Abstract
Magnetic resonance imaging has emerged as a preferred modality in pediatric imaging because of its high soft-tissue contrast and the lack of ionizing radiation. It is important to recognize that despite its many advantages, several challenges to performing neonatal MRI arise from the lack of patient compliance and the small size of the anatomy. This manuscript presents the approach to patient preparation used at the authors’ institution, summarizes general principles of image optimization and hardware selection, and reviews common indications across various organ systems. This manuscript also incorporates input from our pediatric-trained MRI technologists, in an attempt to compile a practical guideline covering all major aspects of neonatal MRI, from its execution to its interpretation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The noninvasive nature of MRI, its multiplanar capabilities and its lack of ionizing radiation have positioned this modality as a cornerstone of diagnostic imaging in pediatrics. Despite its widespread adoption in older children and teenagers, MRI remains somewhat underutilized in newborns. This age group presents a series of technical and biological challenges that complicate MRI acquisition and interpretation, such as the small size of the anatomy of interest, development-related changes (e.g., marrow composition, fast and shallow respiration) and distinct set of medical indications. Familiarity with patient-preparation strategies, age-appropriate MRI hardware and basic principles of image optimization can help navigate these barriers and harness the potential of MRI in neonates.
This manuscript synthesizes our cumulative experience across a variety of subspecialties and emphasizes practical aspects that are important for image optimization and interpretation in this age group. The manuscript also incorporates input from our pediatric-trained MRI technologists, integrating a highly practical perspective on image acquisition. We review operational and safety considerations and appropriate MRI hardware selection (e.g., receiver coils) and discuss common indications for MRI in newborns. The intent of this review is to compile a comprehensive guideline for practicing pediatric radiologists and other members of the medical imaging team who might face challenges inherent to performing MRI in neonates.
Operational and safety considerations
Many institutions attempt non-sedated MRIs with a high success rate. Though still performed, the routine use of sedation in neonatal MRI has been losing popularity because it increases the cost of the exam, adds to the logistical complexity of the visit and carries medical risk. Although traditionally considered safe when performed by experienced providers, anesthesia still carries a risk for cardiopulmonary complications, particularly for neonates [1]. Furthermore, some debate remains regarding the potential for negative long-term neurocognitive effects of exposure to anesthetics early in life, although data from the recent “General anaesthesia or awake-regional anaesthesia in infancy (GAS)” study appear to support that a single short exposure (<1 h) to anesthesia carries no substantial risk [2, 3].
We use a feed-and-swaddle strategy as the first option, attempting to image all non-critically ill newborns without sedation. For these studies, the baby is fed in close coordination with an MRI slot. We generally recommend keeping the neonate awake for a few hours prior to the exam and withholding one feed, so that the baby is awake and hungry prior to the timed feed. Upon arrival to the MRI department, the infant is brought to a scanner room with dim lights and fed to encourage natural postprandial sleep. We use pre-recorded mock MRI gradient noises over the built-in intercom speakers in the room while the neonate is being fed by the parent in the rocking chair; we find that falling asleep to noises increases the chance of success. The infant is then swaddled in an immobilizing device such as a Med-Vac vacuum immobilization bag (CFI Medical Solutions, Fenton, MI), provided with acoustic protection (ear putty and earmuffs), and placed into the scanner (Fig. 1). The success rate of neonatal MRI using the feed-and-swaddle technique exceeds 80% for a broad range of clinical indications [4, 5]. Based on our experience, failure rate is higher when the neonate is brought into the radiology department already asleep.
For inpatients undergoing MRI, monitoring of vital signs does not need to be adjusted and can continue as ordered by the primary team. For outpatients, pulse oximetry is usually sufficient for most MRI exams, assuming that monitoring is even indicated. To avoid hypothermia, MRI-compatible incubators have been designed for neonatal MRI; however, prewarmed blankets are an excellent substitute and allow use of standard MRI scanner coils. Pre-term neonates might require special care because they have a propensity for apnea and their ability to thermoregulate is even less developed than that of term neonates, increasing the risk for hypothermia [6].
As with any other medication, intravenous gadolinium-based contrast agent should only be administered to children when there is a clear diagnostic benefit. It is known that contrast agent can cause adverse reactions (both physiological and allergic-like) and be retained in the brain and body of the neonate. However, the risk is considered very low given that there are no documented adverse neurologic outcomes from deposition in the brain, and no cases of nephrogenic systemic fibrosis have been reported in this age group [3, 7, 8]. We exclusively use macrocyclic agents because they have the most favorable safety profile and retain reasonable T1 relaxivity properties. It is generally considered safe to administer gadolinium-based contrast agent to newborns with normal renal function, despite the theoretical immaturity of their kidneys [9, 10]. Note that not all gadolinium-based contrast agents are approved by the United States Food and Drug Administration to be used for children younger than 2 years (Magnevist and Omniscan are approved only for those age ≥2 years), although they are often used off-label. It is also important to emphasize the practical advantage of non-contrast MRIs in terms of the success of non-sedated MRIs. Venipuncture for catheter placement or intravenous injection carries the risk of waking the child and potentially not being able to obtain diagnostic contrast-enhanced images. In addition, some neonates with body wall edema have very difficult intravenous access. Additional details on the use of intravenous contrast agents in pediatric patients are covered in a separate issue of this supplement.
