Abstract
Background
Fetal MRI at 3 T is associated with increased acoustic noise relative to 1.5 T.
Objective
The goal of this study is to determine if there is an increased prevalence of congenital hearing loss in neonates who had a 3-T prenatal MR vs. those who had it at 1.5 T.
Materials and methods
We retrospectively identified all subjects who had 3-T fetal MRI between 2012 and 2016 and also underwent universal neonatal hearing screening within 60 days of birth. Fetuses with incomplete hearing screening, magnetic resonance imaging (MRI) studies at both field strengths or fetuses affected by conditions associated with hearing loss were excluded. A random group of controls scanned at 1.5 T was identified. Five subjects had repeat same-strength MRIs (one at 3 T and four at 1.5 T). The pass/fail rate of the transient otoacoustic emissions test and auditory brainstem response test were compared using the Fisher exact test. A logistic regression was performed to assess the effects of other known risk factors for congenital hearing loss.
Results
Three hundred forty fetal MRI examinations were performed at 3 T, of which 62 met inclusion criteria. A control population of 1.5-T fetal MRI patients was created using the same exclusion criteria, with 62 patients randomly selected from the eligible population. The fail rates of transient otoacoustic emissions test for the 1.5-T and 3-T groups were 9.7% and 6.5%, respectively, and for the auditory brainstem response test were 3.2% and 1.6%, respectively. There was no significant difference in the fail rate of either test between groups (P=0.74 for transient otoacoustic emissions test, and P=0.8 for auditory brainstem response test). The median gestational age of the 3-T group was 30 weeks, 1 day, significantly higher (P<0.001) than the 1.5-T group (median gestational age: 20 weeks, 2 days).
Conclusion
Our findings suggest that the increase in noise associated with 3 T does not increase the rate of clinically detectable hearing abnormalities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Congenital abnormalities complicate approximately 1.5–3% of pregnancies and constitute a major cause of perinatal morbidity and mortality [1,2,3]. Many of these abnormalities are detected on routine prenatal ultrasound (US) screening and are subsequently characterized by detailed sonographic anatomical surveys and/or followed longitudinally [4]. Over the past decades, fetal magnetic resonance imaging (MRI) has emerged as a valuable complementary imaging modality to characterize fetal abnormalities detected on prenatal US, by virtue of its high soft-tissue contrast, large field of view and ability to reconstruct images in multiple planes [5,6,7,8].
Until recently, fetal MRI had been limited to 1.5-Tesla (T) scanners. However, the challenge of imaging smaller structures, the need for faster scanning and the known signal-to-noise ratio benefits associated with imaging at higher field strengths led to the utilization of 3-T MRI systems for clinical fetal imaging [9]. Previous work has shown improved delineation of fetal anatomy at 3 T compared with 1.5 T that is thought to result in improved diagnostic performance [10, 11]. However, although 3-T MRI is routinely used in the clinical setting for imaging children and adults, its use for fetal imaging has been more limited. This is in large part due to potential safety concerns to the fetus, including higher acoustic noise levels associated with higher strength magnets, which can theoretically affect neonatal hearing [12, 13]. Several retrospective studies have evaluated the effects of prenatal exposure to MRI scanner-related noise at 1.5 T; these studies did not identify any adverse effects on neonatal hearing as determined by the results of neonatal hearing screening tests [14, 15]. However, the biological effects of acoustic noise at 3 T have not been reported.
In the United States, all newborns undergo universal neonatal hearing screening, which consists of a battery of tests that can be used individually or in combination to evaluate hearing function. The most commonly utilized tests are the transient otoacoustic emissions test and the auditory brainstem response test. In normal newborns, the fail rate of the transient otoacoustic emissions test is approximately 7% and that of the auditory brainstem response is less than 1% [16,17,18,19,20]. In high-risk populations, these tests are often used in conjunction to independently assess various aspects of hearing and to increase sensitivity of the screening.
The purpose of this study is to determine if the increased acoustic noise associated with higher field strength fetal MRI has a clinically significant effect on neonatal hearing by comparing the results of the universal neonatal hearing screening in fetuses scanned at 3 T and 1.5 T.
