Abstract
Background
Recent reports have suggested residual gadolinium deposition in the brain in subjects undergoing multiple contrast-enhanced MRI exams. These findings have raised some concerns regarding gadolinium-based contrast agent (GBCA) usage and retention in brain tissues.
Objective
To summarize findings of hyperintense brain structures on precontrast T1-weighted images in 21 children undergoing multiple GBCA MRI exams.
Materials and methods
This retrospective study involved 21 patients, each of whom received multiple MRI examinations (range: 5-37 exams) with GBCA over the course of their medical treatment (duration from first to most recent exam: 1.2-12.9 years). The patients were between 0.9 and 14.4 years of age at the time of their first GBCA exam. Regions of interest were drawn in the dentate nucleus and the globus pallidus on 2-D fast spin echo images acquired at 1.5 T. The signal intensities of these two structures were normalized by that of the corpus callosum genu. Signal intensity ratios from these patients were compared to control patients of similar ages who have never received GBCA.
Results
Signal intensity ratios increased between the first and the most recent MRI exam in all 21 patients receiving GBCA, with an increase of 18.6%±12.7% (range: 0.5% to 47.5%) for the dentate nucleus and 12.4%±7.4% (range: -1.2% to 33.7%) for the globus pallidus (P<0.0001). Signal intensity ratios were also higher in GBCA patients than in controls (P<0.01). The degree of signal intensity enhancement did not correlate with statistical significance to the cumulative number or volume of GBCA administrations each patient received, the patient’s age or the elapsed time between the first and most recent GBCA MRI exams.
Conclusion
These results in children are consistent with recent findings in adults, suggesting possible gadolinium deposition in the brain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past year, multiple independent reports have described similar observations of hyperintense brain structures in adults, namely in the dentate nucleus and the globus pallidus, on precontrast unenhanced T1-weighted images [1, 2]. Many of these patients had normal renal function and exhibited no contraindications toward receiving gadolinium contrast. It has been postulated that the apparent increases in signal intensity observed in these brain structures are likely due to the residual deposition of gadolinium (Gd)-based contrast agents (GBCA) from repeated prior administrations. Kanda et al. [3], Quattrocchi et al. [4], Errante et al. [5] and Adin et al. [6] have demonstrated that the progressive increases in signal intensities were statistically correlated with the cumulative number of GBCA administrations a patient received. McDonald et al. [7] and Kanda et al. [8] have provided additional histological evidence in postmortem specimen studies of intracranial GBCA deposition and bio-distribution using mass spectrometry and electron microscopy. Radbruch et al. [9], Kanda et al. [10] and Ramalho et al. [11] have further provided results suggesting that GBCAs with linear molecular structures were the source of the residual deposition and consequent hyperintensity. However, similar findings with macrocyclic GBCAs are also emerging, as described by Stojanov et al. [12].
A few animal studies further support the image-based observations reported in human adults. Robert et al. [13] provided evidence in healthy rats that serial administration of linear GBCA was associated with progressive and persistent T1 signal hyperintensity in the deep cerebellar nuclei, in contrast to minimal effects with repeat macrocyclic GBCA administration [13]. Wáng et al. [14] performed an extensive study in rats and found considerable gadolinium retention in the skin, the femur and the liver of animals who were administered linear versus macrocyclic GBCAs. While a majority of these aforementioned studies have involved animal and human adults, limited data in the current literature exist to support whether similar observations also manifest in children. Roberts et al. [15] recently reported supportive findings in the dentate nucleus and globus pallidus from a case study involving a 13-year-old girl who received six MRI exams with a linear GBCA [15]. Miller et al. [16] similarly described a case of a patient who received 35 GBCA administrations over the course of 12 years. The work by Adin et al. [6], which included a total of 184 patients, involved 20 subjects younger than the age of 18 years who exhibited hyperintense dentate nucleus, findings that were similar to those in adults from the study’s cohort. We provide additional imaging evidence and summarize our complementary findings of hyperintense signal intensities in the dentate nucleus and the globus pallidus in 21 children. The patient previously described by our group was not included in this study [16].