The MRI environment poses additional theoretical risks to newborns, although available evidence and our experience suggest that these are minimal and that the benefits of the MRI almost invariably outweigh the risks [11, 12]. The noise associated with the MRI gradients can exceed 120 dB for 3-tesla (T) scanners, which is above the threshold for discomfort and acoustic-induced damage. Age-appropriate auditory protection can completely mitigate this risk. A recent study by Jaimes et al. [13] showed that the increase in noise associated with 3-T scanners does not increase the rate of clinically detectable hearing abnormalities. A similar consideration arises from the higher energy deposition (specific absorption rate [SAR]) that takes place when scanning at 3 T. A recent study by Malik et al. [14] indicated that energy deposition is generally lower in neonates relative to adults undergoing comparable MRI exams at 1.5 T and 3 T [14]. Furthermore, the biological consequence of increased energy deposition at 3 T is elevation of core temperature; considering that newborns have a high surface area–to-volume ratio and that they are inherently prone to hypothermia, the potential for hyperthermia is not a practical concern.
Magnetic resonance imaging hardware: scanner field strength and coils
The most obvious technical challenge associated with neonatal imaging is the small size of the anatomy, which requires high spatial resolution. This is generally achieved by decreasing slice thickness and increasing in-plane resolution through increasing imaging matrix or decreasing field of view (FOV) (Fig. 2). While advantageous from the perspective of anatomical detail, this results in a corresponding drop in the signal-to-noise ratio (SNR), which is directly proportional to the voxel volume [15]. One way to compensate for the lower SNR is to increase the number of excitations (NEX), but this lengthens the duration of the MRI. We strongly favor imaging neonates at 3 T to harness the benefits of a higher B0, which nearly doubles the SNR.
Spatial resolution considerations in neonatal imaging. A common pitfall is to use field of view and matrix that are appropriate for imaging an adult or older child. a, b Axial T2-W MR image of the upper abdomen in a 2-week-old girl acquired using an 8-channel coil with a 30-cm field of view (FOV) (a) and a similar image acquired using a 20-cm FOV (b) show improvements in spatial resolution in (b). c, d Sagittal magnetization-prepared rapid gradient echo (MP-RAGE) acquired in a 4-day-old girl using a 31-cm × 24-cm FOV (c) demonstrates limited spatial resolution and encoding of air surrounding the head into the image. Repeat MP-RAGE image obtained a week later with a 20-cm × 17-cm FOV (d) offers superior spatial resolution and anatomical detail
A second component of the MRI hardware that plays a crucial role is the receiver coil [16, 17]. The overwhelming majority of coils are designed to fit the anatomy of adults. Identifying a coil that appropriately couples with the anatomy of the child and minimizes the space between the region-of-interest and the receiving elements is vital. A second advantageous attribute of coils is the presence of multiple receiving elements. Multi-channel phased-array coils should be used whenever possible because they result in higher SNR and enable use of higher parallel imaging acceleration factors. A multichannel adult head coil (e.g., 64-channel head coil) offers a robust and versatile alternative for neurologic and non-neurologic indications (e.g., lower extremity imaging, brachial plexus imaging), if the anatomy can be adequately positioned within the coil-array [18, 19].
Imaging various organ systems
Body imaging
Common indications for neonatal abdominal MRI include evaluation of congenital masses, cysts, vascular anomalies, malformations and congenital hemochromatosis. Common indications for thoracic MRI include intrathoracic vascular anomalies and malformations, mediastinal masses, paraspinal masses and, in recent years, bronchopulmonary dysplasia.
Coil selection
A variety of coils are available depending on the size of the baby, including an 18-channel adult knee coil, an 18-channel body coil or a 64-channel head/neck coil (Fig. 3). Other options include commercially available neonatal body coil and flex coil.
Coil configurations for body imaging. The goal is to cover the area of interest with a series of coil arrays that conform to the child’s anatomy. One common configuration is to use the 64-channel head and neck coil (64-ch H&N) in conjunction with an 18-channel body (18-ch B) coil. Another common configuration is to use an 18-channel extremity (18-ch Ex) coil with the chest or abdomen centered within the coil, as shown in the images
Protocol considerations
The mainstay of abdominal imaging consists of a combination of fluid-sensitive sequences and post-contrast imaging, with modest contributions from non-enhanced T1-weighted sequences and diffusion-weighted imaging. To mitigate the artifacts from respiratory motion, abdominal MRI uses respiratory-triggering, rapid acquisitions or a high number of signal averages. The shallow breathing of newborns poses an additional challenge because it leads to poor triggering from the bellows/belt, and therefore either navigator-based approaches or radial imaging is preferred [6].