Materials and methods
Study participants
This single institution retrospective study was approved by the institutional review board and executed in compliance with the Health Insurance Portability and Accountability Act (HIPAA). A search engine (Softek Illuminate; Softek Solutions, Overland Park, KS) was used to query a radiology report database during the years 2012–2016 (with 2012 being the first year that clinical 3-T fetal MRIs were performed at our institution) to identify subjects who underwent fetal MRI using 1.5-T and 3-T MRI scanners. Our query identified some subjects who underwent more than one MRI as part of their clinical care; these subjects were included in the analysis provided that the MRIs were performed at the same field strength. Electronic medical record queries were then performed to identify patients with available neonatal records including hearing screening results performed within 60 days of birth. Fetuses with primary neurological abnormalities (e.g., myelomeningocele, ventriculomegaly, absent corpus callosum), systemic diseases and syndromes known to be associated with hearing loss were excluded.
MRI examinations
Fetal MRIs at 3 T were performed on Magnetom Skyra, Prisma and Verio clinical scanners, and 1.5-T studies were performed on Magnetom Avanto scanners (Siemens Healthcare, Erlangen, Germany).
Fetal imaging studies at 1.5 T and 3 T were performed according to standard protocols that have been previously been published [9, 10]. The 3-T protocol included these tri-plane sequences: a) T2-weighted half-Fourier acquisition single-shot turbo spin-echo (HASTE; repetition time [TR]/echo time [TE] of 1,100/76 ms; 1 signal average; flip angle 180°; matrix 320 × 256, field of view [FOV] 280–300 mm), b) T1-weighted fast low-angle shot (FLASH; TR/TE of 180/4.76 ms; 1 signal average; flip angle 60°; matrix 256 × 192, FOV 280–300 mm), and c) true fast imaging with steady-state precession (TrueFISP; TR/TE 4.66/1.93 ms; 1 signal average; flip angle 90°; matrix 320 × 256, FOV 280–300 mm). The 1.5-T protocol utilized the same pulse sequences with slightly different imaging parameters: a) single-shot T2-weighted (TR/TE of 1,100/76 ms; 1 signal average; flip angle 180°; matrix 256 × 256, FOV 280–300 mm), b) T1-weighted fast low flip angle gradient echo (TR/TE of 202/4.76 ms; 1 signal average; flip angle 60°; matrix 256 × 166, FOV 280–300 mm), and c) balanced steady-state free precession (SSFP) (TR/TE 4.05/1.65 ms; 1 signal average; flip angle 70°; matrix 256 × 256, FOV 280–300 mm).
Hearing screening tests in newborns
Since 2001, state government legislation has required hearing screening of all newborns [21]. At our institution, the hearing screening program consists of transient otoacoustic emissions test screening and auditory brainstem response testing. The transient otoacoustic emissions test measures sound generated by the outer hair cells of the cochlea in response to acoustic stimuli, and is sensitive to causes of hearing loss located between the external auditory canal and the cochlea. The auditory brainstem response test measures electric activity in the brainstem in response to sound and evaluates the integrity of the entire auditory system. This protocol adheres to the American Speech-Language-Hearing Association’s recommendations, which require that all high-risk children have an auditory brainstem response test as part of their newborn hearing screening, whether as a stand-alone test or as part of a transient otoacoustic emissions test/auditory brainstem response test battery [22]. Transient otoacoustic emissions testing was performed using a hearing-screening machine (Madsen AccuScreen; Otometrics, Taastrup, Denmark) and reported as positive or negative. Auditory brainstem response testing was considered normal if air-conducted 2,000 Hz wave V responses were recorded at 30 dB at normalized hearing level. Smart EP equipment (Intelligent Hearing Systems, Miami, FL) was used. Electronic medical records were queried to retrieve hearing test results on all patients in the study.