Materials and methods
This retrospective, HIPAA-compliant, image review study was approved by the local institutional review board and informed consent was waived. Table 1 summarizes the study cohort of GBCA patients, including gender, age at the time of the patient’s first GBCA MRI exam, the reason for the initial exam, the total number and volume of GBCA administrations the patient has received to date, and the time between the first and the most recent GBCA MRI exam. Table 2 summarizes 21 control patients of similar ages who have not previously received GBCA. They will be subsequently referred to as controls.
Precontrast (i.e. unenhanced) axial 2-D multi-slice nonfat/fluid-suppressed fast spin echo T1-weighted MRI data acquired with standard head coil receiver arrays on two 1.5-T scanners (Intera and Achieva platforms; Philips Healthcare, Best, The Netherlands) were reviewed in this study. All GBCA patients from Table 1 had normal renal function test results prior to each MRI exam and exhibited no contraindications to receiving GBCA. For each of their MRI exams, all patients in Table 1 were intravenously administered GBCA (gadopentetate dimeglumine; Magnevist; Bayer Healthcare Pharmaceuticals, Whippany, NJ) at a standard dose of 0.1 mmol/kg. Typical imaging parameters were an in-plane pixel size of 0.8-1.0 mm, 5-mm slice thickness, 20-30 slices, a TR range of 450-600 ms, and TE range of 10-15 ms.
Region of interests (ROIs) were manually drawn by a pediatric neuroradiologist (J.H.M.) with 10 years of clinical experience using Osirix MD 64-bit software. ROIs were individually placed in the left and right dentate nucleus and the left and right globus pallidus to obtain mean signal intensities. The ROI procedure was adopted from previous methodological approaches described by Kanda et al. [3], McDonald et al. [7] and Stojanov et al. [12]. These mean values were then normalized by the mean signal intensity of the corpus callosum genu, a structure that did not exhibit any visible signal intensity enhancement features. The ROI measurement procedure was performed on images from every GBCA exam for the patients in Table 1 and in the baseline images from every control in Table 2. For each GBCA patient in Table 1, the reviewing neuroradiologist was also asked to record the first exam number where either hyperintense dentate nucleus or globus pallidus became visually evident (Table 1).
STATA software (version 13; StataCorp LP, College Station, Texas) was used to characterize trends in the measured dentate nucleus and globus pallidus ratios. Statistical significance was set a priori to a P-value less than 0.05. Similar to the analysis carried out by Radbruch et al. [9], a one-sample t-test was used to examine whether the difference in dentate nucleus and globus pallidus ratios between the most recent and the first GBCA examination in each GBCA patient was different from 0 (i.e. no signal intensity enhancement trend). Since several recent studies have further investigated signal intensity ratios between patients who have received six or more GBCA administrations and those who have received five or fewer [4–6, 9], we similarly applied the one-sample t-test to results from each GBCA patient’s first and fifth examinations to assess potential statistical significant increases in signal intensity ratios. A two-sample t-test and a Mann Whitney U-test were used to compare signal intensity ratios between the GBCA patients in Table 1 and controls in Table 2.
Correlation analyses using Spearman rank test were performed to determine whether any trends existed between the measured differences in dentate nucleus and globus pallidus ratios and the total number of GBCA MRI examinations and the total GBCA volume a patient had received. The age of each patient at their first GBCA exam and the elapsed time between the first and most recent GBCA exam were also considered.