T2-weighted imaging plays a crucial role in the characterization of pathology because most lesions and fluid collections result in T2 prolongation. Single-shot T2-W sequences provide the fastest alternative to survey the abdomen. While their speed is advantageous to avoid respiratory artifacts, the long echo train length results in a substantial decrease in tissue contrast that might hamper lesion detection (Fig. 4). Two-dimensional (2-D) fast spin-echo T2-W sequences offer a superior tissue contrast at the expense of longer scan time. Fast spin-echo (FSE) or turbo spin-echo (TSE) T2-W images can be acquired by sampling k-space in either Cartesian or non-Cartesian radial patterns with PROPELLER (periodically rotated overlapping parallel lines with enhanced reconstruction; GE Healthcare, Chicago, IL), BLADE (Siemens Medical Solutions, Erlangen, Germany) or MultiVane (Philips Healthcare, Best, the Netherlands) [20]. The benefit of radial acquisitions is a reduction of motion artifacts because of the dispersion of phase encoding aliasing in multiple radial directions, although they also result in longer scan times [21]. Fat-suppressed sequences optimize abdominal imaging by allowing for identification of fluid or inflammatory change in the mesenteric fat and removing background fat signal, which can aid in identifying subtle lesions. The T2-weighted Dixon method, if available, can be used for homogeneous fat suppression in the abdomen with advantages of both speed and uniformity, although frequency selective and inversion recovery methods can also be utilized (Fig. 5) [22]. Three-dimensional (3-D) heavily T2-W FSE magnetic resonance cholangiopancreatography (MRCP) sequences with high echo times (TEs>500 ms) can be used to evaluate the biliary tree and pancreatic ducts [23]. On occasion, it is valuable to add 3-D isotropic T2-weighted FSE in the evaluation of complex genitourinary or gastrointestinal malformations such as cloacal exstrophy because the ability to perform various multiplanar reformations is vital, and isotropic imaging saves time compared to multiple individual plane acquisitions (Fig. 6). The disadvantage of using isotropic imaging in the abdomen and pelvis is the potential for motion artifacts that would degrade images in all planes.
Varying tissue contrast on T2-W sequences acquired in a 5-week-old boy with a focal liver lesion. a, b Axial single-shot fast spin-echo (45 s) MR image (a) is devoid of respiratory artifact but shows low lesion-to-background contrast (arrow) relative to (b), a radial T2-weighted sequence (4 min, 30 s) (arrow)
Three-dimensional imaging in a 1-month-old girl with a covered cloacal exstrophy. Coronal three-dimensional image acquired using T2-W SPACE (sampling perfection with application-optimized contrasts using different flip-angle evolution) allows for multiplanar reformations, which are vital for understanding the complex anatomy of the malformation
The main role of non-enhanced T1-W imaging is providing greater anatomical detail and baseline for post-contrast imaging. The presence of intrinsic T1 shortening is indicative of fat, blood products, proteins or melanin. The recently developed radial 3-D T1-W gradient echo (GRE) sequences that use radial stack-of-stars non-Cartesian sampling allow free-breathing, motion-robust imaging that is ideal for neonates at the cost of relatively longer scan time and loss of multiphase enhancement information [24, 25]. Some vendors allow Dixon fat–water separation, which enables multiple contrast images (e.g., in-phase, opposed-phase, water-only and fat-only images) in a single acquisition. The water-only sequence can be used as the pre-contrast fat-suppressed T1 sequence, and the baseline for evaluating enhancement after contrast administration. A recently developed motion-robust T1-W imaging technique (GRASP, or golden-angle radial sparse parallel, by Siemens; 4D FreeBreathing by Philips; DISCO star, or differential sub-sampling with Cartesian ordering and star, by GE) allows free-breathing post-contrast imaging with temporal-resolved arterial-, venous- and delayed-phase images through a combination of radial k-space sampling, parallel imaging and compressed sensing reconstruction [26].
Musculoskeletal imaging
The most common indication for neonatal musculoskeletal imaging is suspected infection. The subject of musculoskeletal infection in the newborn is covered in greater detail in a separate issue of this supplement. Other relatively common indications include the study of skeletal malformations, vascular lesions (e.g., venous or lymphatic malformations), congenital masses (fibrous tumors or sarcomas), brachial plexus injury and spica cast MRI following hip reduction.
