Mariano E, Trivelin PCO, Vieira MX, Leite JM, Otto R, Franco HCJ (2012) Ammonia losses estimated by an open collector from urea applied to sugarcane straw. Rev Bras Cienc Solo 36:411–419. https://doi.org/10.1590/S0100-06832012000200010
Martins MR, Jantalia CP, Polidoro JC, Batista JN, Alves BJR, Boddey RM, Urquiaga S (2015) Nitrous oxide and ammonia emissions from N fertilization of maize crop under no-till in a Cerrado soil. Soil Till Res 151:75–81. https://doi.org/10.1016/j.still.2015.03.004
Meier EA, Thorburn PJ, Wegener MK, Basford KE (2006) The availability of nitrogen from sugarcane trash on contrasting soils in the wet tropics of North Queensland. Nutr Cycl Agroecos 75:101–114. https://doi.org/10.1007/s10705-006-9015-0
Mira AB, Cantarella H, Souza-Netto GJM, Moreira LA, Kamogawa MY, Otto R (2017) Optimizing urease inhibitor usage to reduce ammonia emission following urea application over crop residues. Agric Ecosyst Environ 248:105–112. https://doi.org/10.1016/j.agee.2017.07.032
Mosier AR, Halvorson AD, Reule CA, Liu XJ (2006) Net global warming potential and greenhouse gas intensity in irrigated cropping systems in northeastern Colorado. J Environ Qual 35:1584–1598. https://doi.org/10.2134/jeq2005.0232
Ng Kee Kwong KF, Deville J (1987) Residual fertilizer nitrogen as influenced by timing and nitrogen forms in a silty clay soil under sugarcane in Mauritius. Fert Res 14:219–226. https://doi.org/10.1007/bf01050666
Otto R, Castro SAQ, Mariano E, Castro SGQ, Franco HCJ, Trivelin PCO (2016) Nitrogen use efficiency for sugarcane-biofuel production: what is next? Bioenergy Res 9:1272–1289. https://doi.org/10.1007/s12155-016-9763-x
Otto R, Zavaschi E, Souza GJM, Machado BA, Mira AB (2017) Ammonia volatilization from nitrogen fertilizers applied to sugarcane straw. Rev Cienc Agron 48:413–418. https://doi.org/10.5935/1806-6690.20170048
Pan B, Lam SK, Mosier A, Luo Y, Chen D (2016) Ammonia volatilization from synthetic fertilizers and its mitigation strategies: a global synthesis. Agric Ecosyst Environ 232:283–289. https://doi.org/10.1016/j.agee.2016.08.019
Paredes DS, Lessa ACR, Sant’Anna SAC, Boddey RM, Urquiaga S, Alves BJR (2014) Nitrous oxide emission and ammonia volatilization induced by vinasse and N fertilizer application in a sugarcane crop at Rio de Janeiro, Brazil. Nutr Cycl Agroecosys 98:41–55. https://doi.org/10.1021/acs.est.5b01504
Pinheiro PL, Recous S, Dietrich G, Weiler DA, Giovelli RL, Mezzalira AP, Giacomini SJ (2018) Straw removal reduces the mulch physical barrier and ammonia volatilization after urea application in sugarcane. Atmos Environ 194:179–187. https://doi.org/10.1016/j.atmosenv.2018.09.031
Pitombo LM, Cantarella H, Packer APC, Ramos NP, Carmo JB (2017) Straw preservation reduced total N2O emissions from a sugarcane field. Soil Use Manage 33:583–594. https://doi.org/10.1111/sum.12384
Rawlings JO, Pantula SG, Dickey DA (1998) Applied regression analysis: a research tool, 2nd edn. Springer, New York
Roche L, Forrestal PJ, Lanigan GJ, Richards KG, Shaw LJ, Wall DP (2016) Impact of fertiliser nitrogen formulation, and N stabilisers on nitrous oxide emissions in spring barley. Agric Ecosyst Environ 233:229–237. https://doi.org/10.1016/j.agee.2016.08.031
Rochette P, Macdonald JD, Angers DA, Chantigny MH, Gasser MO, Bertrand N (2009) Banding urea increased ammonia volatilization in a dry acidic soil. J Environ Qual 38:1383–1390. https://doi.org/10.2134/jeq2008.0295
Rochette P, Angers DA, Chantigny MH, Gasser MO, MacDonald JD, Pelster DE, Bertrand N (2013) Ammonia volatilization and nitrogen retention: how deep to incorporate urea? J Environ Qual 42:1635–1642. https://doi.org/10.2134/jeq2013.05.0192
