Abstract
Gadolinium has been used as a base for contrast agents in MRI for the last three decades. Numerous studies over the last 4 years have reported increased signal intensity in deep brain nuclei in non-contrast MRI images following gadolinium-based contrast agent (GBCA) administration. Pathology studies performed on adults and children, and rodent necropsy studies have also shown gadolinium deposition in brain and other tissues after GBCA administration. The purpose of this review was to summarize and discuss the knowledge gained from these reports and the relevance for imaging pediatric patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
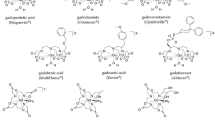
Gadolinium (Gd) is a highly paramagnetic lanthanide-series heavy metal used as a base for intravenous contrast agents in MRI. While the free gadolinium ion (Gd3+) itself is intrinsically toxic [1, 2], gadolinium-based contrast agents (GBCAs) contain organic ligands that bind Gd3+ to form stable molecular complexes that can be administered intravenously and then subsequently excreted from the body [3, 4]. The seven GBCAs on the market differ by the configuration of their chelating agents, which can be either linear or macrocyclic, and are further subdivided as ionic or nonionic [1, 3, 4] (Table 1). Macrocyclic GBCAs have higher structural stability than linear agents, and ionic agents tend to bind Gd3+ more tightly than nonionic agents [1, 4, 5].
The first GBCA to be approved by the United States Food and Drug Administration (FDA) in 1988 was Magnevist (gadopentetate dimeglumine; Bayer, Leverkusen, Germany), a linear ionic agent. Overall, GBCAs have been considered safe during the last three decades of their clinical use [2, 6]. In fact, during the first 15 years after their introduction, contrast-enhanced MRI was considered safer than CT for imaging children with impaired renal function because of a perceived risk of iodine contrast-induced nephropathy. However in 2006 an association between GBCAs and nephrogenic systemic fibrosis (NSF) was reported [7, 8].
Further research into the association of gadolinium exposure and development of NSF revealed that nearly all cases of NSF occurred following administration of linear GBCAs in patients with renal impairment. This led to a series of practice guidelines limiting use of GBCAs in patients with renal impairment [9], as well as a general shift toward use of macrocyclic agents [3]. Still, GBCAs continued to be used in patients with normal renal function without concern for adverse effects. Literature regarding gadolinium retention in the body was sparse and did not gain significant attention from the medical community until 4 years ago.
In 2014, Kanda et al. [10] reported a new observation of increased T1 signal intensity in the globi pallidi and the dentate nuclei on non-contrast brain MRI examinations of patients who had previously received multiple doses of GBCAs. In their discussion, the authors highlighted two older articles that reported on gadolinium deposition after GBCA administration: one described bone deposition in patients who had undergone contrast-enhanced MRI before hip replacement (specimens included resected femoral heads) [11], while the other described retention in mice (specimens included liver) [12]. Kanda’s report received wide attention from the medical community and led to a broad effort to further investigate the mechanism and clinical relevance of gadolinium retention in otherwise healthy patients. Our specific purpose in this article was to review the abundant recent literature on gadolinium retention and to summarize the knowledge that has been gained from these reports.
Studies reporting high T1 signal intensity in the brain
The breakthrough article by Kanda et al. [10] was the first study that demonstrated signal changes occurring in the brain parenchyma of patients who previously received GBCAs. This article led to extensive research on GBCAs over the last 4 years, evaluating different agents and their effect on T1 signal on unenhanced MR studies on various regions in the brain in a variety of patient cohorts, including adults and children, groups with normal versus impaired renal function, and groups with diseases such as multiple sclerosis that might affect the integrity of the blood–brain barrier. In this section we discuss reports on signal changes in the brain parenchyma of adults who received GBCAs (Table 2; [10, 13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]).
Prior intravenous (IV) administration of the linear ionic agent Magnevist and the linear nonionic agent Omniscan (gadodiamide) in patients with normal renal function was associated with increase in T1 signal intensity in the dentate nuclei and globi pallidi in two studies, with linear correlation between the administered dose and the signal intensity that persisted for several years following at least five administrations [10, 13]. A study by Zhang et al. [14] demonstrated that following multiple (39–59) administrations of both these GBCAs, there was increased signal in other regions of the brain, as well, including the posterior thalamus, substantia nigra, red nucleus, cerebellar peduncles and colliculi.
Other studies have evaluated the linear ionic agent MultiHance (gadobenate dimeglumine). One study by Weberling et al. [15] on 50 patients who received at least five administrations of MultiHance demonstrated increased signal in the dentate nuclei. They compared their results to published literature and found no significant difference between the dentate nuclei T1 signal intensity changes in comparison to changes observed after administrations of Magnevist, also a linear ionic agent. However there was a statistically significant higher dentate nucleus/pons signal intensity ratio associated with exposure to MultiHance as compared to Dotarem (gadoterate meglumine), a macrocyclic ionic agent. On the other hand, a study by Ramalho et al. [16] from about the same time on 69 patients, of whom 23 received the linear nonionic agent Omniscan and 46 received the linear ionic agent MultiHance (3–11 doses in each group), found that when the T1 signal intensity was compared between the last and first administrations there was a statistically significant increase in the globi pallidi and dentate nuclei signal intensity in the Omniscan group. They also reported a trend toward a significant increase in the dentate nuclei signal intensity but no increased signal intensity in the globi pallidi in the MultiHance group [16].