Protocol considerations
Evaluation of the bones, cartilage and soft tissues in the newborn requires a combination of high-resolution, high-SNR and high contrast-to-noise (CNR) sequences. Given the high prevalence of infectious and inflammatory pathology, fluid-sensitive sequences with fat suppression are essential. Fat suppression can be achieved through the Dixon method, frequency-selective fat suppression or inversion recovery imaging (Fig. 7). A major advantage to inversion recovery imaging is that it achieves robust fat suppression even in the presence of magnetic field inhomogeneities. An illustrative example is the assessment of small joints (e.g., hands or feet); however, the SNR is substantially lower compared to non-fat-suppressed images. Frequency-selective fat suppression offers the advantage of faster imaging and is, therefore, favored in cases where multiple slices are needed, such as axial imaging through a long bone [27]. Intermediate weighted or proton-density (PD) weighted sequences allow for optimal differentiation of fluid, hyaline cartilage, and fibrocartilage.
Robust fat suppression in musculoskeletal imaging in a 60-day-old boy with fever of unknown origin. a Coronal large field-of-view inversion recovery MR image clearly delineates edema about the left knee, as well as a joint effusion (arrow). b Sagittal post-contrast T1-weighted MR image with frequency-selective fat suppression shows severe signal drop (*) as a result of field inhomogeneity. c Sagittal T1-weighted post-contrast MR image with fat suppression using Dixon enables identification of a large joint effusion with marked synovitis (arrow), proven to represent septic arthritis
Bone marrow imaging is also challenging in newborns because of high water content and increased cellularity in the hematopoietic red marrow, which render the marrow hypointense on T1-weighted imaging. A combination of bilateral assessment, fat suppression and post-contrast imaging might be required to characterize focal osseous lesions, with the latter often carrying the highest specificity.
When evaluating a joint, appropriate sequences to delineate the various types of cartilage (articular, epiphyseal, physeal) are paramount, and this is best achieved with fluid-sensitive sequences and PD sequences [28]. In cases of complex skeletal malformations, such as complex polydactyly–syndactyly spectrum or amniotic band syndrome, the use of 3-D spin-echo PD sequences can be helpful. In addition, high-resolution GRE imaging (e.g., MEDIC, or multiple echo data image combination, by Siemens; MERGE, or multiple echo recombined gradient echo, by GE; M-FFE, or merged fast field echo, by Philips) or double echo steady state (DESS; Siemens) can also be used to outline the cartilaginous anlage of an unossified epiphysis, small bones (e.g., carpal, tarsal), or those with malformation or congenital dislocations. Depending on the clinical indication, contrast agent can be administered to evaluate for tumoral enhancement, normal epiphyseal perfusion, synovitis or cases of focal chondral lesions (e.g., chondritis) [29, 30].
Upper extremity
Positioning and coil selection
To evaluate the supraclavicular region, brachial plexus and shoulder, a 64-channel head and neck coil with or without spine coil can be used with the head placed deeply into the coil. For more distal upper extremity, a flex coil could be wrapped around the area of interest.
Imaging protocol
For brachial plexus injury, 3-D imaging is particularly useful because it allows multiplanar reconstructions to be generated from a single acquisition. Coronal inversion recovery 3-D spin-echo allows optimal visualization of the brachial plexus, with high SNR and CNR. This sequence exquisitely delineates the nerve roots and any associated inflammation (Fig. 8). A 3-D T2-W spin-echo sequence or a 3-D gradient echo sequence (CISS, or constructive interference steady state; Siemens) can be concurrently acquired to better outline the contour of the thecal sac and the intradural course of the nerve roots. Utilizing a wide field that includes the cervical spine and both shoulders is advantageous to provide a comparison and profits from the robust fat suppression achieved with the inversion recovery technique.
Brachial plexus injury in a 10-day-old boy. a, b Coronal (a) and sagittal (b) reformatted three-dimensional spin-echo inversion recovery MR images show thickening of the brachial plexus nerve roots (arrows). c A coronal wide-field-of-view two-dimensional inversion recovery image shows a healing left humeral fracture sustained at birth (arrow)
Hip and spica cast magnetic resonance imaging
Positioning and coil selection
Infants are placed in the scanner feet first and supine on the table. A pillow is placed under the infant’s legs to support the bend of the hard cast. A flex coil is placed directly over the hip joints.
Imaging protocol
The primary goal of MRI of the hips after spica cast placement is to assess concentric placement of the femoral head within the acetabulum after closed or open reduction. Additional important imaging findings include identification of interposed soft-tissue structures that impede concentric hip reduction. The protocol should be short enough that the image acquisition can be performed in the immediate postoperative period using the same anesthesia event of the reduction procedure, or it can be performed without sedation. When planning the MRI protocol for this indication, it is important to recognize the forced abduction position of the hips and dysmorphic appearance of the femoral head, which can hinder imaging acquisition of the orthogonal planes. Protocols for hip and spica cast MRI are variable among institutions, with some advocating for only axial and coronal T2-W fat-saturated (FS) sequences [31] and others recommending axial and coronal T1- and T2-weighted images [32]. We use 3-D coronal T2-W MEDIC and coronal PD sequences, which result in approximately 8 min of imaging time; the short imaging time facilitates completion of this protocol immediately after surgery, without the need to separately sedate the infant (Fig. 9). The use of 3-D T2 MEDIC allows for triplanar reformation and provides excellent discrimination between the non-ossified femoral head cartilage and the bony acetabulum. The addition of a coronal PD sequence allows visualization of fibro-cartilaginous structures such as labral or limbus tissue that can be interposed. Although rare, motion artifacts sometimes degrade images, and in these cases we use radial fast spin-echo images to acquire diagnostic images (BLADE, PROPELLER). We do not routinely perform contrast-enhanced imaging because the enhancement pattern of the femoral head does not predict the development of avascular necrosis [32].