Obstetric and newborn clinical data
For all subjects included in the study, the gestational age at the time of the MRI and delivery, gender, weight at birth and diagnosis at birth were obtained from the electronic medical records. The gestational age at the time of the MRI and delivery were estimated based on biometric data reported on an obstetric US performed within 72 h of the MRI, as part of the routine clinical assessment of patients at the institutional fetal medicine center. Subjects who were part of a twin pregnancy or who underwent two fetal MRIs during pregnancy were also recorded. Admission to the neonatal intensive care unit (NICU) for longer than 5 days was also assessed as this is a known risk factor for the development of hearing impairment [23]. After analyzing the entire data set, we performed a subset analysis excluding subjects who were affected by the possible confounding variables of preterm birth and a NICU admission.
Statistical analysis
For the descriptive analysis percentages, medians, ranges and interquartile ranges were used. The Fisher exact test was used to evaluate differences in the distribution of nominal data, while the Mann-Whitney U test was used to evaluate differences in continuous variables. Multivariate logistic regression was also used to evaluate for potential predictors of hearing test performance, with model significance assessed by Wald Z-statistic. Statistical significance was defined by P-values <0.05. Statistical analyses were performed using SPSS version 20 (IBM, Armonk, NY).
Results
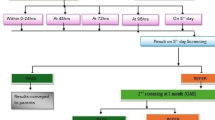
A radiology report database query yielded 340 fetal MRI studies performed at 3 T in the years 2012–2016. Of the 340 3-T fetal MRI studies available, 278 studies were excluded for the following reasons: a) no neonatal medical records available for review (fetal demise or lost to follow-up) (n=118), b) no hearing screening results available in the medical records (n=89) or hearing screening could not be tested after several attempts (n=7), c) fetuses underwent MRI studies at both 1.5 T and 3 T (n=25), d) hearing screening performed more than 60 days after birth (n=12), e) primary neurological abnormalities (Chiari malformation, ventriculomegaly, callosal agenesis) (n=16), and f) systemic diseases and syndromes known to be associated with hearing loss, including 22q11.2 deletion syndrome (n=4), trisomy 21 (n=3), Pierre Robin sequence (n=1), cleft lip and palate (n=1), arthrogryposis (n=1) and microtia (n=1). A total of 62 neonates were included in our study population. A control population of 1.5-T fetal MRI patients was created using the same exclusion criteria, with 62 patients randomly selected from the eligible population (Fig. 1). In total, 124 newborns who underwent fetal MRI and neonatal hearing testing were included in our study population.
The demographic information for the patients included in the study is summarized in Table 1. The gender, median gestational age at birth, median gestational age at transient otoacoustic emissions testing, weight at birth, number of twin pregnancies, and number of subjects who had repeat MRIs at the same field strength were not significantly different between the 1.5-T and the 3-T fetal MRI groups (P values >0.05). The median gestational age at the time of MRI was significantly lower for fetuses scanned at 1.5 T (25 weeks, 2 days) relative to those scanned at 3 T (30 weeks 1 day; P<0.001). The degree of pathology affecting specific organ systems was not significantly different between both groups for any of the categories evaluated. The relative percentages of disease categories affecting the subjects included in this study are listed in Table 2.
Fetal and neonatal risk factors
Next, we focused on potential risk factors for neonatal hearing loss. The first was prolonged NICU admission of more than 5 days, which was observed in 85/124 (68.5%) patients in the overall population, including 45/62 (72.6%) of 1.5-T MRI patients and 40/62 (64.5%) of 3-T MRI patients. The second was prematurity; 34/124 (27.4%) patients overall were born preterm, including 16/62 (25.8%) 1.5-T MRI patients and 18/62 (29.0%) 3-T MRI patients. There was no significant difference in the rate of either prolonged NICU admission or prematurity between the 1.5-T and 3-T groups (P-values >0.4).