Schoninger EL, González-Villalba HA, Bendassolli JA, Ocheuze Trivelin PC (2018) Fertilizer nitrogen and corn plants: not all volatilized ammonia is lost. Agron J 110:1111–1118. https://doi.org/10.2134/agronj2017.07.0372
Siqueira Neto M, Galdos MV, Feigl BJ, Cerri CEP, Cerri CC (2016) Direct N2O emission factors for synthetic N-fertilizer and organic residues applied on sugarcane for bioethanol production in Central-Southern Brazil. Global Change Biol Bioenergy 8:269–280. https://doi.org/10.1111/gcbb.12251
Snyder CS, Bruulsema TW, Jensen TL, Fixen PE (2009) Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric Ecosyst Environ 133:247–266. https://doi.org/10.1016/j.agee.2009.04.021
Snyder CS, Davidson EA, Smith P, Venterea RT (2014) Agriculture: sustainable crop and animal production to help mitigate nitrous oxide emissions. Curr Opin Env Sust 9–10:46–54. https://doi.org/10.1016/j.cosust.2014.07.005
Soares JR, Cantarella H, Vargas VP, Carmo JB, Martins AA, Sousa RM, Andrade CA (2015) Enhanced-efficiency fertilizers in nitrous oxide emissions from urea applied to sugarcane. J Environ Qual 4:423–430. https://doi.org/10.2134/jeq2014.02.0096
Soares JR, Cassman NA, Kielak AM, Pijl A, Carmo JB, Lourenço KS, Laanbroek HJ, Cantarella H, Kuramae EE (2016) Nitrous oxide omission related to ammonia-oxidizing bacteria and mitigation options from N fertilization in a tropical toil. Sci Rep 6:30349. https://doi.org/10.1038/srep30349
Systat Software (2006) Inc—SSI Sigmaplot for windows, version 12.5
Tenuta M, Beauchamp EG (2003) Nitrous oxide production from granular nitrogen fertilizers applied to a silt loam soil. Canadian J Soil Sci 83:521–532. https://doi.org/10.4141/S02-062
van Raij B, Cantarella H, Quaggio JA, Furlani AMC (1996) Sugarcane. In: Recommendations for lime and fertilizer for the State of São Paulo Campinas: Instituto Agronômico (in Portuguese)
van Raij B, Andrade JC, Cantarella H, Quaggio JA (2001) Chemical analysis for evaluation of fertility of tropical soils. Campinas: Instituto Agronômico (in Portuguese)
Venterea RT, Clough TJ, Coulter JA, Breuillin-Sessoms F (2015) Ammonium sorption and ammonia inhibition of nitrite-oxidizing bacteria explain contrasting soil N2O production. Sci Rep 5:12153. https://doi.org/10.1038/srep12153
Verburg K, Zhao Z, Biggs JS, Thorburn PJ (2016) Controlled release fertilisers—lessons from a review and early results characterising release, synchrony and nitrogen losses. Proc Aust Soc Sugar Cane Technol 38:159–169
Vitti AC, Trivelin PCO, Gava GJC, Franco HCJ, Bologna IR, Faroni CE (2007) Sugarcane yield related with the nitrogen fertilizer placement over residues of sugarcane harvest without burning (in Portuguese). Rev Bras Cienc Solo 31:491–498. https://doi.org/10.1590/S0100-06832007000300009
Vitti AC, Franco HCJ, Trivelin PCO, Ferreira DA, Otto R, Fortes C, Faroni CE (2011) Nitrogênio proveniente da adubação nitrogenada e de resíduos culturais na nutrição da cana-planta. Pesq Agropec Bras 46:287–293. https://doi.org/10.1590/S0100-204X2011000300009
Acknowledgment
This work was supported in part by the CAPES Interuniversity exchange doctorate (Process: PDSE - 88881.134105/2016-01), CAPES Scholarship (Process 88882.157081), FAPESP Project 2013/50.365-5, CNPq Grant 310 478/2017-0, and Yara Fertilizers (Project Fundag 01054-IA Greenhouse).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Degaspari, I.A.M., Soares, J.R., Montezano, Z.F. et al. Nitrogen sources and application rates affect emissions of N2O and NH3 in sugarcane. Nutr Cycl Agroecosyst 116, 329–344 (2020). https://doi.org/10.1007/s10705-019-10045-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-019-10045-w