Abstract
Objective
To investigate the performance of contrast-enhanced MRI for predicting avascular necrosis (AVN) of the treated femoral head after surgical reduction for developmental dysplasia of the hip (DDH) using qualitative and quantitative methods.
Methods and materials
This IRB-approved, HIPAA compliant retrospective study included 47 children who underwent same-day contrast-enhanced MRI following unilateral surgical hip reduction between April 2009 and June 2018. Blinded to the clinical outcome, 3 reviewers (2 pediatric radiologists and 1 pediatric orthopedist) independently categorized the enhancement pattern of the treated femoral head. Signal intensities, measured using regions of interest (ROI), were compared between treated and untreated hips and percent enhancements were compared between hips that developed and did not develop AVN. Post-reduction radiographs were evaluated using Salter’s criteria for AVN and Kalmachi and MacEwen’s classification for growth disturbance. Non-parametric tests and Fisher exact test were used to compare enhancement values between AVN and non-AVN hips. Bonferroni correction was used for multiple comparisons.
Results
Ten (21%) out of the 47 children (7 boys and 40 girls; mean age 9.0 ± 4.7 months) developed AVN. Age at surgical reduction was significantly higher (p = 0.03) for hips that developed AVN. No significant differences were found in gender (p = 0.61), laterality (p = 0.46), surgical approach (p = 0.08), history of pre-operative bracing (p = 0.72), abduction angle (p = 0.18–0.44), enhancement pattern (p = 0.66–0.76), or percent enhancement (p = 0.41–0.88) between AVN and non-AVN groups.
Conclusion
Neither enhancement pattern nor percent enhancement predicted AVN, suggesting that post-reduction conventional MRI does not accurately distinguish between reversible and permanent vascular injury.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Developmental dysplasia of the hip (DDH) is the leading cause of hip disease in infants with an estimated incidence ranging between 1.5 and 20 per 1000 newborns [1,2,3]. Early detection and intervention are advocated by the American College of Radiology, the American Academy of Pediatrics, and the American Academy of Orthopedic Surgeons [4,5,6,7] because unrecognized and untreated DDH can produce pain and disability and lead to premature osteoarthritis [8]. While Pavlik harness or abduction brace treatment is generally successful for younger infants, surgical hip reduction followed by rigid spica cast immobilization is necessary for older children and those who have failed conservative treatment [9, 10]. The goals of treatment are to improve hip joint congruity in order to facilitate growth and remodeling of the developing hip while minimizing the most concerning complication of treatment, namely iatrogenic avascular necrosis (AVN) and permanent vascular injury [11].
The precise pathophysiology behind the development of AVN remains unclear and the published rates vary between 4 and 47% [12,13,14,15,16,17,18]. This is because the unequivocal diagnosis of AVN is often delayed for months to years due to the abundance of unossified epiphyseal cartilage of the immature femoral head and the ossific growth that is necessary for growth deformity to become apparent on follow-up radiographs [19]. The increasing use of same-day post-reduction MRI examinations to confirm restored anatomic alignment without use of radiation has led some authors to suggest the use of enhancement images to detect abnormal tissue perfusion and the use of abnormal enhancement patterns as surrogate markers for predicting future development of AVN [16, 20,21,22]. However, the existing published literature used sample sizes that contained only a small number of AVN-positive cases that were diagnosed using predominantly short-term post-reduction radiographs [16, 20, 22]. Using a piglet model, Jaramillo and colleagues demonstrated correlation between abduction-induced ischemia on MRI and the absence of regional red blood cells on histopathology, but the relative short duration of the study period did not allow for the development of osteonecrosis [23]. Therefore, it is uncertain whether enhancement on MRI reliably distinguishes between ischemia (that is reversible) and AVN (that is permanent) as the latter often leads to growth deformity and impacts long-term prognosis and clinical outcome. Therefore, the purpose of this study was to investigate the performance of contrast-enhanced MRI for predicting the development of AVN of the treated femoral head following surgical reduction for DDH using qualitative and quantitative methods.