Results
Overall, the percent changes in the signal intensity ratios of the dentate nucleus and globus pallidus between the first and most recent GBCA exam were 20.3%±13.2% (range: 4.4% to 47.5%) and 17.0%±12.4% (range: 0.5% to 45.7%) for the right and left dentate nucleus, respectively, and 12.6%±7.9% (range: -1.2% to 33.7%) and 12.1%±7.1% (0.2% to 25.2%) for the right and left globus pallidus, respectively. There were no statistically significant differences in enhancement patterns between the left and right structures. The absolute change in signal intensity ratios for the right and left dentate nucleus combined was 0.16±0.11 (range: 0.0 to 0.4) and 0.12±0.07 (range: -0.01 to 0.33) for the globus pallidus. For the dentate nucleus ratio difference, the one-sample t-test yielded a P-value less than 0.0001, with a 95% confidence interval (CI) 0.13, 0.19. For the globus pallidus ratio difference, the one-sample t-test also yielded a similar P-value less than 0.0001 and a 95% CI 0.10, 0.14. Note that the confidence intervals of the change of both ratios are positive and do not contain the value of zero. From Table 1, note from the seventh column that hyperintensity in either the dentate nucleus or the globus pallidus was visually noticed within the first 10 GBCA exams in all patients.
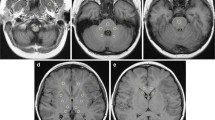
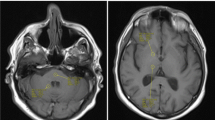
Figures 1 and 2 illustrate representative examples from two girls who each received more than 20 total administrations of GCBA to date, and where hyperintensity in the dentate nucleus and globus pallidus in contrast to surrounding tissues was visually prominent. Figure 3 illustrates an interesting example from GBCA patient 20, a girl who has to date received only five GBCA exams but already exhibits hyperintensity in the dentate nucleus on the second exam. Within our cohort, patient 20 also has one of the longest time gaps between two successive GBCA exams, at nearly 4.4 years. The images in Fig. 3 show that hyperintensity in the dentate nucleus persists during the lengthy time span between the third and fourth GBCA exams. According to Table 1, hyperintense structures were already noticed by the evaluating neuroradiologist on her second GBCA exam. Figure 4 illustrates a representative example from GBCA patient 12, showing the time course of the signal intensity ratios as a function of elapsed time (and GBCA administrations), similar to the approach shown by Miller et al. [16]. A gradual increase in the signal intensity ratio within the dentate nucleus and globus pallidus can be observed.
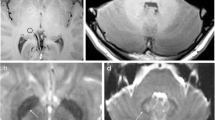
Representative precontrast unenhanced T1-weighted 2-D fast spin echo images from gadolinium-based contrast agent (GBCA) patient 9 (female) (a, c) show comparison images from the patient’s first GBCA MRI examination, at the age of 3.9 years and images (b, d) from the patient’s most recent (23rd) GBCA MRI examination, approximately 6.7 years later at the age of 10.6 years. Image appearances of the dentate nucleus (solid arrows) and globus pallidus (dashed arrows) structures are typically isointense with surrounding gray and white matter at the baseline and become more prominent with repeated GBCA administration
Representative precontrast unenhanced T1-weighted 2-D fast spin echo images from gadolinium-based contrast agent (GBCA) patient 19 (female) illustrate data from (a) the 1st GBCA MRI examination (day 1, age 14.4 years), (b) the 5th (day 6, age 14.4 years), (c) the 11th (day 574, age 16.0 years), (d) 20th (day 1353, age 18.1 years) and (e) the 26th and most recent (day 2494, age 21.2 years). Hyperintensity in the dentate nucleus (solid arrows) is visually evident on the (c) 11th, (d) 20th and (e) 26th images. The inset in (c) shows a zoomed region of the dentate nucleus, displayed at a narrower gray-scale window/level. Subtle signal intensity enhancement in the dentate nucleus can be appreciated 19 months after the initial GBCA MRI exam