Hearing test performance
Overall, 10 out of 124 newborns (8.1%) failed the transient otoacoustic emissions hearing test in at least one ear, and 3 out of 124 subjects (2.4%) failed the auditory brainstem response test in at least one ear. Two of the three subjects who failed the auditory brainstem response test also failed the transient otoacoustic emissions test. The clinical data of patients who failed the transient otoacoustic emissions test and/or auditory brainstem response test are listed in Table 3. Six of the newborns who failed the transient otoacoustic emissions test had 1.5-T MRI (9.7% incidence) while the other four had 3-T MRI (6.5% incidence). Of the three patients who failed the auditory brainstem response test, two had 1.5-T MRI (3.2% incidence) compared with one who had 3-T MRI (1.6% incidence). No significant difference was observed in test failure rates at 3 T compared with 1.5 T for both the transient otoacoustic emissions test (P=0.74) and auditory brainstem response test (P=0.80) tests. These results are summarized in Table 4.
Logistic regression analyses did not demonstrate a significant relationship among the variables of MRI field strength, prolonged NICU admission or prematurity with hearing test failure, assessed by either the transient otoacoustic emissions test or auditory brainstem response tests (Table 4). None of the subjects who were part of a twin pregnancy or who had repeat MRIs failed either the transient otoacoustic emissions test or the auditory brainstem response test.
We performed subset analyses of the groups based on the trimester of gestation in which the children had their MRIs. Sixty-five patients underwent MRI in the second trimester (42 at 1.5 T and 23 at 3 T) and 59 underwent MRI in the third trimester (20 at 1.5 T and 39 at 3 T). The fail rate for the transient evoked otoacoustic emissions (TEAOE) for fetuses who underwent MRI in the second trimester was 5/65 (7.7%). The five subjects who failed the test had MRI at 1.5 T. The fail rate of TEAOE in the third trimester 5/59 (8.4%); four had MRI at 3 T and one at 1.5 T (P>0.05). The fail rate of the auditory brainstem response test for subjects who underwent second trimester MRI was 1/65 (1.5%); the subject who failed the auditory brainstem response test in the second trimester had a 1.5-T MRI. The fail rate of the auditory brainstem response test for subjects who underwent third trimester MRI was 2/59 (3.4%); one subject had MRI at 1.5 T and one at 3 T (P>0.1).
Subset analysis of subjects without clinical risk factors
We performed a subset analysis after excluding subjects with known risk factors including preterm birth (n=6), NICU admission (n=57) or both (n=28). Of the remaining 33 subjects, 14 had MRIs at 1.5 T and 19 had MRIs at 3 T. Two subjects (6.6%) failed the transient otoacoustic emissions test in this subgroup, one of which had MRI at 1.5 T (7.1%) and one at 3 T (5%). No significant difference in the rate of failure was observed between groups (P=0.83). None of the subjects in this subgroup failed the auditory brainstem response screen.
Discussion
Fetal MRI is a well-established imaging modality used to characterize prenatal pathology, determine prognosis and guide treatment [8, 24]. With the recent implementation of 3-T fetal MRI, the concern for acoustic injury to the fetus related to the noise generated by the gradients in the scanner has been a subject of controversy [12, 13]. Our study compared the results of neonatal transient otoacoustic emissions test and auditory brainstem response test in born subjects who had 1.5-T and 3-T fetal MRIs. Our results did not identify an increase in the overall fail rate of either test in patients who were imaged at 3 T, suggesting that the increase in acoustic noise associated with higher field strength does not result in clinically significant acoustic injury. These findings are consistent with results from a small study on hearing screening of infants whose mothers had significant occupational exposure to noise during their pregnancy (>80 dB for 8 h per day), which did not identify an increase in hearing impairment [25].
The mechanism of noise-induced acoustic damage to the fetus is not completely elucidated, but it has been associated with injury to the hair cells of the cochlea, predominantly affecting the cochlear basal turn [26,27,28,29,30]. Histopathological analysis of experimentally induced acoustic injury in a fetal lamb (exposure to broadband noise with 130 dB peak sound pressure or Lpeak [highest peak levels in decibels] for 16 consecutive h) revealed hair cell injury [31]; however, there was more severe involvement of the apical and mid turns of the cochlea (which have a lower association with clinical hearing impairment in humans) compared with the basal turn [31].