Materials and methods
Study group
This study was performed in compliance with Health Insurance Portability and Accountability Act (HIPAA) regulations with the approval from our Institutional Review Board (IRB) at the Children’s Hospital of Philadelphia and with a waiver of written informed consent. MRI examinations were identified through two methods, a retrospective review of the imaging database (Nuance®; Montage Healthcare Solutions; Eden Prairie, MN) using the search term “spica” and a surgical database query using the Current Procedural Terminology (CPT) codes for closed and open reduction for DDH (27257, 27258, 27259). In total, the search yielded 108 patients, who both underwent surgical reduction and same-day contrast-enhanced MRI examinations at our institution between April 1, 2009, and June 30, 2018. Patients who underwent surgical reduction for both hips and those with neuromuscular or teratological dislocations were excluded. Patients with a history of prior hip surgery, incomplete or motion-degraded non-diagnostic MRI examinations, incomplete medical records, or most recent post-reduction radiographs that were less than 11 months after surgical reduction were also excluded, which produced the final study group of 47 children.
Electronic medical records were reviewed to collect demographic information, surgical approach, and clinical outcome. The diagnosis of DDH was determined according to established guidelines [4,5,6,7, 11]. Surgical reduction is the standard of treatment at our institution for patients with DDH who have failed conservative treatment with harnesses/braces or who were diagnosed late (> 6 months of age). In all cases, closed reduction is attempted first. If a concentric reduction cannot be achieved by closed means, then an open reduction is performed. In either case, an adductor tenotomy is almost always performed, and infants are immobilized postoperatively in a one-and-a-half or a two-legged spica cast. Abduction angle within the spica cast was recorded using two methods: the traditional method [22, 23] and a new adjusted method using the recently proposed trigonometric model [24]. Spica cast changes are maintained for 6–12 weeks depending on the surgical approach. The patients are then transitioned into a nighttime abduction orthosis until approximately 2 years of age [16].
MRI examination
At our institution, all same-day contrast-enhanced pelvic MRI examinations were performed within 6 h after surgical hip reduction and preferentially on a 3.0-Telsa magnet (Magnetom Prisma; Siemens Healthineers, Munich, Germany; n = 40) over a 1.5-Telsa magnet (Avanto; Siemens Healthineers, Munich, Germany, n = 7). All subjects were imaged supine and within the spica cast using a multi-channel phased-array posterior torso coil (Body 18; Siemens Healthineers, Munich, Germany) that is centered over the hips.
The MRI protocol included T1-weighed and T2-weighted turbo spin echo pulse sequences, performed in axial and coronal planes. Imaging parameters were as follows: field-of-view of 16–20 cm, matrix of 256 × 256, slice thickness of 3–4 mm and without an interslice skip. Repetition time and echo time were 550–750 msec and 8–12 msec for T1-weighed images and 3500–5000 msec and 60-75 msec for T2-weighted images, respectively. Contrast agent gadobenate dimeglumine (MultiHance; Bracco Diagnostics, Monroe Township, NJ) was given at a dose of 0.1 mmol/kg. If needed, the injection volume was diluted with normal saline to a minimum volume of 3 mL and injected at a rate of approximately 0.5 mL/s. Post-contrast images were acquired following the complete injection of the contrast agent. Due to the relatively delayed enhancement of the unossified epiphyseal cartilage of the immature femoral head that occurs over 5–10 min [20, 25], a minimum of 3 post-contrast series were acquired, typically with two series performed in the axial plane and one series performed in the coronal plane.
Qualitative assessment of enhancement pattern on MRI
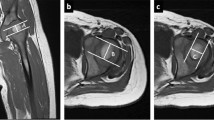
MRI enhancement patterns were assessed by three reviewers, who were blinded to the clinical history and patient outcome. Two board-certified pediatric radiologists, one with 7 years of clinical experience and one with 6 years of clinical experience and with additional fellowship-training in musculoskeletal radiology and one board-certified pediatric orthopedic surgeon with 10 years of clinical experience, independently reviewed the selected images from all patients. Due to the small size of the femoral heads, best visualization was often on only a single image with adjacent consecutive images containing volume-averaging effects. To ensure direct comparison between qualitative assessment and quantitative measurements, the pediatric musculoskeletal radiologist selected the most representative images for each hip (treated and untreated sides), which included the pre-contrast and post-contrast T1-weighted images. Two sets of post-contrast images were selected, one from the first series (early enhancement) and the other from the last series (late enhancement) (Fig. 1). The enhancement pattern of the treated femoral head was classified into normal, globally decreased, focally decreased, or near-absent enhancement [20, 22]. Normal enhancement was defined as relatively symmetric enhancement between treated and untreated femoral heads. Globally decreased enhancement was defined as diffusely decreased enhancement of the treated femoral head. Focally decreased enhancement was defined as heterogeneous enhancement with areas of focally decreased enhancement of the treated femoral head. Near-absent enhancement was defined as essentially no discernible enhancement of the treated femoral head (Fig. 2). If there were disagreements among the independent reviews, then the majority consensus was accepted as the final interpretation. All pre-contrast images were reviewed by a pediatric musculoskeletal radiologist to identify any signal abnormalities in the regional cartilage and bone marrow.