To the best of our knowledge, only one study, by Conte et al. [17], evaluated Eovist (gadoxetate disodium), a linear ionic liver-specific GBCA with up to 50% hepatobiliary excretion in the normal liver. This study analyzed a small sample of 18 patients with melanoma who received 2–18 doses of Eovist and had MR imaging of their brain and abdomen. The authors found a trend (P=0.09) toward increased globi pallidi signal intensity between the last and first administrations but no significant difference in the dentate nuclei signal intensities.
Several studies comparing T1 signal intensities after multiple administrations of the linear agents Magnevist and Omniscan to the macrocyclic agents Dotarem, Gadovist (gadobutrol) and ProHance (gadoteridol) demonstrated increased signal intensity in the globi pallidi and dentate nuclei following administration of the linear agents, but no increase in signal intensity was found following administration of macrocyclic agents [18,19,20,21]. In one of these studies, Bae et al. [21] evaluated 122 patients and demonstrated that even following multiple (between 15 and 65) administrations of either linear (Magnevist, Omniscan) or macrocyclic (Gadovist, Dotarem) agents, there was no increased signal intensity associated with the macrocyclic agents, but there was a significant increase in signal intensity in the globi pallidi and dentate nuclei following administration of linear agents.
Other studies evaluated T1 signals in patients who received only macrocyclic agents. Two studies, one by Radbruch et al. [22] on 30 patients and one by Langner et al. [23] on 217 patients, evaluated T1 signal intensities in brains of patients who received at least five doses of Gadovist, a macrocyclic nonionic agent, and found no increased parenchymal signal. A study by Kromrey et al. [24] evaluated 271 healthy volunteers 5 years after a single 1.5-fold dose (1.5 mmol/kg) administration of Gadovist and found no increased signal intensity in the globi pallidi or dentate nuclei. In one study by Lee at al. [25], who evaluated 385 patients who received 2–52 doses of Dotarem, the majority of patients showed no T1 signal change between the first and last scans; in 28 with mildly impaired renal function, with glomerular filtration rate (GFR) ranging 45–60 mL/min, there was increased signal intensity in the dentate nuclei but not in the globi pallidi. Nevertheless a study by Bjørnerud et al. [26] demonstrated increased T1 signal intensity in the dentate nuclei in 17 patients following multiple administrations (10–44) of Gadovist. However all of the patients in this study were diagnosed with a high-grade glioma, which might alter the blood–brain barrier by itself or secondary to radiation therapy.
Several studies were performed on patients with multiple sclerosis and found increased signal in the dentate nuclei and globi pallidi after multiple administrations of both linear and macrocyclic agents [27,28,29]. One study by Forslin et al. [27] found an association between increased T1 signal in the dentate nuclei and globi pallidi, and lower verbal fluency scores; this association remained significant after corrections for several aspects of multiple sclerosis disease severity.
In summary, there is clearly increased T1 signal intensity in the globi pallidi and dentate nuclei following multiple administrations of linear GBCAs; this finding is dose-dependent and is more significant with linear nonionic versus ionic agents, likely secondary to the higher overall kinetic stability of the latter in physiological conditions. Many studies demonstrated no increased T1 signal following multiple administrations of all three macrocyclic GBCAs that are on the market in subjects with normal renal function and intact blood–brain barrier. Studies that demonstrated increased T1 signal intensity in association with macrocyclic GBCAs were performed either on patients with disorders affecting the integrity of the blood–brain barrier (e.g., multiple sclerosis and high-grade gliomas) or on patients with renal function impairment. Nonetheless evaluation of signal intensities is probably not a sensitive method for detecting gadolinium deposits in tissues. Consequently tissue biopsy and autopsy studies were performed, as well, and are discussed in the following section.
Tissue biopsy and autopsy studies
In 2004 Gibby et al. [30] and in 2006 White et al. [11] reported deposition of gadolinium in femoral bone tissue excised during hip replacement surgeries in patients who underwent MRI examinations with IV GBCA several days prior to the surgery. They compared the linear nonionic agent Omniscan and the macrocyclic nonionic agent ProHance and found that gadolinium was retained in the bones with both agents, with 2.5- to 4-fold higher intraosseous concentrations associated with Omniscan as compared to ProHance. Another study, by Darrah et al. [31], that also evaluated femoral samples found retention of gadolinium 8 years following IV administration of either Omniscan or ProHance, with no significant difference in the concentration of gadolinium between the agents. They also found higher concentrations of GBCA in trabecular bone than in cortical bone, and lower concentrations in patients with osteoporosis or fractures in contrast to patients with osteoarthritis, and they concluded that gadolinium might be released from the bone in the context of demineralization. In 2010, a study by Xia et al. [32] demonstrated gadolinium deposits in biopsied brain tumors, with higher concentration in specimens from patients who received the linear nonionic agent Omniscan when compared to MultiHance, a linear ionic agent. The authors concluded that this deposition occurs secondary to loss of the integrity of the blood–brain barrier, and is lower with MultiHance because of its greater chemical stability. In 2011, a study by Christensen et al. [33] demonstrated gadolinium deposition in the skin biopsied from patients with NSF.