During fetal MRI, the sound from the scanner is attenuated by the maternal soft tissues and by amniotic fluid, resulting in a substantial decrease in the intensity of the sound that reaches the fetus. Even though there is no data regarding attenuation of sound by the human gravid abdomen, a study using pregnant ewes showed a decrease of nearly 40 dB as a result of amniotic fluid and maternal soft-tissue attenuation [32]. Another study performed in human volunteers who weren’t pregnant reported a decrease in approximately 30 dB on measurements obtained with a microphone placed in the volunteer’s fluid-filled stomach relative to those obtained at the surface [33]. We believe that similar acoustic attenuation may occur during the second and third trimesters of pregnancy, largely shielding the fetus from noise.
Additional mechanisms that contribute to lowering the noise exposure to the fetus include the preferential attenuation of higher frequency sounds and the duration of the exam. The acoustic noise associated with MRI scanning results from motion of the gradient coils related to the application of an activating electric current [34, 35]. The reported frequency of the sound produced by the gradients at 3 T is above 1 kHz [10, 36, 37]. Direct measurements of attenuation of high frequency sounds (>1 kHz) by the gravid abdomen in sheep reported a reduction of approximately 45 dB, as compared to a 15-dB attenuation for a sound with a frequency of 0.25 kHz [38]. In addition, during prenatal life, conduction of sounds occurs preferentially through the temporal bone rather than the middle ear ossicles, which provides additional attenuation for high-frequency sounds [39]. Another important consideration is related to the duration of the exposure. Most of the data on experimentally induced hearing damage in animal models used very long exposure times (16 h) [31]. Given that the extent of cochlear hair cell loss has been linked to the duration of the exposure and considering that clinical fetal MRI takes an average 30–45 min [27, 28], the potential deleterious effects reported by these experiments are not directly translatable to clinical fetal MRI or would be markedly diminished [31].
Another potential mechanism for noise-induced congenital hearing loss is impairment of development of the hearing apparatus, which is generally considered unlikely given the early gestational age at which this occurs. For instance, the cochlea is formed by the 8th to 10th post-conceptional week and reaches adult size by the 17th to 19th week of post-conceptional age [40, 41]. Similarly, the cartilaginous anlage of the ossicles morphologically resembles the adult structures by the end of the 8th post-conceptional week and ossification progresses rapidly between the 19th and 24th weeks [42, 43]. Thus, it is unlikely that MRI performed in the late 2nd or 3rd trimester will adversely impact the development of the hearing apparatus.
For our assessment of newborn hearing, we adhered to the guidelines of the American Speech-Language-Hearing Association and used a combined battery of transient otoacustic emission tests and auditory brainstem response tests, given the inherently elevated risk of hearing impairment in our population [44]. Even though performance characteristics may vary with the population analyzed, data from Wolff et al. [45], estimated the sensitivity and specificity of a combined approach to be 91.7% and 98.5%, respectively. In addition, some reports suggest that auditory brainstem response testing, utilizing the hearing level of 30 dB that was used in our project, can have sensitivity and specificity as high as 100% and 91%, respectively, relative to the gold standard of behaviorally confirmed hearing status at 4 years of age [46]. Consequently, we feel that our approach would be able to reliably identify hearing loss, despite the lack of longitudinal follow-up.
The lower fail rate of the auditory brainstem response test relative to the transient otoacoustic emissions test is expected given the known higher rate of false-positive studies (lower specificity) that result from transient otoacoustic emissions test -based screening [47]. These false-positive results in transient otoacoustic emissions tests are attributed to debris in the external auditory canal, fluid in the middle ear and ambient noise [48]. The fail rate of the transient otoacoustic emissions test in our population (8.1%) was only slightly higher than the reported fail rate for a single-time otoacoustic emissions assessment (approximately 7%) in healthy newborns, and was similar to the fail rate observed in our subset analyses that excluded children born preterm and those requiring NICU admission (6.1%) [16, 17]. Importantly, 8 of the 10 patients who failed the transient otoacoustic emissions test passed the auditory brainstem response test, suggesting that most of these subjects had normal hearing even though clinical follow-up was not available in the electronic medical record for any of them. While the fail rate of the auditory brainstem response test in our study (2.4%) is above the reported fail rate of the auditory brainstem response test in healthy infants (<1%), it is similar to that observed by other authors in high-risk populations, including infants with congenital diaphragmatic hernia (2–4%) [18,19,20]. It should also be noted none of the subjects who was born at term and did not require NICU admission failed the auditory brainstem response test screen. These findings support our hypothesis that the increased rate of hearing test failure is probably related to the underlying comorbidities and associated risk factors in our population. Although our experimental design cannot exclude an increase in the fail rate as a consequence of fetal MRI (independent of field strength), we believe prior studies have substantiated the acoustic safety of fetal MRI at 1.5 T [14, 15].