Representative precontrast unenhanced T1-weighted 2-D fast spin echo images from gadolinium-based contrast agent (GBCA) patient 20 (female) illustrate data from (a) the 3rd (day 833, age 14.0 years) and (b) 4th (day 2444, age 18.4 years) GBCA MRI examinations. Among the study cohort, these two time points represent the longest separation in time between two consecutive GBCA administrations. Hyperintensity in the dentate nucleus (solid arrows) is visually evident in (a) and persists in (b) after nearly 4.4 years
Figure 5 compares the group average signal intensity ratios of the dentate nucleus and globus pallidus between the controls and the GBCA patients. For the GBCA patients, the ratios are plotted at their first exam, their fifth exam and their most recent GBCA exam. There were no statistically significant differences between the signal intensity ratios for both structures between the controls and the GBCA patients at their first exam (P=0.77 for dentate nucleus; P=0.19 for globus pallidus). For the dentate nucleus, the ratio distribution was 0.93±0.03 (range: 0.88 to 0.99) in the controls and 0.90±0.05 (range: 0.84 to 1.01) in the GBCA patients. For the globus pallidus, the ratio distribution was 0.98±0.04 (range: 0.87 to 1.04) in the controls and 0.99±0.04 (range: 0.92 to 1.06) in the GBCA patients. At the fifth examination for the GBCA patient group, the dentate nucleus signal intensity ratio distribution was 0.96±0.07 (range: 0.84 to 1.09) while that of the globus pallidus was 1.05±0.06 (range: 0.94 to 1.18). Comparing these values to the signal intensity ratios of these patients at their first GBCA exam yields statistically significant results for both structures (P<0.01). Namely, the absolute change in signal intensity ratio for the dentate nucleus was 0.06±0.08 and 0.05±0.07 for the globus pallidus. For the dentate nucleus ratio difference, the one-sample t-test yielded a 95% CI 0.04, 0.06. For the globus pallidus ratio difference, the 95% CI was 0.03, 0.05.
At the most recent examination for the GBCA patient group, the dentate nucleus signal intensity ratio distribution was 1.07±0.08 (range: 0.93 to 1.22) while that of the globus pallidus was 1.12±0.06 (range: 1.02 to 1.28). Comparing these values to the signal intensity ratios at the previous fifth GBCA exam yielded similarly statistically significant results for both structures (P<0.01). The absolute change in signal intensity ratio for the dentate nucleus was 0.15±0.24 and 0.12±0.24 for the globus pallidus. The 95% CI were 0.08, 0.22 and 0.05, 0.19 for the dentate nucleus and globus pallidus, respectively.
Lastly, from the Spearman rank correlation analysis, our current data did not suggest a statistically significant trend between signal intensity ratios versus the number of GBCA doses received or the total volume of GBCA administered. Changing the dependent variable to the age of each patient at their first GBCA exam and the elapsed time between the first and most recent GBCA exams also did not yield any statistically significant correlations.
Discussion
In this work, we have provided additional MRI-based data in a cohort of 21 children demonstrating hyperintense dentate nucleus and globus pallidus structures on pre-gadolinium unenhanced T1-weighted MR images. Our findings exhibit trends similar to existing literature data in adults and represent a unique contribution as it involved children who received their first GBCA MRI exam at a very young age (average: 7.5±4.3 years). Furthermore, these patients also received a significant number of subsequent GBCA MRI exams (range: 5-37 exams) in a relatively short span of time (average: 7.1±3.7 years). To the best of our knowledge, patients in our study cohort did not have any previous exposure to GBCA at another institution prior to their first exam at our hospital. However, we cannot guarantee this with absolute certainty and recognize the potential confounder of prolonged GBCA retention from prior applications. In our GBCA cohort, the absolute changes in signal intensity ratios 0.16±0.11 (for dentate nucleus) and 0.12±0.07 (for globus pallidus) were similar to the numbers previously reported in Fig. 2 of the study by Radbruch et al. [9].