After the breakthrough article by Kanda et al. [10] in 2014, several published autopsy studies strengthened the recognition of gadolinium deposits in brain and other tissues in healthy subjects with normal renal function and intact blood–brain barrier. The first known autopsy study that followed Kanda’s article was by McDonald et al. [34]. They analyzed brain tissues from autopsies of 13 subjects with normal renal function who received 1–29 administrations of Omniscan, with a wide range of timing between the last contrast administration and death, ranging from several days to years [34]. They found gadolinium deposits in capillary endothelium and neural interstitium in a dose-dependent relationship that correlated with T1 signal intensities on pre-contrast MRI scans [34]. They concluded that “intravenous GBCA exposure is associated with neuronal tissue deposition in the setting of relatively normal renal function” [34]. Shortly after, another autopsy study by Kanda et al. [35] evaluated brain tissue of five subjects who had received the linear ionic agent Magnevist alone or in combination with other GBCAs, and found brain deposits with the highest concentrations in the globi pallidi and dentate nuclei. Another autopsy study by McDonald et al. [36] found deposits in brains of five patients with no known blood–brain barrier abnormalities after 4–18 doses of Omniscan, with higher concentrations in the globi pallidi as compared to the dentate nuclei. Tissue localization performed with transmission electron microscopy showed gadolinium deposits present in the endothelial wall and neuronal interstitium but also in neuronal nuclei [36]. However they found no evidence of gadolinium-mediated histological changes to suggest a toxic effect.
A study by Murrata et al. [37] evaluated brain, skin and bone tissues from autopsies in nine subjects who received either macrocyclic agents (ProHance and Gadovist) or linear ionic agents (MultiHance and Eovist). Interestingly, they found the highest concentrations in two patients who had received Gadovist (of which one died several days after the administration with multi-organ failure). They also found that bone concentrations were 23-fold higher than brain concentrations. Low concentrations of gadolinium were found in the skin in three of the autopsies from whom the skin was sampled. These patients had received either MultiHance or ProHance [37].
In summary, gadolinium deposits in the brain, bone, skin and liver and possibly other tissues as well. Brain, bone and skin depositions were demonstrated with both linear and macrocyclic agents. The concentrations in osseous tissues are significantly higher than those in the brain, which might be explained by the similarity of the gadolinium ion to ionic calcium [3]. Autopsy studies show that gadolinium retention in the brain occurs independent of renal function and blood–brain barrier integrity. The concentrations of retained gadolinium are cumulative, proportionally related to the administered doses, and small concentrations of gadolinium appear to persist for years.
Gadolinium-based contrast agents in pediatric patients
Like in adults, GBCAs are frequently employed in pediatric patients for characterizing and staging tumors, evaluating inflammatory and infectious processes, and assessing vascular structures, and GBCAs are used in approximately 40% of the total number of MRI examinations performed annually in children in the United States [38].
Despite being safely used in pediatric patients since their introduction in clinical practice in the late 1980s [39,40,41,42,43,44], GBCAs are often an off-label indication, although well supported by clinical standard of care [45, 46]. NSF has been reported in few pediatric patients, all 8 years or older, despite the known renal functional immaturity characteristic for children younger than 2 years [46, 47].
With the new safety concerns regarding gadolinium retention, multiple studies documenting signal changes in pediatric brain structures after the use of GBCAs have been published, following the trend and overall mirroring the findings described in adults (Table 3; [46, 48,49,50,51,52,53,54,55,56]).
Two initial case reports by Miller et al. [48] and Roberts and Holden [49] described increased T1 signal intensity on unenhanced MR images in the dentate nuclei and globi pallidi after 35 and 6 doses, respectively, of the linear ionic agent Magnevist. This same agent was further evaluated in several subsequent studies, documenting dose-dependent increases in T1 signal intensity in the dentate nuclei [46] and in the dentate nuclei and globi pallidi [50]. Statistically significant increase in dentate nucleus/pons signal intensity ratio after 12 or more doses of Magnevist as well as the linear nonionic agent Omniscan was described by Kasper et al. [51]. Interestingly, in one study, by Flood et al. [52], no significant T1 signal change was found in the globi pallidi despite the dose-dependent T1-weighted hyperintensity changes in the dentate nuclei documented in this series of patients after exposure to Magnevist.
Another linear ionic agent, MultiHance, however, did not show any statistically significant differences in mean signal intensity in the dentate nuclei, globi pallidi, pons or thalami after 5–15 doses of GBCA versus age- and weight-matched gadolinium-naïve controls [53]. Possible explanations for these results, which appear to contradict prior studies of linear ionic agents, include a different patient population with no neurological abnormalities, as well as the presence of the aromatic substituent component in the gadobenate dimeglumine (MultiHance) molecule, which might influence the elimination profile by increasing the kinetic inertia [5].