The main limitations of this study are its small sample size, its retrospective nature and its bias in selection. We were only able to include a small number of patients in our study due to the fact 3-T fetal MRI is not yet as widespread as 1.5-T fetal MRI. Also included in the bias in selection is the relative difference in gestational age between the control and subject groups. This difference owes to the fact that when we started scanning at 3 T at our institution, we did so in a more mature fetal population (25 weeks and older) to insure that organogenesis was well underway and that the mineralization of the ossicles, which occurs between 19 and 24 weeks, was largely complete. The retrospective nature of the study also affects our ability to confirm definitive hearing impairment, given that the false-negative rate of universal neonatal hearing screening can be as high as 23% in cases of mild sensorineural hearing loss [49]. When a newborn fails the universal neonatal hearing screening, this prompts a referral to a definitive diagnostic audiological evaluation; these data are not available for any of our patients. However, the tests we used to assess hearing in our population are identical to neonatal screening protocols utilized in prior studies looking at postnatal hearing outcomes in subjects who underwent fetal MRI [14, 15]. Finally, all the subjects included in our study were referred for known or suspected congenital anomalies and many of them were born preterm and/or required NICU admissions. While these two variables constitute confounding factors when assessing hearing impairment, we believe that by choosing a control population with a similar level of complexity, conducting multivariate analysis and performing subset analyses excluding subjects with these comorbidities we have mitigated these effects. Further studies with larger sample size and with prospective enrollment of healthy subjects are needed to definitely determine the potential impact of MRI-related noise on fetal hearing.
Conclusion
We did not observe increased prevalence of clinically significant hearing loss in subjects who underwent fetal MRI at 3 T relative to those who had MRI at 1.5 T.
References
Munim S, Nadeem S, Khuwaja NA (2006) The accuracy of ultrasound in the diagnosis of congenital abnormalities. J Pak Med Assoc 56:16–18
Rankin J, Pattenden S, Abramsky L et al (2005) Prevalence of congenital anomalies in five British regions, 1991-99. Arch Dis Child Fetal Neonatal Ed 90:F374–F379
Zimmer EZ, Avraham Z, Sujoy P et al (1997) The influence of prenatal ultrasound on the prevalence of congenital anomalies at birth. Prenat Diagn 17:623–628
American Institute of Ultrasound in Medicine (2013) AIUM practice guideline for the performance of obstetric ultrasound examinations. J Ultrasound Med 32:1083–1101
Patek KJ, Kline-Fath BM, Hopkin RJ et al (2012) Posterior fossa anomalies diagnosed with fetal MRI: associated anomalies and neurodevelopmental outcomes. Prenat Diagn 32:75–82
Bebbington M, Victoria T, Danzer E et al (2014) Comparison of ultrasound and magnetic resonance imaging parameters in predicting survival in isolated left-sided congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 43:670–674
Li Y, Sansgiri RK, Estroff JA et al (2011) Outcome of fetuses with cerebral ventriculomegaly and septum pellucidum leaflet abnormalities. AJR Am J Roentgenol 196:W83–W92
Griffiths PD, Bradburn M, Campbell MJ et al (2016) Use of MRI in the diagnosis of fetal brain abnormalities in utero (MERIDIAN): a multicentre, prospective cohort study. Lancet 389:538–546
Victoria T, Jaramillo D, Roberts TP et al (2014) Fetal magnetic resonance imaging: jumping from 1.5 to 3 tesla (preliminary experience). Pediatr Radiol 44:376–386