Time-dependent progressive enhancement of the dysplastic left femoral head in a 7-month-old girl. a Axial T1-weighted fat-suppressed pre-contrast, b first post-contrast (early enhancement), and c last post-contrast (late enhancement) images demonstrate progressive enhancement within the femoral head from near-absent (b block arrow) to focal enhancement (c chevron). Note the enhancing greater trochanter (triangles) and partial contrast washout within the metaphysis (circles). An anterior soft tissue hematoma (stars) is present
MRI enhancement patterns of the treated femoral head. Axial contrast-enhanced T1-weighted fat-suppressed images from 4 different patients demonstrate normal enhancement (a dashed arrow) in a 10-month-old girl, globally decreased enhancement (b arrow) in a 15-month-old girl, focally decreased enhancement (c arrowhead) in a 6-month-old girl, and near-absent enhancement (d block arrow) in an 8-month-old girl
Quantitative measurement of enhancement on MRI
Using the institution’s Picture Archiving and Communication System (PACS; Philips IntelliSpace, Pleasanton, CA, USA), regions of interest (ROI) was placed on both treated and untreated sides over the femoral heads, greater trochanters, and proximal femoral metaphyses using the pre-contrast, early enhancement, and late enhancement images. The ROI was selected to maximally include the area of interest while avoiding volume-averaging effects and keeping the size of the sampled regions constant between pre-contrast (PC) and contrast-enhanced (CE) images and similar between treated and untreated sides. The percent enhancement was calculated using the previously published formula [23]: Enhancement (%) = (CE − PC) / PC × 100. The signal intensities were compared between treated and untreated sides and the calculated percent enhancements were compared between AVN and non-AVN groups.
Reference standard for AVN
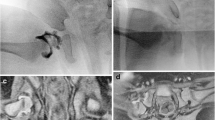
In consensus, two reviewers, a pediatric musculoskeletal radiologist and a pediatric orthopedic surgeon, retrospectively assessed all available post-reduction pelvic radiographs. In patients who only had the 12-month radiograph (defined as the post-reduction radiograph obtained at or around 12 months) AVN was diagnosed using the Salter’s criteria [26]. In patients who had longer radiographic follow-up, which is defined as the latest post-reduction radiograph after the 12-month radiograph, AVN was diagnosed using a combination of Salter’s criteria and the Kalmachi and MacEwen’s Classification [19] (Fig. 3).
Radiographic findings of avascular necrosis (AVN) and remodeling in a boy who underwent right hip surgical reduction at 4 months of age. At 14 months after surgical reduction, the right ossific nucleus (a arrow) appeared small, irregular, and sclerotic (Salter’s classification for AVN, grade 4). At 32 months after surgical reduction, there was only mild residual flattening (b dashed arrow) of the right ossific nucleus with relatively preserved morphology and alignment of the femoral neck (Kalmachi and MacEwen’s criteria for growth disturbance, grade 1)
Statistical analysis
All data analysis was performed with SPSS Statistics (v.23.0, IBM, Chicago, Illinois). Continuous variables were presented as means ± standard deviation (SD). Categorical variables were presented as percentages and counts. A Fisher exact test was used to evaluate differences for categorical demographic variables and Mann-Whitney U test for the continuous variables between patients who developed and did not develop AVN.
Chi-square was used to assess the MRI enhancement patterns between AVN and non-AVN groups. This analysis was performed initially using all four enhancement patterns described in Table 1. A second analysis was performed following the methodology described by Tiderius and colleagues [20] as follows: all MRI examinations that received normal, diffusely decreased, and focally decreased enhancement patterns were grouped together and compared against those that received a near-absent enhancement pattern using a Fisher exact test. Fleiss’ kappa statistic was used to assess inter-observer agreement between the 3 reviewers on the determination of enhancement patterns. Agreement was categorized as follows: less than 0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; and greater than 0.81, almost perfect agreement [27].