Serial injections of macrocyclic agents, both ionic and nonionic, were not found to cause MR signal changes in deep brain nuclei, or signal intensity ratios (dentate nucleus/pons, globus pallidus/thalamus) differences between patient cohorts and matched controls [54], and between first and last GBCA administration in several studies on pediatric patients [51, 55]. However a study by Rossi Espagnet et al. [56] found increased signal intensity ratios of globus pallidus/thalamus and dentate nucleus/pons after >6 serial administrations of the macrocyclic ionic agent Dotarem, with no visible T1 hyperintensity. Controversies following the publication of this study [57, 58] emphasized that in all the published studies, there was no clear explanation of the origin of the T1 hyperintensity present in brain structures, either visible or demonstrated by measurements of signal intensity changes. Soon after, a study by Tamrazi et al. [59] in 2017 showed that associated conditions also appear to play a role in signal changes in the deep brain nuclei. Brain radiation appeared to cause increased signal intensity in the dentate nuclei, independent of GBCA administration, at less than 10 doses of Magnevist, while primary brain tumors were associated with an increase of signal intensity in the globi pallidi [59].
In summary, several studies on pediatric patients showed T1 signal hyperintensity in deep brain nuclei after exposure to linear GBCAs, suggestive of gadolinium deposition. On the other hand, no significant differences in T1 signal intensities of these brain areas could be documented after administration of macrocyclic agents in three studies, with a single paper showing an increased signal intensity ratio without visible T1 hyperintensity. This favors the conclusion that macrocyclic GBCAs are less likely to deposit in the body and are therefore safer for use. As a consequence, most pediatric practices switched or were planning to switch to macrocyclic agents according to a survey of the pediatric providers in North America published in 2017 [60].
The first pathology studies in pediatric patients were published in 2016–2017, documenting gadolinium presence in brain and liver tissue samples. A study by Maximova at al. [61] evaluated liver tissue biopsied from 21 children following allogeneic bone marrow transplant; these children had 1–6 prior administrations of a macrocyclic agent, and 8 of them also received deferoxamine for chelation of iron overload. Liver gadolinium deposits were found in all children, with linear correlation between the concentration and the administered dosage [61]. Nevertheless there were significantly lower concentrations of gadolinium in the children who received the chelating agent deferoxamine [61].
In 2017, Roberts et al. [62] published a case report documenting a distribution map of gadolinium deposits after four linear GBCA doses, using laser ablation inductively coupled plasma mass spectroscopy, in a 17-year-old decedent. The highest levels of gadolinium were found in the dentate nuclei and cerebellar cortex. Interestingly, despite the heavy gadolinium load in the dentate nuclei, there was no corresponding T1 hyperintensity on unenhanced MRI [62]. This was followed by a study from McDonald et al. [38] that confirmed gadolinium brain deposition in three pediatric decedents with normal renal function who underwent multiple MR examinations with the linear nonionic agent Omniscan.
In summary, gadolinium deposition has been demonstrated pathologically in pediatric brain and liver tissue after both linear and macrocyclic GBCA exposure, replicating the findings in adults.
Rodent studies
Several rodent studies assessing gadolinium deposition have been performed since 2014 [63,64,65,66], although gadolinium dissociation in vivo in rats was first shown in 1992 by Wedeking et al. [67] without eliciting much attention from the medical community at that time.
In recent studies, multiple IV injections of linear or macrocyclic agents were administered to rodents over a period of several days to several weeks, followed by necropsies. In some cases, MRI brain examinations were performed prior to the necropsy. All studies that compared linear to macrocyclic agents found significantly higher concentrations of deposited gadolinium associated with linear GBCAs when compared to macrocyclic GBCAs.
An article by McDonald et al. [63] demonstrated that the concentration of gadolinium in all examined rat tissues was 2- to 4-fold higher with linear agents than with macrocyclic agents. Furthermore they found that concentrations in visceral organs, including the liver, spleen and kidneys, were 100 times higher than in the brain.
Two articles, one by Gianolio et al. [64] and the other by Frenzel et al. [65], found that the deposited gadolinium in the cases of linear agents consists largely of insoluble macromolecules, which are different chemically from the chelated ion form. The Frenzel group also reported that most of the deposited gadolinium in the rats injected with macrocyclic GBCAs consisted of low molecular weight soluble form, which is similar chemically to the injected GBCA [65].
A study by Kartamihardja et al. [66] reported that there was a significantly higher clearance rate of macrocyclic agents from various parts of the brain when compared to linear agents. They evaluated the concentration of gadolinium in brain tissues after 3 days from the final administration and found it to be 2- to 5-fold higher with linear than with macrocyclic agents. However when they evaluated a different group of rats that were sacrificed 45 days after the final administration of GBCA, they demonstrated this difference to be substantially higher — 15–75 times higher gadolinium concentrations were found in the brains of rats injected with linear agents than in those injected with macrocyclic agents [66]. Moreover there was significant clearance of macrocyclic agents in rats with renal failure but no significant clearance of linear agents [66].
A recent study by Bussi et al. [68] compared the three macrocyclic agents Dotarem, ProHance and Gadovist in rats that were sacrificed 28 days following multiple administrations of one of these GBCAs. The authors found significantly lower concentrations of gadolinium in rats injected with Dotarem when compared to the other two agents, in the cerebrum, cerebellum, femur and renal tissues.