Spica MRI in a 6-week-old girl status post closed hip reduction. a Coronal three-dimensional T2-weighted gradient echo MEDIC (multiple echo data image combination) MR image shows shallow coverage of both femoral heads, worse on the left. Both femoral heads are in concentric position within the acetabula. b Coronal proton-density-weighted MR image with BLADE acquisition shows a prominent left-side fatty pulvinar (arrow)
Lower extremity
Positioning and coil selection
For imaging of the lower extremity, a multi-channel head and neck coil could be used with the feet deeply positioned within the coil, or an 18-channel knee coil could be used with both lower extremities placed within the coil. A flex coil can also be wrapped around the area of interest.
Imaging protocol
Lower extremity imaging often requires coverage of long segments of anatomy, which are prone to field inhomogeneity. When imaging in the axial plane, a time-efficient approach such as frequency-selective fat suppression has advantages; combining this technique with slightly thicker slices or simultaneous multi-slice acquisition can result in substantial time savings (Fig. 10) [33]. If imaging is done around the foot or ankle, either Dixon or inversion recovery often results in better image quality because of its more robust homogeneous fat suppression. Post-contrast imaging can also be performed with Dixon when incomplete fat suppression artifacts obscure important findings.
Efficient imaging in the axial plane of the legs in a 90-day-old girl with an extensive venous malformation. a, b Axial fat-saturated T2-weighted MR images show venous malformation involving the right perineal region (a), the right medial thigh and leg (b); image acquisition is faster than with inversion recovery images, although some inhomogeneous fat suppression is apparent (arrow in a). c, d Fat-suppressed gradient echo T1-weighted post-contrast MR images after contrast cover the same extent of the anatomy with the gradient echo technique, resulting in a fast acquisition
Neuroradiology
Common reasons for brain imaging in newborns include neonatal encephalopathy, seizures, infection (meningoencephalitis) and the study of prenatally detected congenital abnormalities. Head and neck MRI is substantially less common but is occasionally performed to characterize complex malformations and congenital lesions, in cases of infection and in the evaluation of sensorineural hearing loss. Spinal MRI occurs most commonly in the setting of a known or suspected spinal dysraphism.
Coil selection
We find that the 64-channel head and neck coil (Siemens, Erlangen, Germany), in conjunction with a 3-T MRI scanner, offers optimal SNR for brain MRI and head and neck MRI (Fig. 11). Purpose-built pediatric head coils are a suitable alternative for brain MRI, but in our experience they do not outperform higher-density adult head coils. Spinal imaging is routinely performed with the built-in spinal coil of the MRI table; we do not recommend using an anterior abdominal coil because respiratory motion often introduces artifacts.
Clinical photographs of a 64-channel head and neck coil (64-ch H&N). a, b Given the small size of the newborn, the coil provides satisfactory coverage of the brain and craniofacial structures. Despite the relatively poor anatomical coupling between the small head size and the coil, the high number of receiving elements provides satisfactory image quality
Imaging approach
Brain imaging
The first consideration in obtaining high-quality imaging is to position the infant’s head as close as possible to the center of the coil, thereby taking full advantage of the array of receiving elements. Second, the FOV should be adjusted to the head size of the newborn (e.g., 170 mm × 170 mm with matrix size allowing for submillimeter in-plane resolution). Third, the parameters of T2-weighted sequences should be adjusted to account for the watery composition of the unmyelinated brain (repetition time/echo time [TR/TE] = >12,000/>100 ms).
Our neonatal brain imaging protocols are built from a base of five sequences: sagittal 3-D T1-W MP-RAGE (magnetization-prepared rapid acquisition with gradient echo), axial/coronal T2-W sequences with near 0.5-mm in-plane resolution and 2.5-mm z-axis resolution, axial T1-W fluid-attenuated inversion recovery (FLAIR) (again with nearly 0.5-mm in-plane resolution and 2.5-mm z-axis resolution), diffusion tensor imaging (DTI; 1.5-mm in-plane resolution and 2-mm z-axis resolution) and susceptibility-weighted imaging (SWI) (Fig. 12). We favor DTI over DWI because the higher number of diffusion encoding directions in DTI increases the SNR, reduces distortions around bones and enables construction of fractional anisotropy (FA) and color FA maps, which aid in the evaluation of the fascicular architecture of the white matter. This protocol takes approximately 26 min, but abridged versions of this protocol can be performed in as few as 12 min with technical adjustments (e.g., shortened TR, decreased NEX, simultaneous multi-slice acquisition). The 2-D T1-W FLAIR sequence is employed in addition to the 3-D T1-W gradient echo sequence to allow for greater in-plane resolution and tissue contrast (e.g., gliosis, myelination). As mentioned, the T2-W sequences are also optimized to account for the long T2 relaxation of neonatal tissues. SWI sequences are always acquired in neonates because hemorrhagic injuries are extremely common.