Wilcoxon signed-rank test was used to compare signal intensities between treated and untreated hips. Mann-Whitney U test was used to evaluate the difference in the enhancement percentages between hips with and without AVN. Bonferroni correction was implemented if a significant p value was found in multiple comparison. A p value < 0.05 was considered significant.
Results
Study group
The study group consisted of 47 MRI examinations from 47 children (7 boys and 40 girls; mean age at reduction, 9.0 ± 4.7 months; range, 3–23 months). All children had 12-month radiographs (14.5 months + 3.5 from surgical reduction; range 11–24 months) and 36 (75%) children had longer radiographic follow-up (48.1 months + 24.0, range 17–110 months). Ten (21%) hips (9 girls and 1 boy) developed AVN, which consisted of 3 hips with abnormal 12-month radiographs, 2 hips with abnormal later radiographs, and 5 hips with abnormal 12-month and later radiographs. As shown in Table 1, there was significant association between older age at surgical reduction (p = 0.03) and the development of AVN. However, no significant differences were found in gender (p = 0.61), laterality (p = 0.46), surgical approach (p = 0.08), history of prior harness treatment (p = 0.72), and abduction angle in spica cast (traditional method, p = 0.44; adjusted method, p = 0.18) between AVN and non-AVN groups.
Qualitative assessment of enhancement pattern
Table 2 summarizes the distribution of enhancement patterns using early and late enhancement images. No cartilage or marrow signal abnormality was identified on the pre-contrast images. Inter-observer agreement among the 3 reviewers was substantial (early enhancement, κ = 0.74; late enhancement, κ = 0.72). Between AVN and non-AVN hips, the distribution in the enhancement patterns was also not significantly different (early enhancement, p = 0.76; late enhancement, p = 0.66). Near-absent enhancement pattern was uncommon and only observed when early enhancement images were used. Furthermore, when normal, globally decreased, and focally decreased patterns were combined and compared to near-absent enhancement pattern, the difference remained non-significant (p = 0.40) between AVN and non-AVN hips.
Quantitative assessment of relative enhancement
No significant differences were found in the percent enhancement of the treated hips between those that developed and did not develop AVN (early enhancement, p range = 0.41–0.88; late enhancement, p range = 0.53–0.74) (Table 3). Furthermore, as shown in Table 4, there were no significant differences in the signal intensities between treated and untreated sides for both AVN (p range = 0.09–1.00) and non-AVN groups (p range = 0.54–1.00). The mean ROI sizes for the treated femoral head, greater trochanter, and metaphysis were 0.57 + 0.21 cm2, 0.28 + 0.08 cm2, and 0.72 + 0.24 cm2, respectively, and for the untreated side were 0.83 + 0.32 cm2, 0.30 + 0.09 cm2, and 0.77 + 0.26 cm2, respectively.
Discussion
We investigated the performance of same-day contrast-enhanced MRI to predict the future development of AVN in surgically reduced hips in children with DDH. Between AVN and non-AVN groups, we did not find a significant difference in the enhancement characteristics using qualitative or quantitative methods. While differences were observed between early and late enhancement images and between treated and untreated hips, these enhancement characteristics did not reach statistical significance. These results suggest that the perceived enhancement patterns and measured signal intensities of the surgically reduced hip are influenced by post-surgical changes in the regional hemodynamics (such as venous congestion) and imaging technique (timing of the post-contrast image acquisition) and, thus, is not a specific marker for predicting AVN.
Between AVN and non-AVN groups, we did not find a significant difference in the distribution of enhancement patterns. This finding is not in agreement with previously published smaller case series that found global or near-global decreased or absent enhancement to associate with the development of AVN [20, 21]; however, these previously published reports only provided limited information on the imaging parameters pertaining to the acquisition of the post-contrast images. We did notice that the enhancement pattern can change over time and the single hip that initially appeared as near-absent enhancement on early enhancement images improved on the late enhancement images. This observation is due to the unique vascular anatomy of the unossified cartilage where blood vessels run within vascular channels and nutrients (and contrast agent) must diffuse from the vascular lumen into the intracannalicular perivascular tissue and then into the surrounding cartilage, a process that can take up to 5–10 min [23, 25, 28]. This accounts for the slow uptake and washout of the enhancement signal intensity curve of unossified cartilage [23], making it the least enhancing structure in the region [25]. Therefore, in order to ensure adequate cartilage enhancement, our study evaluated both early and late enhancement images.