In summary, gadolinium retention occurs in rats’ abdominal viscera at substantially (hundreds-fold) higher concentrations than in the brain. There is evidence of a chemical change of GBCAs’ molecules that occurs in vivo at a considerably higher rate with linear agents secondary to their relative chemical instability. This seems to prevent clearance of gadolinium from the body. At the same time, there is evidence that clearance of gadolinium from the body continues to occur over time with macrocyclic agents because of their chemical stability, which allows them to remain in their chelated form for longer periods. Furthermore there are differences between macrocyclic agents that are related to their chemical stability, with a higher clearance rate of Dotarem, which is ionic and hence more stable than ProHance and Gadovist.
Discussion
While numerous studies demonstrated no brain signal changes in association with macrocyclic GBCAs, tissue, human autopsy and rodent necropsy studies demonstrated that these agents do indeed deposit in brain and other tissues. The evidence suggests that all GBCAs are incompletely cleared from the body and that small amounts are retained in the brain, bones and other tissues of healthy subjects with normal renal function and intact blood–brain barrier. The concentrations of tissue gadolinium deposits are cumulative and are dependent on the chemical stability of the agent. Deposition rates are higher in patients with impaired renal function, which has also been demonstrated in experimental rodent studies. Brain deposition is probably more significant in patients with diseases affecting the integrity of the blood–brain barrier. However, even in healthy subjects gadolinium concentrations are significantly higher in tissues other than neuronal tissue, particularly the skeleton, which might become a reservoir of gadolinium from which it could be released when there is increased bone turnover [31]. This is not surprising because the free gadolinium ion bears chemical similarity to ionic calcium and other metals that might substitute for calcium and deposit in bone tissue [3, 69]. This raises the question whether gadolinium toxicity manifested with NSF or with other clinical presentations occurs in patients long after the GBCA administration. This is conceivable in certain clinical scenarios, leading to osteoporosis and increased bone turnover with subsequent release of gadolinium deposited in the bones [70], particularly in association with renal failure that would delay the clearance of this released gadolinium.
We are aware of no study to date that has delineated the exact chemical structure of gadolinium deposits in human or animal tissues. Specifically, it is not clear whether the deposited gadolinium is the highly toxic free gadolinium ion, it remains bound to its chelating molecule, or it is otherwise bound to competitor ligands. Current methods of gadolinium detection in tissue including inductively coupled plasma mass spectroscopy, while being highly sensitive, can only detect elemental gadolinium because the analyzed tissue is usually destroyed in the process [71]. As a result, most of the studies measured total concentration of gadolinium in tissue, without delineating the chemical form (i.e. chelated versus non-chelated). However, from rat studies [64, 65] we have learned that an in vivo chemical change occurs in the majority of deposited molecules of linear agents after a relatively short time. While this is indicative of de-chelation, it does not necessarily mean that free gadolinium ions are released. Nevertheless, this chemical change does not occur with macrocyclic agents in the short term. Because rodent studies have all been relatively short in duration, there is no current information with regard to the molecular structure of retained gadolinium in the long term. Of note, no definite histological evidence of cytotoxic effects of gadolinium deposits have been proved [63].
To the best of our knowledge, although the term “gadolinium deposition disease” was introduced [72], there are no scientifically proven clinical long-term adverse effects from GBCAs in subjects with normal renal function. A recent study by Robert J. McDonald, MD, presented at the 103rd Scientific Assembly and Annual Meeting of the Radiological Society of North America (RSNA 2017), did not identify any significant association between GBCA exposure and cognitive decline, dementia, diminished neuropsychological or motor performance [73]. Although there is not sufficient knowledge regarding the long-term effects, gadolinium deposition raises a potentially greater concern in childhood when tremendous brain development and rapid skeletal growth occur. In pediatric patients, we have witnessed a significant increase in contrast-enhanced MR imaging over the last decade in an attempt to avoid ionizing radiation, an effort that gained momentum around the year 2007 when the “Image Gently” campaign was launched [74, 75]. Long-term, large cohort studies would be required to determine the safety of GBCAs in children.
Because long-term effects of GBCAs are not known, they should be administered with caution and with the lowest dose necessary to answer the clinical question, and each case should be evaluated and protocoled individually. In some cases, radiologists need to review pre-contrast images in real time while the child is being scanned to determine the necessity a GBCA, rather than protocoling a study based on the clinical indication alone. For example, when assessing for osteomyelitis, if the initial T1- and T2-weighted images do not demonstrate abnormal bone marrow signal, osteomyelitis can be safely excluded without using GBCAs. Macrocyclic agents are preferred over linear agents when possible. The radiologist should always evaluate whether the administration of gadolinium would add value, especially in neonates in whom glomerular filtration rate is inherently lower, as well as in children who are typically exposed to multiple GBCA administrations (e.g., those with optic pathway gliomas, tuberous sclerosis). A recent study by Maloney et al. [76] demonstrated that changes in the pattern of enhancement in pediatric patients with optic pathway gliomas did not cause a change in management, and as such, GBCA administration might not be needed for follow-up in these pediatric patients who often undergo a significant number of MRI examinations during their lifetime. Future considerations might include alternatives to GBCAs and administration of chelating agents such as deferoxamine following GBCA injection because it was shown in at least one study to decrease gadolinium retention in the liver [61].