Victoria T, Johnson AM, Edgar JC et al (2016) Comparison between 1.5-T and 3-T MRI for fetal imaging: is there an advantage to imaging with a higher field strength? AJR Am J Roentgenol 206:195–201
Priego G, Barrowman NJ, Hurteau-Miller J, Miller E (2017) Does 3T fetal MRI improve image resolution of nbrain structures between 20 and 24 weeks' gestational age? AJNR Am J Neuroradiol 38:1636–1642
Bulas D, Egloff A (2013) Benefits and risks of MRI in pregnancy. Semin Perinatol 37:301–304
Tocchio S, Kline-Fath B, Kanal E et al (2015) MRI evaluation and safety in the developing brain. Semin Perinatol 39:73–104
Reeves MJ, Brandreth M, Whitby EH et al (2010) Neonatal cochlear function: measurement after exposure to acoustic noise during in utero MR imaging. Radiology 257:802–809
Strizek B, Jani JC, Mucyo E et al (2015) Safety of MR imaging at 1.5 T in fetuses: a retrospective case-control study of birth weights and the effects of acoustic noise. Radiology 275:530–537
Yousefi J, Ajalloueyan M, Amirsalari S, Hassanali Fard M (2013) The specificity and sensitivity of transient otoacustic emission in neonatal hearing screening compared with diagnostic test of auditory brain stem response in Tehran hospitals. Iran J Pediatr 23:199–204
Maxon AB, White KR, Behrens TR, Vohr BR (1995) Referral rates and cost efficiency in a universal newborn hearing screening program using transient evoked otoacoustic emissions. J Am Acad Audiol 6:271–277
Hille ET, van Straaten HI, Verkerk PH, Dutch NICU Neonatal Hearing Screening Working Group (2007) Prevalence and independent risk factors for hearing loss in NICU infants. Acta Paediatr 96:1155–1158
van Straaten HL, Hille ET, Kok JH et al (2003) Implementation of a nation-wide automated auditory brainstem response hearing screening programme in neonatal intensive care units. Acta Paediatr 92:332–338
Partridge EA, Bridge C, Donaher JG et al (2014) Incidence and factors associated with sensorineural and conductive hearing loss among survivors of congenital diaphragmatic hernia. J Pediatr Surg 49:890–894
Pennsylvania Department of Health (2013) Newborn hearing screening program guidelines. Pennsylvania Department of Health, http://www.paearlyhearing.org/images/attachments/PA_Newborn_Hearing_Screening_Guidelines_-_March_2013.pdf. Accessed 28 Feb 2018
American Speech-Language-Hearing Association. (2013). Expert panel recommendations on newborn hearing screening. Available from: www.asha.org
American Academy of Pediatrics, Joint Committee on Infant Hearing (2007) Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics 120:898–921
Victoria T, Bebbington MW, Danzer E et al (2012) Use of magnetic resonance imaging in prenatal prognosis of the fetus with isolated left congenital diaphragmatic hernia. Prenat Diagn 32:715–723
Rocha EB, Frasson de Azevedo M, Ximenes Filho JA (2007) Study of the hearing in children born from pregnant women exposed to occupational noise: assessment by distortion product otoacoustic emissions. Braz J Otorhinolaryngol 73:359–369
Le TN, Straatman LV, Lea J, Westerberg B (2017) Current insights in noise-induced hearing loss: a literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J Otolaryngol Head Neck Surg 46:41
Chen GD, Fechter LD (2003) The relationship between noise-induced hearing loss and hair cell loss in rats. Hear Res 177:81–90
Harding GW, Bohne BA (2009) Relation of focal hair-cell lesions to noise-exposure parameters from a 4- or a 0.5-kHz octave band of noise. Hear Res 254:54–63
Hawkins JE Jr, Johnsson LG, Stebbins WC et al (1976) Hearing loss and cochlear pathology in monkeys after noise exposure. Acta Otolaryngol 81:337–343