Our study used percent enhancement to quantify change after injection of the contrast agent. This method was previously proposed by Jaramillo and colleagues to quantify enhancement of the femoral head using a piglet model [23]. Applying this method to treated hips in children, we found that the enhancement of the unossified cartilage (femoral head and greater trochanter) increased slowly between early and late enhancement images, while the adjacent highly vascular metaphysis demonstrated brisk enhancement with early washout observed on the late enhancement images. These findings reflect the differences in the underlying vascular anatomy, which is relatively sparse within the cartilage and densely packed within the metaphysis [25, 28].
Between AVN and non-AVN groups, we did not find a significant difference in the percent enhancement for all three anatomic locations (femoral head, greater trochanter, and metaphysis) and on both early and late enhancement images. Furthermore, no significant differences were found between treated and untreated sides for both AVN and non-AVN groups. The slight (non-significant) differences in the signal intensities between treated and untreated sides likely reflect alterations in the microenvironment as the result of the surgery and changes in the regional hemodynamics. Since AVN is the end product of irreversible vascular injury that depends on both the severity of the hypoperfusion and the duration of the insult, the findings from our study support the hypothesis that the same-day (single time point) conventional MRI may not adequately capture the underlying complex and dynamic vascular anatomy or take into account the immediate post-instrumentation venous congestion [16, 20, 21, 23].
The etiology and pathogenesis that underlie the development of AVN remain unknown and thus, there is no consensus on the optimal timing for surgical correction. Some authors advocate for delaying the surgical reduction until the secondary ossification center has formed as the latter can increase tissue stiffness and allows the dysplastic femoral head to better withstand the increased compressive forces during and immediately after surgical reduction [19, 29,30,31]. However, the appearance of the secondary ossification center may be delayed for up to 17 months in a dysplastic hip [20, 32]. Since the potential for acetabular modeling progressively declines with time, other authors have favored earlier surgical reduction in order to avoid the need for future corrective acetabular surgery [20, 32,33,34]. Our study found a positive association between older age at surgical reduction and the development of AVN, which supports previously published reports that advocated for earlier surgical reduction [20, 35].
There were several limitations. One limitation is the retrospective nature of the study that prevented the standardization of image acquisition after contrast material injection and did not allow the collection of additional clinical information and images, such as quantitative T1Rho or T2 relaxation time maps. In particular, the timing of the contrast agent inject was not recorded, which prevented more precise correlation between enhancement characteristics and the timing of the post-contrast images. Additionally, since the milder cases of AVN may not be conspicuous until months to years later, it is possible that we may be under-diagnosing these patients with the cross-sectional design of our study; thus, future prospective studies are needed to validate our findings. Second is the relatively small study size with only a minority of the children who went on to develop AVN. The size limitation is due to the overall low incidence of DDH that ultimately required surgical reduction and our use of stringent criteria to exclude patients with incomplete medical records and those with concomitant bilateral hip reductions, with less than 11 months of follow-up, and without immediate post-operative contrast-enhanced MRI. However, our overall study size remains large when compared to the existing published reports [20,21,22]. Another limitation is the selection of the ROI, which is not a volumetric inclusion of the entire structure of interest. However, this method allows the comparison of relatively equal sized sample regions between treated and untreated sides, which are often slightly discrepant in size with the dysplastic hip smaller than the normal hip.
AVN of the proximal femur is one of the major uncommon complications of treatment for DDH and the resulting growth deformity of the proximal femur is an important determinant of clinical outcome and long-term prognosis. Although enhancement characteristics can reflect regional changes in the hemodynamics, our study did not find a significant difference between hips that developed and did not develop AVN using both qualitative and quantitative methods. Thus, same-day post-reduction contrast-enhanced MRI could not predict the development of avascular necrosis, suggesting that conventional imaging parameters may not reliably distinguish between reversible and permanent vascular injury of the treated femoral head. However, future prospective studies are necessary to validate the results from this study, particularly as it relates to the time-dependent changes in the perfusion of the recently reduced dysplastic femoral head.