Existing literature reports to date have largely involved adults. Kanda et al. [3] studied a cohort of 381 patients (229 men, 152 women) with an average age of 67.4±11.2 years. Quattrocchi et al. [4] studied 46 meningioma patients (6 men, 40 women; age range: 46-87 years). Errante et al. [5] studied 38 patients (11 men, 27 women; age: 47±15 years) with multiple sclerosis and 37 patients (19 men, 18 women; age: 62±13 years) with brain metastases [5]. McDonald et al. [7] studied 13 postmortem subjects with an average age distribution of 52.4±13.8 years. Radbruch et al. [9] studied 100 patients (57 men, 43 women) in two groups, with an average age of 50±14.1 years for those receiving a macrocyclic GBCA and 46.8±15.2 years for those receiving a linear GBCA, respectively [9]. Stojanov et al. [12] studied 58 patients (21 men, 37 women) with an average age of 42.4±8.1 years. Most recently, Ramalho et al. [11] studied 69 patients (26 men, 43 women) in two groups with an age distribution of 51.1±14.9 years and 60.2±13.9, respectively, with the former group containing subjects as young as 17 years. Roberts et al. [15] detailed one of the youngest patients so far published in the literature, a 13-year-old girl. Similarly, the case report by Miller et al. [16] describes an 8-year-old patient who received 35 GBCA exams over the course of 12 years. The study by Adin et al. [6] involved 184 patients (101 males, 83 females) with an average age of 43.3±16.8 years, although the age range spanned from 3 to 73 years and included 20 patients under the age of 18 years.
Our current study included mostly children (19 out of 21) who received more than six serial GBCA MRI exams. This selection was motivated by the previous results of Errante et al. [5], who demonstrated that the dentate nucleus-to-pons ratio between the first and last GBCA MRI exams were significantly different in patients who received more than six GBCA administrations, whereas the difference in ratio was not statistically different in patients who received fewer than six GBCA administrations. Quattrocchi et al. [4] reported non-statistically significant differences in the dentate nucleus-to-pons ratio in patients who had received one to five GBCA administrations, while statistically significant signal intensity enhancement was observed in patients who had received more than six GBCA MRI exams. Adin et al. [6] also demonstrated a greater likelihood of signal intensity enhancement in the dentate nucleus in patients with a history of more than six GBCA administrations. The hyperintensity effect was most noticeable in the 92 patients who had received 12 to 60 GBCA MRI exams. The study further showed no evidence of hyperintense dentate nucleus in 53 control subjects (41.9±11.8 years) who have never received GBCA. Radbruch et al. [9] illustrated more visually apparent hyperintensity in the dentate nucleus after the sixth administration in patients receiving a linear GBCA than in those receiving a macrocyclic GBCA. However, as illustrated in Table 1 and Fig. 3, our results in children suggest that hyperintensity in the dentate nucleus and globus pallidus can manifest after only a few GBCA administrations. We believe this finding in children is the most important contribution of our work.
A variety of GBCAs have been evaluated in the literature. Kanda et al. [3] described their results in patients who received gadodiamide (Omniscan, linear nonionic) or gadopentetate dimeglumine (Magnevist, linear ionic). Similarly, the postmortem study by McDonald et al. [7] was performed in patients who were administered Omniscan. The studies by Quattrocchi et al. [4] and Errante et al. [5] also involved Omniscan. Radbruch et al. [9] investigated the influences of gadoterate meglumine (Dotarem, macrocyclic ionic) and Magnevist and reported significant signal intensity effects with the latter. This differential finding between linear and macrocyclic GBCA was further supported by two extensive animal studies in rats by Robert et al. [13] and Wáng et al. [14] comparing Omniscan and Dotarem. Stojanov et al. [12] has reported visual evidence of GBCA deposition in the brain with gadobutrol (Gadavist, macrocyclic nonionic). Ramalho et al. [11] reported their comparison results in patients who either received either gadodiamide or gadobenate dimeglumine (MultiHance, linear ionic) and noted greater signal intensity effects with the former nonionic GBCA. The recent study by Adin et al. [6] primarily involved Magnevist, but also included a small proportion of cases involving Omniscan, MultiHance, gadoteridol (ProHance, macrocyclic nonionic), gadoversetamide (OptiMARK, linear nonionic), and Gadavist. Our current study and that of Roberts et al. [5] in children have further assessed the effects of Magnevist.