Routine neonatal MRI brain protocol in a 2-week-old boy with prenatally diagnosed septo-optic dysplasia. a Sagittal magnetization-prepared rapid acquisition with gradient echo (MP-RAGE). b Axial T2-W fast spin echo (FSE). c Thirty-five-direction diffusion tensor imaging (DTI). Images show various tissue contrasts in the immature brain and an absence of the septal leaflets
To these base sequences, modifications can be made for specific indications. For low-risk trauma evaluations (e.g., dropped from changing table, no suspicion of inflicted trauma), we simply add a single-shot T2-W sequence at the beginning to ensure that we get usable images, and we add fat-suppressed T2-W FLAIR (typically noncontributory for parenchymal evaluation in a neonate) to increase visibility of extra-axial hemorrhage as well as scalp trauma. We also electively add black-bone sequences to increase visibility of fractures, though referring physicians must be aware that MRI is generally outperformed by CT for evaluating fractures and assigning acuity to hemorrhage. For suspected abusive head trauma, we also add a high-resolution isotropic 3-D T2-W spin-echo sequence for detecting bridging vein avulsions, synechiae and injuries to stabilizing ligaments at the cervicocranial junction; we also typically add MR venography, sagittal short tau inversion recovery (STIR) images of the cervical spine, and (assuming the infant is still asleep) sagittal T1/T2 FS sequences to screen the spinal column. For infection, we complement axial diffusion imaging with fat-suppressed T2-W FLAIR for detecting inflammatory exudate, and post-contrast 3-D T1-W spin-echo sequence.
If there is a concern for hypoxic–ischemic injury or a metabolic disorder, we frequently acquire MR spectroscopy. We employ 3-D chemical shift imaging (CSI) spectroscopy, which enables simultaneous acquisition of data emanating from a grid of voxels in the brain, rather than a single-voxel sample [34]. The spectra are routinely acquired using a combination of short (30 ms) and intermediate (135 ms) TEs. The short TE spectrum aids in the evaluation of small molecules, which have a short decay time, while the intermediate TE spectrum allows for better evaluation of the dominant peaks. In addition, the acquisition of both spectra allows for reliable identification of lactate as an upward pointing doublet at 1.3 ppm in the short TE spectrum, with corresponding inversion on the intermediate TE (Fig. 13).
Diffusion and spectroscopic abnormalities in a 4-day-old boy with hypoxic–ischemic injury (HII). a Axial apparent diffusion coefficient (ADC) map shows decreased diffusivity in the ventrolateral thalami (arrow), consistent with a central pattern of HII. b, c MR spectroscopy acquired using a short echo time (30 ms; b) shows an upward pointing doublet at 1.3 ppm (arrow in b), which shows inversion (arrow in c) in the intermediate echo time spectrum (135 ms; c)
For hydrocephalus evaluations in a neonate, we typically add a heavily T2-weighted sequence: preoperatively, a steady-state sequence such as fast imaging employing steady-state acquisition (FIESTA) or CISS, and postoperatively, a flow-sensitive sequence such as T2-SPACE (sampling perfection with application optimized contrasts using different flip angle evolution).
Head and neck imaging
Protocols for evaluating head and neck pathology observe similar principles. A high in-plane resolution is highly desired and, consequently, 2-D FSE imaging is widely used. T1-W sequences allow for evaluation of bone marrow and for a general anatomical survey because they clearly outline tissue planes around the orbits and in the suprahyoid and infrahyoid neck. T2-W sequences with fat suppression contribute to the evaluation of cystic lesions and edema. Occasionally, the field inhomogeneity in the submandibular space and supraclavicular fossa results in incomplete fat suppression; inversion recovery sequences and Dixon-based fat-suppression techniques can be used to overcome this challenge. Diffusion-weighted images are used to evaluate the cellularity of lesions, to evaluate for purulent contents or to diagnose dermoid cysts. Many institutions have incorporated segmented read-out for echoplanar diffusion sequences (readout segmentation of long variable echo-trains [RESOLVE]) as a strategy to mitigate geometric distortions in the head and neck. Post-contrast imaging can help characterize solid lesions, identify drainable collections and determine the extent of inflammatory pathology (Fig. 14).