References
Patel H. Preventive health care, 2001 update: screening and management of developmental dysplasia of the hip in newborns. Cmaj. 2001;164(12):1669–77.
Shipman SA, Helfand M, Moyer VA, Yawn BP. Screening for developmental dysplasia of the hip: a systematic literature review for the US Preventive Services Task Force. Pediatrics. 2006;117(3):e557–76.
Lee MC, Eberson CP. Growth and development of the child’s hip. Orthop Clin North Am. 2006;37(2):119–32 v.
American Academy of Orthopaedic Surgeons. Detection and nonoperative management of pediatric developmental dysplasia of the hip in infants up to six months of age. Evidence-based clinical practice guideline. Rosemont: American Academy of Orthopaedic Surgeons; 2014.
American College of Radiology. AIUM-ACR-SPR-SRU practice parameter for the performance of an ultrasound examination for detection and assessment of developmental dysplasia of the hip. J Ultrasound Med. 2018;37(11):E1–e5.
American Academy of Pediatrics. Clinical practice guideline: early detection of developmental dysplasia of the hip. Committee on Quality Improvement, Subcommittee on Developmental Dysplasia of the Hip. American Academy of Pediatrics. Pediatrics. 2000;105(4 Pt 1):896–905.
American College of Radiology. ACR appropriateness criteria - developmental dysplasia of the hip (DDH)–Child. 2019.
Wenger D, Duppe H, Tiderius CJ. Acetabular dysplasia at the age of 1 year in children with neonatal instability of the hip. Acta Orthop. 2013;84(5):483–8.
Sankar WN, Nduaguba A, Flynn JM. Ilfeld abduction orthosis is an effective second-line treatment after failure of Pavlik harness for infants with developmental dysplasia of the hip. J Bone Joint Surg Am. 2015;97(4):292–7.
Ucar DH, Isiklar ZU, Kandemir U, Tumer Y. Treatment of developmental dysplasia of the hip with Pavlik harness: prospective study in Graf type IIc or more severe hips. J Pediatr Orthop B. 2004;13(2):70–4.
Nguyen JC, Dorfman SR, Rigsby CK, Iyer RS, Alazraki AL, Anupindi SA, et al. ACR appropriateness criteria((R)) developmental dysplasia of the hip-child. J Am Coll Radiol. 2019;16(5s):S94–s103.
Haruno LS, Kan JH, Rivlin MJ, Rosenfeld SB, Schallert EK, Zhu H, et al. Spica MRI predictors for epiphyseal osteonecrosis after closed reduction treatment of dysplasia of the hip. J Pediatr Orthop B. 2019.
Sankar WN, Gornitzky AL, Clarke NMP, Herrera-Soto JA, Kelley SP, Matheney T, et al. Closed reduction for developmental dysplasia of the hip: early-term results from a prospective. Multicenter Cohort J Pediatr Orthop. 2019;39(3):111–8.
Novais EN, Hill MK, Carry PM, Heyn PC. Is age or surgical approach associated with osteonecrosis in patients with developmental dysplasia of the hip? A Meta-analysis. Clin Orthop Relat Res. 2016;474(5):1166–77.
Thomas IH, Dunin AJ, Cole WG, Menelaus MB. Avascular necrosis after open reduction for congenital dislocation of the hip: analysis of causative factors and natural history. J Pediatr Orthop. 1989;9(5):525–31.
Gornitzky AL, Georgiadis AG, Seeley MA, Horn BD, Sankar WN. Does perfusion MRI after closed reduction of developmental dysplasia of the hip reduce the incidence of avascular necrosis? Clin Orthop Relat Res. 2016;474(5):1153–65.
Novais EN, Kestel LA, Carry PM, Meyers ML. Higher Pavlik harness treatment failure is seen in Graf type IV Ortolani-positive hips in males. Clin Orthop Relat Res. 2016;474(8):1847–54.
Brougham DI, Broughton NS, Cole WG, Menelaus MB. Avascular necrosis following closed reduction of congenital dislocation of the hip. Review of influencing factors and long-term follow-up. J Bone Joint Surg British Vol. 1990;72(4):557–62.
Kalamchi A, MacEwen GD. Avascular necrosis following treatment of congenital dislocation of the hip. J Bone Joint Surg Am. 1980;62(6):876–88.