Although previous studies have frequently used the thalamus and the pons as the structures to normalize the signal intensities of the globus pallidus and dentate nucleus, respectively, in order to account for intrasubject and intersubject variations in T1 tissue signal contrast weighting due to magnetic field strength as well as TR and TE settings, we opted in this work to utilize a common value from the corpus callosum genu to compute the signal intensity ratios. The selection of a particular normalizing structure is arbitrary, as long as the selected tissue does not show any GBCA-related enhancement. For example, Ramalho et al. [11] used the middle cerebellar peduncle to normalize the signal intensity of the dentate nucleus. McDonald et al. [7] employed the signal intensity of cerebrospinal fluid to account for intra- and interpulse sequence variations. Radbruch et al. [9] have also used signal intensity from the cerebrospinal fluid and cerebellum as additional validators. Miller et al. [16] utilized both the corpus callosum genu and the temporalis muscle, positing that the thalamus and pons may also be subjected to GBCA retention and hyperintense signals. It is worth mentioning that in all of the ratio-based approaches described in the literature so far, including in this study, potential confounding effects from non-uniform radiofrequency transmit and coil receiver fields that cause signal intensity shading across the image are not considered or addressed. However, these effects are likely minimal at 1.5 T, given the collective visual evidence of hyperintense structures presented so far in literature.
In contrast to predominant findings in adult studies, our current pediatric data, when considered as a group, did not yield any statistically significant correlations between the number of GBCA administrations and the degree of dentate nucleus and globus pallidus signal intensity enhancement nor were there any notable trends when the age at which each patient first received GBCA administration, the GBCA dose (i.e. total volume), or the duration between their first and most recent GBCA were considered. Our lack of these particular findings in children does not preclude their potential existence and is possibly a reflection of our limited cohort size. Another drawback of our study is that we did not investigate and compare signal intensity enhancement effects from a matching patient group that had received serial macrocyclic GBCA administrations. Such assessment has not yet been reported in children and is a direction of future work at our institution. Although our study included controls who were similar in age to the GBCA patients at their first MRI exam, a more appropriate choice would have been to age-match the controls to the age of the GBCA patients at their most recent MRI exam. We do not believe the different choice in controls will significantly alter the study’s findings. Although our current study did not match for gender, current evidence in the literature does not suggest dependence between males and females. Finally, histological analyses will likely continue to serve as the ground truth in elucidating the source of these hyperintense brain structures observed in imaging data. While histological analyses have been implemented in rodent studies and in the reports involving adults by McDonald et al. [7] and Kanda et al. [8], similar results have not been reported in children and are impractical.
Our retrospective image-review-based findings parallel existing observations in adult and animal investigations. Collectively, while these findings, particularly in children, should raise concerns for safety regarding GBCA usage among all health care practitioners, additional studies are needed to determine the significance, the risk-to-benefit ratios, the potential adverse health effects, the potential impact on practice of care, and the long-term effects, if any, of intracranial GBCA deposition [17–19]. Nevertheless, our findings are supplemental to the studies presented by Adin et al. [6], Roberts et al. [15] and Miller et al. [16] that have involved children, and support the notion of possibly adopting a subset of GBCAs (e.g., macrocyclic) with higher stability as the standard of care in children [20–22].
Conclusion
In this preliminary work, we have provided data in 21 children that support the growing evidence of potential gadolinium deposition in the brain. In our pediatric patients who have received multiple administrations of gadolinium-based contrast agents, the observation of signal intensity increases in the dentate nucleus and the globus pallidus on unenhanced T1-weighted MR images are consistent with prior studies in adults. Additional studies are warranted to determine whether intracranial gadolinium deposition is the source responsible for these hyperintense structures and whether changes in standard practice of care are needed.