Magnetic resonance imaging of the neck in a pre-term boy obtained 14 days after birth. a, b Coronal T2-weighted (a) and post-contrast T1-weighted (b) images using Dixon-based fat suppression show a large collection with rim enhancement in the submental space, consistent with an abscess (arrows). c Apparent diffusion coefficient (ADC) map obtained with readout segmentation of long variable echo-trains (RESOLVE) diffusion shows decreased diffusivity, consistent with pus
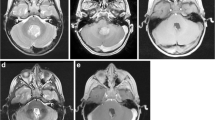
Evaluation of sensorineural hearing loss requires special considerations. MRI of the temporal bone, in addition to high in-plane resolution FSE, requires the addition of heavily T2-weighted (cisternographic) sequences for evaluating cranial nerve VIII and the inner ear structures (Fig. 15). The most commonly used sequence is a 3-D T2-W spin-echo sequence (e.g., SPACE by Siemens; CUBE by GE; VISTA [volume isotropic T2 acquisition] by Philips) or a steady-state free precession sequence (e.g., FIESTA, CISS). These are typically acquired with slice thickness in the range of 0.5–0.7 mm to enable characterization of the cisternal segments of cranial nerve VIII, the cochlear aperture, the cochlear partition and the internal cochlear structure. These sequences can also be used to evaluate the cisternal segments of other cranial nerves. It is worth noting that with some frequency, the 3-D T2-W spin-echo sequences can be degraded by cerebrospinal fluid (CSF) pulsation, which can obscure the nerves of interest; steady-state free precession sequences are less sensitive to these artifacts and can be used to increase diagnostic yield.
Incomplete partition type 2 with enlarged vestibular aqueduct in a 2-week-old boy who failed the auditory brainstem response screen. Axial T2-W SPACE (sampling perfection with application optimized contrasts using different flip angle evolution) MR image shows loss of the interscalar ridge between the mid and apical cochlear turns (arrow) as well as enlargement of both vestibular aqueducts (arrowheads)
Spine imaging
Magnetic resonance imaging of the spine consists predominantly of T1-W and T2-W sequences to characterize the anatomy. T1-W sequences are used to evaluate for lipomatous elements of spinal dysraphisms, and T2-W sequences are used to evaluate the cord, CSF spaces and cauda equina nerve roots (Fig. 16). While the vast majority of the spinal pathology in this age range affects the lumbar spine, it is important to ensure that the interpreting neuroradiologist has sufficient imaging available to accurately determine the complement of vertebrae; this can be done by imaging the entire spine or by purposefully acquiring whole-spine localizers that include the craniocervical junction.
Magnetic resonance imaging of the spine in a 1-month-old girl with a sacral dimple and a tuft of hair in the lumbosacral region. a Sagittal T1-W image shows a closed spinal dysraphism with an intradural lipoma (arrow). b Sagittal T2-W image shows fibrous elements in association with the lipoma (arrow), which also appears inseparable from the low-lying spinal cord
Conclusion
Magnetic resonance imaging is a versatile modality that can be used to study complex pathology in newborns. Despite challenges related to the small size of the patients and the limited compliance, a systematic approach to patient preparation, coil selection and protocol design enables high-quality examinations in the large majority of newborns.
References
Cravero JP, Beach ML, Blike GT et al (2009) The incidence and nature of adverse events during pediatric sedation/anesthesia with propofol for procedures outside the operating room: a report from the Pediatric Sedation Research Consortium. Anesth Analg 108:795–804
Davidson AJ, Disma N, de Graaff JC et al (2016) Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet 387:239–250
McCann ME, de Graaff JC, Dorris L et al (2019) Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet 393:664–677
Windram J, Grosse-Wortmann L, Shariat M et al (2012) Cardiovascular MRI without sedation or general anesthesia using a feed-and-sleep technique in neonates and infants. Pediatr Radiol 42:183–187
Antonov NK, Ruzal-Shapiro CB, Morel KD et al (2017) Feed and wrap MRI technique in infants. Clin Pediatr 56:1095–1103
Dillman JR, Tkach JA (2022) Neonatal body magnetic resonance imaging: preparation, performance and optimization. Pediatr Radiol 52:676–684
Sun LS, Li G, Miller TL et al (2016) Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA 315:2312–2320
Bedoya MA, White AM, Edgar JC et al (2017) Effect of intravenous administration of contrast media on serum creatinine levels in neonates. Radiology 284:530–540