Conclusion
Intravenous administration of GBCAs of all classes leads to gadolinium deposition in the body in small amounts, in healthy children and adults. While no clinical long-term adverse effects have been proved, further investigation is required. In the meantime, GBCAs should be administered with caution, particularly in children, who are expected to live long after the administration and in whom organs are still developing.
References
Idée JM, Port M, Medina C et al (2008) Possible involvement of gadolinium chelates in the pathophysiology of nephrogenic systemic fibrosis: a critical review. Toxicology 248:77–88
Huckle JE, Altun E, Jay M, Semelka RC (2016) Gadolinium deposition in humans: when did we learn that gadolinium was deposited in vivo? Investig Radiol 51:236–240
Blumfield E, Moore MM, Drake MK et al (2017) Survey of gadolinium-based contrast agent utilization among the members of the Society for Pediatric Radiology: a quality and safety committee report. Pediatr Radiol 47:665–673
Sherry AD, Caravan P, Lenkinski RE (2009) Primer on gadolinium chemistry. J Magn Reson Imaging 30:1240–1248
Idée JM, Port M, Robic C et al (2009) Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J Magn Reson Imaging 30:1249–1258
Ramalho J, Semelka RC, Ramalho M et al (2016) Gadolinium-based contrast agent accumulation and toxicity: an update. AJNR Am J Neuroradiol 37:1192–1198
Grobner T, Prischl FC (2008) Patient characteristics and risk factors for nephrogenic systemic fibrosis following gadolinium exposure. Semin Dial 21:135–139
Marckmann P (2006) Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 17:2359–2362
Davenport MS, Asch D, Cavallo J et al (2017) ACR manual on contrast media version 10.3. American College of Radiology Committee on Drugs and Contrast Media. https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed 04 Oct 2018
Kanda T, Ishii K, Kawaguchi H et al (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841
White GW, Gibby WA, Tweedle MF (2006) Comparison of Gd (DTPA-BMA) (Omniscan) versus retention in human bone tissue by inductively coupled plasma mass spectroscopy. Investig Radiol 41:272–278
Tweedle MF (1992) Physicochemical properties of gadoteridol and other magnetic resonance contrast agents. Investig Radiol 27:S2–S6
Errante Y, Cirimele V, Mallio CA et al (2014) Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Investig Radiol 49:685–690
Zhang Y, Cao Y, Shih GL et al (2017) Extent of signal hyperintensity on unenhanced T1-weighted brain MR images after more than 35 administrations of linear gadolinium-based contrast agents. Radiology 282:516–525
Weberling LD, Kieslich PJ, Kickingereder P et al (2015) Increased signal intensity in the dentate nucleus on unenhanced T1-weighted images after gadobenate dimeglumine administration. Investig Radiol 50:743–748
Ramalho J, Castillo M, AlObaidy M et al (2015) High signal intensity in globus pallidus and dentate nucleus on unenhanced T1-weighted MR images: evaluation of two linear gadolinium-based contrast agents. Radiology 276:836–844
Conte G, Preda L, Cocorocchio E et al (2017) Signal intensity change on unenhanced T1-weighted images in dentate nucleus and globus pallidus after multiple administrations of gadoxetate disodium: an intraindividual comparative study. Eur Radiol 27:4372–4378
Kanda T, Osawa M, Oba H et al (2015) High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: association with linear versus macrocyclic gadolinium chelate administration. Radiology 275:803–809
Radbruch A, Weberling LD, Kieslich PJ et al (2015) Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 275:783–791
Cao Y, Huang DQ, Shih G, Prince MR (2016) Signal change in the dentate nucleus on T1-weighted MR images after multiple administrations of gadopentetate dimeglumine versus gadobutrol. AJR Am J Roentgenol 206:414–419
Bae S, Lee HJ, Han K et al (2017) Gadolinium deposition in the brain: association with various GBCAs using a generalized additive model. Eur Radiol 27:3353–3361
Radbruch A, Weberling LD, Kieslich PJ et al (2015) High-signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evaluation of the macrocyclic gadolinium-based contrast agent gadobutrol. Investig Radiol 50:805–810
Langner S, Kromrey ML, Kuehn JP et al (2017) Repeated intravenous administration of gadobutrol does not lead to increased signal intensity on unenhanced T1-weighted images — a voxel-based whole brain analysis. Eur Radiol 27:3687–3693
Kromrey ML, Liedtke KR, Ittermann T et al (2017) Intravenous injection of gadobutrol in an epidemiological study group did not lead to a difference in relative signal intensities of certain brain structures after 5 years. Eur Radiol 27:772–778
Lee JY, Park JE, Kim HS et al (2017) Up to 52 administrations of macrocyclic ionic MR contrast agent are not associated with intracranial gadolinium deposition: multifactorial analysis in 385 patients. PLoS One 12:1–15