Tiderius C, Jaramillo D, Connolly S, Griffey M, Rodriguez DP, Kasser JR, et al. Post-closed reduction perfusion magnetic resonance imaging as a predictor of avascular necrosis in developmental hip dysplasia: a preliminary report. J Pediatr Orthop. 2009;29(1):14–20.
Haruno LS, Kan JH, Rivlin MJ, Rosenfeld SB, Schallert EK, Zhu H, et al. Spica MRI predictors for epiphyseal osteonecrosis after closed reduction treatment of dysplasia of the hip. J Pediatr Orthop B. 2019;28(5):424–9.
Jaramillo D, Villegas-Medina O, Laor T, Shapiro F, Millis MB. Gadolinium-enhanced MR imaging of pediatric patients after reduction of dysplastic hips: assessment of femoral head position, factors impeding reduction, and femoral head ischemia. AJR Am J Roentgenol. 1998;170(6):1633–7.
Jaramillo D, Villegas-Medina OL, Doty DK, Dwek JR, Ransil BJ, Mulkern RV, et al. Gadolinium-enhanced MR imaging demonstrates abduction-caused hip ischemia and its reversal in piglets. AJR Am J Roentgenol. 1996;166(4):879–87.
DeFrancesco CJ, Blumberg TJ, Chauvin NA, Sankar WN. An improved method for measuring hip abduction in spica after surgical reduction for developmental dysplasia of the hip. J Child Orthop. 2017;11(4):277–83.
Bedoya MA, Jaimes C, Khrichenko D, Delgado J, Dardzinski BJ, Jaramillo D. Dynamic gadolinium-enhanced MRI of the proximal femur: preliminary experience in healthy children. AJR Am J Roentgenol. 2014;203(4):W440–6.
Salter RB, Kostuik J, Dallas S. Avascular necrosis of the femoral head as a complication of treatment for congenital dislocation of the hip in young children: a clinical and experimental investigation. Can J Surg. 1969;12(1):44–61.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74.
Nguyen JC, Markhardt BK, Merrow AC, Dwek JR. Imaging of pediatric growth plate disturbances. Radiographics. 2017;37(6):1791–812.
Segal LS, Boal DK, Borthwick L, Clark MW, Localio AR, Schwentker EP. Avascular necrosis after treatment of DDH: the protective influence of the ossific nucleus. J Pediatr Orthop. 1999;19(2):177–84.
Roposch A, Odeh O, Doria AS, Wedge JH. The presence of an ossific nucleus does not protect against osteonecrosis after treatment of developmental dysplasia of the hip. Clin Orthop Relat Res. 2011;469(10):2838–45.
Roposch A, Stohr KK, Dobson M. The effect of the femoral head ossific nucleus in the treatment of developmental dysplasia of the hip. A meta-analysis. J Bone Joint Surg Am. 2009;91(4):911–8.
Luhmann SJ, Bassett GS, Gordon JE, Schootman M, Schoenecker PL. Reduction of a dislocation of the hip due to developmental dysplasia. Implications for the need for future surgery. J Bone Joint Surg Am. 2003;85(2):239–43.
Shin CH, Yoo WJ, Park MS, Kim JH, Choi IH, Cho TJ. Acetabular remodeling and role of osteotomy after closed reduction of developmental dysplasia of the hip. J Bone Joint Surg Am. 2016;98(11):952–7.
Luhmann SJ, Schoenecker PL, Anderson AM, Bassett GS. The prognostic importance of the ossific nucleus in the treatment of congenital dysplasia of the hip. J Bone Joint Surg Am. 1998;80(12):1719–27.
Xu M, Gao S, Sun J, Yang Y, Song Y, Han R, et al. Predictive values for the severity of avascular necrosis from the initial evaluation in closed reduction of developmental dysplasia of the hip. J Pediatr Orthop B. 2013;22(3):179–83.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Children’s Hospital of Philadelphia’s institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was not required for this retrospective study according to the Children’s Hospital of Philadelphia’s IRB.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nguyen, J.C., Back, S.J., Barrera, C.A. et al. Developmental dysplasia of the hip: can contrast-enhanced MRI predict the development of avascular necrosis following surgery?. Skeletal Radiol 50, 389–397 (2021). https://doi.org/10.1007/s00256-020-03572-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-020-03572-z