References
Karabulut N (2015) Gadolinium deposition in the brain: another concern regarding gadolinium-based contrast agents. Diagn Interv Radiol 21:269–270
Kanda T, Oba H, Toyoda K (2016) Recent advances in understanding gadolinium retention in the brain. AJNR Am J Neuroradiol 37:E1–E2
Kanda T, Ishii K, Kawaguchi H et al (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841
Quattrocchi CC, Mallio CA, Errante Y et al (2015) Gadodiamide and dentate nucleus T1 hyperintensity in patients with meningioma evaluated by multiple follow-up contrast-enhanced magnetic resonance examinations with no systemic interval therapy. Invest Radiol 50:470–472
Errante Y, Cirimele V, Mallio CA et al (2014) Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol 49:685–690
Adin ME, Kleinberg L, Vaidya D et al (2015) Hyperintense dentate nuclei on T1-weighted MRI: relation to repeat Gadolinium administration. AJNR Am J Neuroradiol 36:1859–1865
McDonald RJ, McDonald JS, Kallmes DF et al (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275:772–782
Kanda T, Fukusato T, Matsuda M et al (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276:228–232
Radbruch A, Weberling LD, Kieslich PJ et al (2015) Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 275:783–791
Kanda T, Osawa M, Oba H et al (2015) High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: association with linear versus macrocyclic gadolinium chelate administration. Radiology 275:803–809
Ramalho J, Castillo M, AlOBaidy M et al (2015) High signal intensity in globus pallidus and dentate nucleus on unenhanced T1-weighted MR images: evaluation of two linear Gadolinium-based contrast agents. Radiology 276:836–844
Stojanov DA, Aracki-Trenkic A, Vojinovic S et al (2015) Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, Gadobutrol. Eur Radiol 26:807–815
Robert P, Lehericy S, Grand S et al (2015) T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of Gadolinium-based contrast agents in healthy rats: difference between linear and macrocyclic agents. Invest Radiol 50:473–480
Wáng Y, Schroeder J, Siegmund H et al (2015) Total gadolinium tissue deposition and skin structural findings following the administration of structurally different gadolinium chelates in healthy and ovariectomized female rats. Quant Imaging Med Surg 5:534–545
Roberts DR, Holden KR (2015) Progressive increase of T1 signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain Dev 38:331–336
Miller JH, Hu HH, Pokorney A et al (2015) MRI brain signal intensity changes of a child during the course of 35 Gadolinium contrast examinations. Pediatrics 136:e1637–e1640
Sato T, Tamada T, Watanabe S et al (2015) Tissue gadolinium deposition in hepatorenally impaired rats exposed to Gd-EOB-DTPA: evaluation with inductively coupled plasma mass spectrometry (ICP-MS). Radiol Med 120:557–562
Huckle JE, Atlun E, Jay M et al (2016) Gadolinium deposition in humans: when did we learn that gadolinium was deposited in vivo? Invest Radiol 51:236–240
Reeder SB, Gulani V (2016) Gadolinium deposition in the brain: do we know enough to change practice? Radiology 279:323–326
Malayeri AA, Brooks KM, Bryant LH et al (2016) National Institutes of Health perspective on reports of gadolinium deposition in the brain. J Am Coll Radiol 13:237–241
Radbruch A, Weberling LD, Kieslich PJ et al (2015) High-signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evaluation of the macrocyclic gadolinium-based contrast agent Gadobutrol. Invest Radiol 50:805–810
Murata N, Gonzalez-Cuyar LF, Murata K et al (2016) Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Invest Radiol. doi:10.1097/RLI.0000000000000252
Acknowledgments
The Department of Medical Imaging and Radiology at Phoenix Children’s Hospital acknowledges research support from Philips Healthcare. The authors also thank librarian Kathy Zeblisky, MLS, of Phoenix Children’s Hospital for literature reference assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Hu, H.H., Pokorney, A., Towbin, R.B. et al. Increased signal intensities in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evidence in children undergoing multiple gadolinium MRI exams. Pediatr Radiol 46, 1590–1598 (2016). https://doi.org/10.1007/s00247-016-3646-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-016-3646-3