Bjørnerud A, Vatnehol SAS, Larsson C et al (2017) Signal enhancement of the dentate nucleus at unenhanced MR imaging after very high cumulative doses of the macrocyclic gadolinium-based contrast agent gadobutrol: an observational study. Radiology 285:170391
Forslin Y, Shams S, Hashim F et al (2017) Retention of gadolinium-based contrast agents in multiple sclerosis: retrospective analysis of an 18-year longitudinal study. AJNR Am J Neuroradiol 38:1311–1316
Stojanov DA, Aracki-Trenkic A, Vojinovic S et al (2016) Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol 26:807–815
Splendiani A, Perri M, Marsecano C et al (2018) Effects of serial macrocyclic-based contrast materials gadoterate meglumine and gadobutrol administrations on gadolinium-related dentate nuclei signal increases in unenhanced T1-weighted brain: a retrospective study in 158 multiple sclerosis (MS) patients. Radiol Med 123:125–134
Gibby WA, Gibby KA, Gibby WA (2004) Comparison of Gd DTPA-BMA (Omniscan) versus Gd HP-DO3A (ProHance) retention in human bone tissue by inductively coupled plasma atomic emission spectroscopy. Investig Radiol 39:138–142
Darrah TH, Prutsman-Pfeiffer JJ, Poreda RJ et al (2009) Incorporation of excess gadolinium into human bone from medical contrast agents. Metallomics 1:479–488
Xia D, Davis RL, Crawford JA, Abraham JL (2010) Gadolinium released from MR contrast agents is deposited in brain tumors: in situ demonstration using scanning electron microscopy with energy dispersive X-ray spectroscopy. Acta Radiol 51:1126–1136
Christensen KN, Lee CU, Hanley MM et al (2011) Quantification of gadolinium in fresh skin and serum samples from patients with nephrogenic systemic fibrosis. J Am Acad Dermatol 64:91–96
McDonald RJ, McDonald JS, Kallmes DF et al (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275:772–782
Kanda T, Fukusato T, Matsuda M et al (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276:228–232
McDonald RJ, McDonald JS, Kallmes DF et al (2017) Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology 285:161595
Murata N, Gonzalez-Cuyar LF, Murata K et al (2016) Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Investig Radiol 51:447–453
McDonald JS, McDonald RJ, Jentoft ME et al (2017) Intracranial gadolinium deposition following gadodiamide-enhanced magnetic resonance imaging in pediatric patients: a case-control study. JAMA Pediatr 171:705–707
Balassy C, Roberts D, Miller SF (2015) Safety and efficacy of gadoteric acid in pediatric magnetic resonance imaging: overview of clinical trials and post-marketing studies. Pediatr Radiol 45:1831–1841
Ball WS, Nadel SN, Zimmerman RA et al (1993) Phase III multicenter clinical investigation to determine the safety and efficacy of godoteridol in children suspected of having neurologic disease. Radiology 186:769–774
Elster AD (1990) Cranial MR imaging with Gd-DTPA in neonates and young infants: preliminary experience. Radiology 176:225–230
Hahn G, Sorge I, Hirsch W et al (2009) Pharmacokinetics and safety of gadobutrol-enhanced magnetic resonance imaging in pediatric patients. Investig Radiol 44:776–783
Lundby B, Gordon P, Hugo F (1996) MRI in children given gadodiamide injection: safety and efficacy in CNS and body indications. Eur J Radiol 23:190–196
Schneider G, Schürholz H (2013) Safety and adverse effects during 24 hours after contrast-enhanced MRI with gadobenate dimeglumine (MultiHance) in children. Pediatri Radiol 43:202–211
Gale EM, Caravan P, Rao AG et al (2017) Gadolinium-based contrast agents in pediatric magnetic resonance imaging. Pediatr Radiol 47:507–521
Roberts DR, Chatterjee AR, Yazdani M et al (2016) Pediatric patients demonstrate progressive T1-weighted hyperintensity in the dentate nucleus following multiple doses of gadolinium-based contrast agent. AJNR Am J Neuroradiol 37:2340–2347
Mendichovszky IA, Marks SD, Simcock CM, Olsen ØE (2008) Gadolinium and nephrogenic systemic fibrosis: time to tighten practice. Pediatr Radiol 38:489–496
Miller JH, Hu HH, Pokorney A et al (2015) MRI brain signal intensity changes of a child during the course of 35 gadolinium contrast examinations. Pediatrics 136:e1637–e1640
Roberts DR, Holden KR (2016) Progressive increase of T1 signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain Dev 38:331–336
Hu HH, Pokorney A, Towbin RB, Miller JH (2016) Increased signal intensities in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evidence in children undergoing multiple gadolinium MRI exams. Pediatr Radiol 46:1590–1598
Kasper E, Schemuth HP, Horry S, Kinner S (2018) Changes in signal intensity in the dentate nucleus at unenhanced T1-weighted magnetic resonance imaging depending on class of previously used gadolinium-based contrast agent. Pediatr Radiol 48:686–693
Flood TF, Stence NV, Maloney JA, Mirsky DM (2017) Pediatric brain: repeated exposure to linear gadolinium-based contrast material is associated with increased signal intensity at unenhanced T1-weighted MR imaging. Radiology 282:222–228
Schneider GK, Stroeder J, Roditi G et al (2017) T1 signal measurements in pediatric brain: findings after multiple exposures to gadobenate dimeglumine for imaging of nonneurologic disease. AJNR Am J Neuroradiol 38:1799–1806