Penfield JG (2008) Nephrogenic systemic fibrosis and the use of gadolinium-based contrast agents. Pediatr Nephrol 23:2121–2129
Nardone B, Saddleton E, Laumann AE et al (2014) Pediatric nephrogenic systemic fibrosis is rarely reported: a RADAR report. Pediatr Radiol 44:173–180
Mathur AM, Neil JJ, McKinstry RC, Inder TE (2008) Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol 38:260–264
Rona Z, Klebermass K, Cardona F et al (2010) Comparison of neonatal MRI examinations with and without an MR-compatible incubator: advantages in examination feasibility and clinical decision-making. Eur J Paediatr Neurol 14:410–417
Jaimes C, Delgado J, Cunnane MB et al (2019) Does 3-T fetal MRI induce adverse acoustic effects in the neonate? A preliminary study comparing postnatal auditory test performance of fetuses scanned at 1.5 and 3 T. Pediatr Radiol 49:37–45
Malik SJ, Beqiri A, Price AN et al (2015) Specific absorption rate in neonates undergoing magnetic resonance procedures at 1.5 T and 3 T. NMR Biomed 28:344–352
Edelstein WA, Glover GH, Hardy CJ, Redington RW (1986) The intrinsic signal-to-noise ratio in NMR imaging. Magn Reson Med 3:604–618
Ditchfield M (2008) 3T MRI in paediatrics: challenges and clinical applications. Eur J Radiol 68:309–319
Hillenbrand CM, Reykowski A (2012) MR imaging of the newborn: a technical perspective. Magn Reson Imaging Clin N Am 20:63–79
Zhang T, Grafendorfer T, Cheng JY et al (2016) A semiflexible 64-channel receive-only phased array for pediatric body MRI at 3T. Magn Reson Med 76:1015–1021
Winkler SA, Corea J, Lechene B et al (2019) Evaluation of a flexible 12-channel screen-printed pediatric MRI coil. Radiology 291:180–185
Jaimes C, Kirsch JE, Gee MS (2018) Fast, free-breathing and motion-minimized techniques for pediatric body magnetic resonance imaging. Pediatr Radiol 48:1197–1208
Pipe JG (1999) Motion correction with PROPELLER MRI: application to head motion and free-breathing cardiac imaging. Magn Reson Med 42:963–969
Delfaut EM, Beltran J, Johnson G et al (1999) Fat suppression in MR imaging: techniques and pitfalls. Radiographics 19:373–382
Gwal K, Bedoya MA, Patel N et al (2015) Reference values of MRI measurements of the common bile duct and pancreatic duct in children. Pediatr Radiol 45:1153–1159
Kozak BM, Jaimes C, Kirsch J, Gee MS (2020) MRI techniques to decrease imaging times in children. Radiographics 40:485–502
Balza R, Jaimes C, Risacher S et al (2019) Impact of a fast free-breathing 3-T abdominal MRI protocol on improving scan time and image quality for pediatric patients with tuberous sclerosis complex. Pediatr Radiol 49:1788–1797
Chandarana H, Feng L, Block TK et al (2013) Free-breathing contrast-enhanced multiphase MRI of the liver using a combination of compressed sensing, parallel imaging, and golden-angle radial sampling. Invest Radiol 48:10–16
Jaramillo D, Laor T (2008) Pediatric musculoskeletal MRI: basic principles to optimize success. Pediatr Radiol 38:379–391
Barnewolt CE, Shapiro F, Jaramillo D (1997) Normal gadolinium-enhanced MR images of the developing appendicular skeleton: part I. Cartilaginous epiphysis and physis. AJR Am J Roentgenol 169:183–189
Laor T, Jaramillo D (2009) MR imaging insights into skeletal maturation: what is normal? Radiology 250:28–38
Boavida P, Muller LS, Rosendahl K (2013) Magnetic resonance imaging of the immature skeleton. Acta Radiol 54:1007–1014
Gould SW, Grissom LE, Niedzielski A et al (2012) Protocol for MRI of the hips after spica cast placement. J Pediatr Orthop 32:504–509
Nguyen JC, Back SJ, Barrera CA et al (2021) Developmental dysplasia of the hip: can contrast-enhanced MRI predict the development of avascular necrosis following surgery? Skeletal Radiol 50:389–397
Benali S, Johnston PR, Gholipour A et al (2018) Simultaneous multi-slice accelerated turbo spin echo of the knee in pediatric patients. Skeletal Radiol 47:821–831
Yazbek S, Prabhu SP, Connaughton P et al (2015) Comparison of accelerated 3-D spiral chemical shift imaging and single-voxel spectroscopy at 3T in the pediatric age group. Pediatr Radiol 45:1417–1422
Acknowledgments
This work was partially supported by the Office of Faculty Development at Boston Children’s Hospital (C.J.), an American Roentgen Ray Society Scholarship (C.J.), the Rosamund Stone Zander Center for Translational Neuroimaging at Boston Children’s Hospital (C.J.) and the Young Investigator Award from the Society for Pediatric Radiology (M.A.B., C.J.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Handa, A., Xu, L., Machado-Rivas, F. et al. Magnetic resonance imaging in neonates: a practical approach to optimize image quality and increase diagnostic yield. Pediatr Radiol 53, 1300–1313 (2023). https://doi.org/10.1007/s00247-022-05550-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-022-05550-0