Clark WW, Bohne BA (1978) Animal model for the 4-kHz tonal dip. Ann Otol Rhinol Laryngol Suppl 87:1–16
Gerhardt KJ, Pierson LL, Huang X et al (1999) Effects of intense noise exposure on fetal sheep auditory brain stem response and inner ear histology. Ear Hear 20:21–32
Gerhardt KJ, Abrams RM, Kovaz BM et al (1988) Intrauterine noise levels produced in pregnant ewes by sound applied to the abdomen. Am J Obstet Gynecol 159:228–232
Glover P, Hykin J, Gowland P et al (1995) An assessment of the intrauterine sound intensity level during obstetric echo-planar magnetic resonance imaging. Br J Radiol 68:1090–1094
Shellock FG, Ziarati M, Atkinson D, Chen DY (1998) Determination of gradient magnetic field-induced acoustic noise associated with the use of echo planar and three-dimensional, fast spin echo techniques. J Magn Reson Imaging 8:1154–1157
Hattori Y, Fukatsu H, Ishigaki T (2007) Measurement and evaluation of the acoustic noise of a 3 tesla MR scanner. Nagoya J Med Sci 69:23–28
Wessinger CM, Buonocore MH, Kussmaul CL, Mangun GR (1997) Tonotopy in human auditory cortex examined with functional magnetic resonance imaging. Hum Brain Mapp 5:18–25
Ravicz ME, Melcher JR, Kiang NY (2000) Acoustic noise during functional magnetic resonance imaging. J Acoust Soc Am 108:1683–1696
Gerhardt KJ, Otto R, Abrams RM et al (1992) Cochlear microphonics recorded from fetal and newborn sheep. Am J Otolaryngol 13:226–233
Gerhardt KJ, Huang X, Arrington KE et al (1996) Fetal sheep in utero hear through bone conduction. Am J Otolaryngol 17:374–379
Jackler RK, Luxford WM, House WF (1987) Congenital malformations of the inner ear: a classification based on embryogenesis. Laryngoscope 97:2–14
Jeffery N, Spoor F (2004) Prenatal growth and development of the modern human labyrinth. J Anat 204:71–92
Hanson JR, Anson BJ, Bast TH (1959) The early embryology of the auditory ossicles in man. Q Bull Northwest Univ Med Sch 33:358–379
Richard C, Courbon G, Laroche N et al (2017) Inner ear ossification and mineralization kinetics in human embryonic development - microtomographic and histomorphological study. Sci Rep 7:4825
United States Department of Labor (2018) Occupational noise exposure. In: Administration OSHA (ed). https://www.osha.gov/SLTC/noisehearingconservation/standards.html. Accessed 28 Feb 2018
Wolff R, Hommerich J, Riemsma R et al (2010) Hearing screening in newborns: systematic review of accuracy, effectiveness, and effects of interventions after screening. Arch Dis Child 95:130–135
Hyde ML, Riko K, Malizia K (1990) Audiometric accuracy of the click ABR in infants at risk for hearing loss. J Am Acad Audiol 1:59–66
Wroblewska-Seniuk KE, Dabrowski P, Szyfter W, Mazela J (2017) Universal newborn hearing screening: methods and results, obstacles, and benefits. Pediatr Res 81:415–422
Akinpelu OV, Peleva E, Funnell WR, Daniel SJ (2014) Response to the letter to the editor regarding “Otoacoustic emissions in newborn hearing screening: a systematic review of the effects of different protocols on test outcomes”. Int J Pediatr Otorhinolaryngol 78:2022–2023
Johnson JL, White KR, Widen JE et al (2005) A multicenter evaluation of how many infants with permanent hearing loss pass a two-stage otoacoustic emissions/automated auditory brainstem response newborn hearing screening protocol. Pediatrics 116:663–672
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Jaimes, C., Delgado, J., Cunnane, M.B. et al. Does 3-T fetal MRI induce adverse acoustic effects in the neonate? A preliminary study comparing postnatal auditory test performance of fetuses scanned at 1.5 and 3 T. Pediatr Radiol 49, 37–45 (2019). https://doi.org/10.1007/s00247-018-4261-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-018-4261-2