Radbruch A, Haase R, Kickingereder P et al (2017) Pediatric brain: no increased signal intensity in the dentate nucleus on unenhanced T1-weighted MR images after consecutive exposure to a macrocyclic gadolinium-based contrast agent. Radiology 283:828–836
Tibussek D, Rademacher C, Caspers J et al (2017) Gadolinium brain deposition after macrocyclic gadolinium administration: a pediatric case-control study. Radiology 285:223–230
Rossi Espagnet MC, Bernardi B, Pasquini L et al (2017) Signal intensity at unenhanced T1-weighted magnetic resonance in the globus pallidus and dentate nucleus after serial administrations of a macrocyclic gadolinium-based contrast agent in children. Pediatr Radiol 47:1345–1352
Radbruch A, Quattrocchi CC (2017) Interpreting signal-intensity ratios without visible T1 hyperintensities in clinical gadolinium retention studies. Pediatr Radiol 47:1688–1689
Rossi-Espagnet MC, Tomà P, Napolitano A (2017) Reply to Radbruch et al.: ‘interpreting signal-intensity ratios without visible T1 hyperintensities in clinical gadolinium retention studies.’ Pediatr Radiol 47:1690–1691
Tamrazi B, Nguyen B, C-SJ L et al (2017) Changes in signal intensity of the dentate nucleus and globus pallidus in pediatric patients: impact of brain irradiation and presence of primary brain tumors independent of linear gadolinium-based contrast agent administration. Radiology 287:171850
Mithal LB, Patel PS, Mithal D et al (2017) Use of gadolinium-based magnetic resonance imaging contrast agents and awareness of brain gadolinium deposition among pediatric providers in North America. Pediatr Radiol 47:657–664
Maximova N, Gregori M, Zennaro F et al (2016) Hepatic gadolinium deposition and reversibility after contrast agent-enhanced MR imaging of pediatric hematopoietic stem cell transplant recipients. Radiology 281:418–426
Roberts DR, Welsh CA, LeBel II DP, Davis WC (2017) Distribution map of gadolinium deposition within the cerebellum following GBCA administration. Neurology 88:1206–1208
McDonald RJ, McDonald JS, Dai D et al (2017) Comparison of gadolinium concentrations within multiple rat organs after intravenous administration of linear versus macrocyclic gadolinium chelates. Radiology 285:161594
Gianolio E, Bardini P, Arena F et al (2017) Gadolinium retention in the rat brain: assessment of the amounts of insoluble gadolinium-containing species and intact gadolinium complexes after repeated administration of gadolinium-based contrast agents. Radiology 285:839–849
Frenzel T, Apte C, Jost G et al (2017) Quantification and assessment of the chemical form of residual gadolinium in the brain after repeated administration of gadolinium-based contrast agents: comparative study in rats. Investig Radiol 52:396–404
Kartamihardja AAP, Nakajima T, Kameo S et al (2016) Distribution and clearance of retained gadolinium in the brain: differences between linear and macrocyclic gadolinium based contrast agents in a mouse model. Br J Radiol 89:20160509
Wedeking P, Kumar K, Tweedle MF (1992) Dissociation of gadolinium chelates in mice: relationship to chemical characteristics. Magn Reson Imaging 10:641–648
Bussi S, Coppo A, Botteron C et al (2018) Differences in gadolinium retention after repeated injections of macrocyclic MR contrast agents to rats. J Magn Reson Imaging 47:746–752
Ramalho M, Ramalho J, Burke LM, Semelka RC (2017) Gadolinium retention and toxicity — an update. Adv Chronic Kidney Dis 24:138–146
Murata N, Murata K, Gonzalez-Cuyar LF, Maravilla KR (2016) Gadolinium tissue deposition in brain and bone. Magn Reson Imaging 34:1359–1365
Ramalho J, Ramalho M (2017) Gadolinium deposition and chronic toxicity. Magn Reson Imaging Clin N Am 25:765–778
Semelka RC, Ramalho M, AlObaidy M, Ramalho J (2016) Gadolinium in humans: a family of disorders. AJR Am J Roentgenol 207:229–233
RSNA Daily Bulletin (2017) No evidence gadolinium causes neurologic harm. Radiological Society of North America. https://rsna2017.rsna.org/dailybulletin/index.cfm?pg=17fri10. Accessed 04 Oct 2018
Goske MJ, Applegate KE, Boylan J et al (2008) The image gently campaign: working together to change practice. AJR Am J Roentgenol 190:273–274
Scheinfeld MH, Moon JY, Fagan MJ et al (2017) MRI usage in a pediatric emergency department: an analysis of usage and usage trends over 5 years. Pediatr Radiol 47:327–332
Maloney E, Stanescu AL, Perez FA et al (2018) Surveillance magnetic resonance imaging for isolated optic pathway gliomas: is gadolinium necessary? Pediatr Radiol 48:1472–1484
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Blumfield, E., Swenson, D.W., Iyer, R.S. et al. Gadolinium-based contrast agents — review of recent literature on magnetic resonance imaging signal intensity changes and tissue deposits, with emphasis on pediatric patients. Pediatr Radiol 49, 448–457 (2019). https://doi.org/10.1007/s00247-018-4304-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-018-4304-8